Muhammad Nabil1,2 , Azman Seeni1
, Azman Seeni1 , Wan Ismahanisa Ismail2
, Wan Ismahanisa Ismail2 , Nurhidayah Ab. Rahim2
, Nurhidayah Ab. Rahim2  and Syarifah Masyitah Habib Dzulkarnain2
and Syarifah Masyitah Habib Dzulkarnain2
1Advanced Medical and Dental Institute, Universiti Sains Malaysia, 13200 Kepala Batas, Pulau Pinang, Malaysia
2Faculty of Health Science, Universiti Teknologi MARA, Cawangan Pulau Pinang, Kampus Bertam, 13200 Kepala Batas, Pulau Pinang, Malaysia
Corresponding Author E-mail: nabilfikrimail@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1796
Abstract
Cervical cancer has been ranked as the third most common cancer among women worldwide. As an alternative to existing preventive and treatment measures, natural plants have been seen to carry potential therapeutic value against cancers. These include Streblus asper which proved to possess anti-cancer effects on several types of cancer. In the present study, we observed that S.asper is able to induce apoptosis on cervical cancer cells through the regulation of several apoptotic proteins. This analysis was performed using both in vitro and in vivo models. The protein expression was analysed using antibody array, 28 protein markers were found differentially expressed in both study models. Based on these findings, we propose that S.asper induces apoptosis on cervical cancer cells through TNF signaling which in turn triggers the activation of SMAC pathway and blockage of NF-κB cascade. It is also suggested that the apoptosis inducement is assisted by the HSP60 downregulation which subsequently results in p53 activation and survivin down-expression. Our study provides a preliminary understanding on selective apoptotic mechanism induced by S.asper on cervical cancer.
Keywords
Anti-cancer; Apoptosis; Cervical cancer; Natural plant; Streblus asper; Tumour Necrosis Factor
Download this article as:| Copy the following to cite this article: Nabil M, Seeni A, Ismail W. I, Rahim Ab, Dzulkarnain S. M. H. Induction of Apoptotic Mechanism by Streblus Asper Root Extract on Cervical Cancer Using in Vitro and in Vivo Models. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Nabil M, Seeni A, Ismail W. I, Rahim Ab, Dzulkarnain S. M. H. Induction of Apoptotic Mechanism by Streblus Asper Root Extract on Cervical Cancer Using in Vitro and in Vivo Models. Biomed Pharmacol J 2019;12(4). Available from: http://biomedpharmajournal.org/?p=29768 |
Introduction
On average, about 570,000 new cases of cervical cancer have been reported globally. It is the third most common cancer affecting women in Malaysia, after breast and colon cancers.1 Early sexual activity, multiple sexual partners, having the history of genital warts, smoking of cigarettes, being infected with Human immunodeficiency Virus (HIV) and being exposed with immunosuppressive agents have been determined to be the risk factors for cervical cancer.2 Nevertheless, the primary risk factors for cervical carcinogenesis are mostly infections of Human papillomavirus (HPV) type 16 and 18. These types of HPV infections were found in most of the invasive cases.3 The preventive measure that has been practised in accordance with this primary risk factor is vaccination. HPV vaccination has been reported to contribute to a risk reduction of 46 per cent for histologically confirmed high-grade cervical abnormalities and 34 per cent for other cervical abnormalities among young women.4 Despite its success rate, vaccination is still not widely practised, and study found that the significant barriers that limit its coverage for high-risk adolescent group include lack of parents’ knowledge about its safety, unawareness of parents on the requirement for multiple doses, and low perceived risk of HPV infection.5 Another preventive measure for cervical cancer is the screening of pre-cancerous lesions through pap smear test. Pap smear has been used increasingly in developing countries as a screening tool for early cancer detection. Reports showed that the use of pap smear has contributed to a reduction of mortality rates due to cervical cancer in women above 30-year old.6 However, cervical cancer screening is practised only by certain groups of society who perceive adequate knowledge and awareness about the importance of it.7 Prevention against cervical cancer would unlikely be effective until necessary steps are taken to address all the barriers identified. A study had elucidated that the success in preventing cervical cancer in developing countries lies in the intervention against multiple obstacles.8
In recent decades, natural product has been portrayed as a potential substitute for existing strategies for cervical cancers. It has been increasingly attracted researcher’s attention across the globe. This shift in paradigm has to date resulted in many natural products that are able to significantly suppress cervical cancer growth have been documented. The inhibition of cervical cancer is commonly evaluated based on its significant responses on common related pathways such as proliferation, apoptosis, cell cycle, and metastasis. For instance, Zhang et al. (2011) proves that black raspberry crude extract significantly triggers apoptosis in cervical cancer cell lines such as HeLa, SiHa, and C-33A.9 The similar inhibitory ability has been seen in Kaffir lime leaf extract, Artocarpus artilis pulp extract, Bryophyllum pinnata leaf extract, Pleurotus tuberregium extract, Thymus kotschyanus extract, and Coryllus avellana L. extracts.10,11,12,13,14,15 However, most of these plants are not locally available. Therefore, we explored the potential of our prospect local plant known as Streblus asper to induce apoptosis on cervical cancer through regulation of related key proteins using in vitro and in vivo models.
Materials and Methods
Plant Extract
Streblus asper plant was purchased from a nursery in Tasek Gelugor, Penang, Malaysia. The plant was authenticated by Associate Professor Dr. Md. Azman Bin Pkm Seeni, (Malaysian Institute of Pharmaceuticals and Neutraceuticals). The roots of the plant were cut into pieces of a minimum of 2 mm size and washed with distilled water and was left to dry in a room for 2 weeks. The roots were ground into powders using Retstch SM 100 grinder. Ground powder samples were boiled with distilled water for 30 minutes and the solution was filtered using 0.75 mm filter size. This procedure was repeated 3 times and filtrate was then freeze-dried. Upon usage, the powder’s weight was measured using analytical balance and diluted with double deionized distilled water according to the need.
Cell Culture
The HeLa cells used in this study were sub-cultured from ATCC with Catalog No. CCL-2 TM purchased from ATCC, Manasus, VA, USA. The cells were maintained in DMEM supplemented with 10% FBS, 1% sodium pyruvate and 1% penicillin-streptomycin. These were purchased from Life Technologies, USA. Cells were incubated in 37°C humidified CO2 incubator, with 5% CO2 and 95% of air. The medium was replaced every 72 hours until it reached 80%-90% of cell confluency before sub-culturing was carried out. The sub-culturing of cells was limited up to ten passages.
Cell Proliferation Assay
After HeLa cells were cultured accordingly and had reached 80-90% of cells confluency, they were detached using 0.25% trypsin/EDTA. The old medium was removed and replaced. To seed cells in 96-well culture plate, a total number of 1×103 cells were pipetted with 100 µl of complete medium in each well. After 24 hours, HeLa cells were treated with varying concentration (0.01 mg/ml, 0.10 mg/ml, 0.15 mg/ml, 0.20 mg/ml, and 0.25 mg/ml) of S.asper root extracts. The cells were incubated in 37°C humidified CO2 incubator, supplemented with 5% CO2 and 95% of air for 72 hours. After 72 hours of treatment, the AlamarBlue® assay was performed according to the manufacturer’s guidelines. The AlamarBlue® solution was prewarmed in 37°C water bath prior to use. All wells including control were prepared in triplicates. The control wells provided the background activity of the AlamarBlue® inside the medium. The plates were incubated for 4 hours at 37°C in a humidified, 5% CO2 atmosphere incubator. Fluorescence (excitation 530 nm, emission 590 nm) was measured using a microplate reader and the background control wells were set as blank. The assay was conducted under minimal light exposure as this solution is light-sensitive. The half maximal inhibitory concentration (IC50) value was determined from this cell proliferation assay.
Animal Study
Ethical approval was applied to the Animal Ethics Committee of Universiti Sains Malaysia (USM) and was approved on 7 October 2015. Ten Severe Combined Immunodeficient (SCID) female mice aged 5 to 6 weeks were purchased from Prima Nexus Sdn. Bhd. All purchased mice were provided with health certificate. They were divided and grouped as untreated and treated (n=5). All mice were housed in an individually ventilated cage (IVC) system with autoclaved woodchips for bedding in an animal room with controlled conditions of temperature (22±2°C), humidity (55±10%) and lighting (12h light/dark), with free access to sterilized food and water. The mice were left for 2 weeks for acclimatization. At week 3, 1×107 of HeLa cells were prepared accordingly and injected subcutaneously at the right flank of mice for both groups using the restraining method. The xenograft model was conducted in accordance to National Institutes of Health guide for the care and use of Laboratory Animals (NIH Publications No. 8023, revised 1978). Water and food consumption, as well as mice’s weights, were recorded every three days. The mice were observed for any clinical signs and symptoms of the health-related problems such as respiratory, skin and growth problems. Early euthanasia was applied if any of the endpoint sign appeared including weight loss of exceeding 20% of the body weight, ulceration/infection at the tumour site, invasion of the surrounding tissues by localised tumour. The mice in the untreated group were left to live freely without any treatment given. As for the treated group, S.asper treatment was started at week 14 and onwards at the dose of 200 mg/kg of body weight in approximately 0.4ml of sterile water administered through oral gavage on the alternate days. The dosage was determined according to the OECD’s (organization of economic corporation and development’s) guidelines as explained by Oghenesuvwe, et al. (2014).16 Post-mortem was performed on both groups at week 16. All mice were sacrificed using carbon dioxide gas. The tumour tissues were stored in liquid nitrogen for protein analysis.
TUNEL Assay
TUNEL assay was conducted on unstained paraffin-embedded-tissue slides using Novus Biologicals APO-BRDU-IHCTM kit. All the protocols provided by the manufacturer were followed with some modification. The slides underwent several staining processes as described in the guidelines. These include deparaffinization, rehydration, permeabilization of specimen, inactivation of endogenous peroxidases, equilibration and end labelling reaction, detection, and counterstain. The prepared slides were observed using a compound microscope.
Total Protein Concentration
Cells and tissue lysates from both groups (untreated and treated) were added with RIPA buffer. The tissues were further homogenised and sonicated for 1 hour in ice. Lysed cells and tissues were centrifuged, and supernatants were collected. The total protein concentration of the samples was measured using Bio-Rad Protein Assay Kit II. This kit contains a dye reagent concentrate and a bovine serum albumin (BSA) standard. This method is a dye-binding assay in which a differential colour change of a dye occurs in response to the concentrations of solubilized protein being detected17. The acidic solution of Coomassie® Brilliant Blue G-250 was used, resulting in an absorbance shift from 465 nm to 595 nm when protein binding occurs. Measurement of protein concentration was obtained by comparing the absorbance change to a standard curve. The method was performed according to the manufacturer’s guidelines with minor modifications.
Antibody Array Analysis
RayBio® C-Series Human Apoptosis Antibody Array C1 kit was used to confirm the involvement of apoptosis in the treatment intervention. The kit was removed from storage and the components were left to equilibrate to room temperature prior to use. The procedures involved in the analysis include sample blocking, sample incubation, first wash, biotinylated antibody cocktail incubation, second wash, HRP streptavidin incubation, third wash, and chemiluminescence detection. The intensity of the spots was analysed using Image J software. The differences of the spot intensities were used to determine relative differences in expression level of each analyte.
Statistical analysis
Body weight, tumour weight, and total protein were analysed as means, standard deviation, and tested for normality of the distribution and homogeneity of variance. The statistical analysis of all these were carried out using IBM Statistical Package for the Social Sciences (SPSS) Version 20. Independent t-test was conducted to calculate significant differences through comparison of mean values between untreated and treated groups.
Results
Inhibitory effects of Streblus asper on cervical cancer proliferation
In cell proliferation assay, five concentrations of Streblus asper extracts were used as a treatment against HeLa cell line. The concentration used were 0.00 mg/ml 0.05 mg/ml, 0.10 mg/ml, 0.15 mg/ml, 0.20 mg/ml, and 0.25 mg/ml. Figure 1 shows the proliferative activity of cells was evaluated using AlamarBlue® and measured by spectrophotometer at 570 nm after 72 hours.
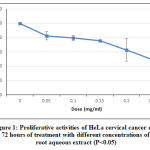 |
Figure 1: Proliferative activities of HeLa cervical cancer cells at after 72 hours of treatment with different concentrations of S. asper root aqueous extract (P<0.05)
|
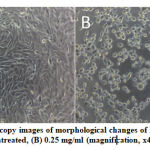 |
Figure 2: Light microscopy images of morphological changes of HeLa cells viability (A) untreated, (B) 0.25 mg/ml (magnification, x40).
Click here to View figure |
The viability of HeLa cell after 72 hours of treatment with S.asper extract at concentrations of 0.00 mg/ml, 0.05 mg/ml, 0.10 mg/ml, 0.15 mg/ml, 0.20 mg/ml, and 0.25 mg/ml were 100.00%, 83.08%, 79.37%, 75.65%, 63.69%, and 49.24% respectively. The half maximal inhibitory concentration (IC50) was found to be 0.25 mg/ml. The treatment induced morphological changes including cell shrinkage, cell rounding, and membrane blebbing. Cell viability was observed lower in the S.asper-treated HeLa cells (Figure 2.B) compared to untreated cells (Figure 2.A). This result suggested that S.asper treatment inhibited the growth of HeLa cells.
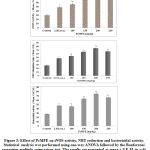 |
Figure 3: Body weight progression in both groups of mice. Independent t-test was conducted to analyse the results. The mean difference of body weight between untreated and treated groups is not significant (p>0.05).
|
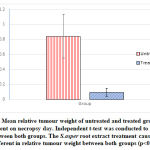 |
Figure 4: Mean relative tumour weight of untreated and treated groups upon measurement on necropsy day. Independent t-test was conducted to analyse the difference between both groups. The S.asper root extract treatment caused significance different in relative tumour weight between both groups (p<0.05). |
The mean body weight of mice in both groups (untreated and treated) increased gradually throughout the experiment. However, the difference of body weight between both groups is not statistically significant. The treatment of S.asper on cervical cancer produces positive effect on the tumour weight. The mean difference of tumour weight between untreated and treated groups was found statistically significant with p<0.05. In addition, no mice were found dead throughout the study protocol until the day of post mortem.
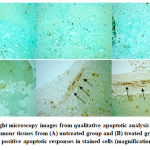 |
Figure 5: Light microscopy images from qualitative apoptotic analysis by TUNEL staining on tumour tissues from (A) untreated group and (B) treated group. Arrows indicate positive apoptotic responses in stained cells (magnification, x40).
|
Expression of Apoptotic Protein Markers
Protein samples prepared from both in vitro and in vivo studies were used for this analysis. The intensity of the spots which represented relative expression of the proteins was evaluated using Image-J software available online.
 |
Figure 6: Representative images of the spots intensities of apoptotic protein markers for the (A) untreated HeLa cells (B) S.asper-treated HeLa cells (C) untreated tumour tissue and (D) S.asper-treated tumour tissue. The panel shows relative expression level of apoptotic protein markers of treated group over untreated group (E) in vitro and (F) in vivo. *Indicates a significant difference from untreated (P<0.05).
|
Figure 6(E) shows 20 apoptotic protein markers are differentially expressed in S.asper extract treatment on HeLa cancer cells. Fifteen of them are upregulated include BID, BIM, IGFBP-3, IGFBP-4, IGFBP-5, p21, p53, SMAC, TNF RI, TNF RII, TNF alpha, TNF beta, TRAIL R1, TRAIL R2, and TRAIL R4. On the other hand, 5 proteins are found downregulated include bcl-w, HSP60, IGF-1, survivin, and XIAP. Based on the results shown in Figure 6(F), 16 apoptotic protein markers are differentially expressed in the S.asper extract treatment on cervical tumour tissues. Five of them are upregulated include Caspase-8, IGFBP-6, SMAC, TNF RI, and TNF RII. On the other hand, 11 of the proteins are found downregulated include bcl-w, CD40 Ligand (TNFRSF5), cIAP-2, HSP27, HSP60, IGF-1, IGF-2, IGFBP-2, livin, survivin, and XIAP.
 |
Figure 7: A Venn diagram comparing the unique and common apoptotic proteins that are differentially expressed in in vitro and in vivo studies.
Click here to View figure |
Eight of the proteins that are common to both in vitro and in vivo include SMAC, TNF RI, TNF RII, bcl-w, HSP60, IGF-1, survivin, and XIAP. Twelve proteins that are unique for in vitro study include BID, BIM, IGFBP-3, IGFBP-4, IGFBP-5, p21, p53, TNF alpha, TNF beta, TRAIL R1, TRAIL R2, TRAIL R4. On the other hand, 8 proteins that are unique for in vivo study include caspase-8, IGFBP-6, CD40 Ligand (TNFRSF5), cIAP-2, HSP27, IGF-2, IGFBP-2, and livin.
Discussion
Numerous studies have revealed the therapeutic potential of Streblus asper on various cancer cell lines such as A549, KB, HOS, and SCC-15 cells.18,19,20 However, none of them had elucidated the S.asper’s potential on cervical cancer. In this study, we observed the ability of S.asper to produce anti-cancer effects on cervical cancer through AlamarBlue® assay which was first developed by Mosmann (1983).21 It is a colourimetric assay used to determine the half maximal inhibitory concentration (IC50) of a treatment. In the present study, S.asper was found able to exert inhibitory effects on HeLa cells. The IC50 value obtained from the AlamarBlue® assay is 0.25mg/ml. S.asper inhibited HeLa cells growth at concentration-dependent manner following 72 hours of treatment.
The treatment produced morphological changes on HeLa cells which include cell rounding, cell shrinkage and membrane blebbing. These characteristics are referred to as morphological changes caused by apoptosis as coined by Kerr, et al. (1972).22 In many occurrences, cell shrinkage is often associated with apoptosis and it is independent of any stimulus. Membrane blebbing is one of the changes that eventually makes up apoptotic bodies.23 Our animal study shows that S.asper seems to exhibit anti-cancer activity against cervical cancer tumour. This is observed from the significant reduction of mice’s tumour weight between untreated and treated groups. The treatment is also observed to trigger apoptotic response in tumour tissues based on its presence on the labelled DNA break (Figure 5).
Apoptosis which is also known as programmed cell death normally occurs as a defence mechanism to maintain cell population. However, this mechanistic activity seems to reduce in most cancers including cervical resulting in cells proliferating indefinitely and uncontrollably. Based on the preliminary findings pertaining to apoptosis involvement, we investigated further the apoptotic markers that trigger this mechanism through antibody array analysis. HeLa cells treated with S.asper demonstrated upregulation of 15 protein markers and downregulation of 5 protein markers (Figure 6). As for in vivo response, cervical tumour-bearing SCID mice treated with S.asper manifested upregulation of 5 proteins and downregulation of 11 proteins. Of all the markers evaluated, TNF alpha expression from in vitro analysis shows the highest overexpression level with 2.8-fold change followed by other markers which related to TNF signalling cascade. These include TNF beta, TRAIL R1, TRAIL R2, TNF RI, TNF RII, BID, SMAC, XIAP, cIAP-2 and caspase 8. The in vivo result seems to support this by showing similar pattern of expression starting from the upregulation of TNFR1 and TNFR2 with slight differences. These differences are within our expectation as different level of biological system complexity would yield different responses.
In general, apoptosis occurs via two pathways; extrinsic and intrinsic. The intrinsic pathway mainly begins with permeabilisation of the mitochondrial membrane. This process causes cytochrome C to be released into the cytoplasm. The release triggers cysteine proteases (caspases) activation and eventually leads to apoptosis.24 The extrinsic pathway on the other hand, involved collective responses of death ligands and receptors. There are several ways that this death ligands and receptors can be induced and one of them is tumour necrosis factor alpha and their cognate receptor (TNF RI).25 Tumour necrosis factor which is also known as tumour necrosis factor alpha is a protein consists of 157 amino acids. It is a form of cytokine that regulates cell survival, proliferation, or death. It involved in many pathological responses of the body such as inflammation, tumour growth, transplant rejection, rheumatoid arthritis, and septic shock.26 TNF alpha has two distinct receptors known as TNF RI and TNF RII. TNF RI is a type of receptor that contains death domain thus making it significant in cell death mechanism. It shares the capability of inducing apoptosis while TNF RII is said to have participated indirectly in the process.27
The TNF signaling begins when the TNF alpha starts to bind with TNF RI. This binding leads to inherent trimerisation process which subsequently results in the formation of complex I. This complex consists of TNF RI, TNF-receptor-associated factor 2 (TRAF2), TNFR1-associated death domain protein (TRADD), and receptor-interacting protein 1 (RIP). This complex serves as a mediator for several other pathways which include NF-κB, MAPK and apoptosis pathway. On one hand, TNF signalling could be observed as proinflammatory inducer while on the other hand it could be seen as proapoptotic inducer. In the present study, we discriminate between these two based on the downregulation of apoptotic inhibitors like cIAP-2 and XIAP (Figure 6E and 6F) which ultimately hinted the blockage of NF-κB pathway. Normally, NF-κB pathway mediates apoptosis suppression through the expression of antiapoptotic protein like BCL-2, A20, cIAP-1, cIAP-2, Bcl-xL, XIAP, and IEX-1L.28,29
Complex I has the capability to form complex II through internalisation process. Complex II consists of RIP, TRADD, FADD and Caspase 8. Caspase 8 autoactivation leads to caspase 3 and caspase 7 activation.30,31 The activation of caspase 8 which eventually results in apoptosis seems to be consistent with our in vivo evidence wherein caspase 8 is upregulated by 1.5-fold change upon S.asper treatment.
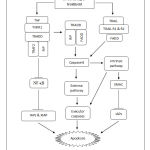 |
Figure 8: Proposed apoptotic mechanism by Streblus asper treatment on cervical cancer
|
Our findings also suggest that S.asper treatment induces the activation of Smac cascade following complex II formation. Smac expression is observed to be upregulated in both of our in vitro and in vivo analyses. Activation of Smac leading to binding with apoptotic inhibitors such as cIAP-1, c-IAP-2, and XIAP. This consequently liberates the apoptosis process to take effect. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) is a member of TNF superfamily. TRAIL was reported to initiate apoptosis in various tumour cell line.32,33 The inducement of apoptosis by TRAIL was found to be due to the interaction with its receptor. These receptors include TRAIL R1 and TRAIL R2 which contain death domain (DD) for apoptosis signalling.34,35 These receptors were observed to be upregulated in S.asper-treated HeLa cells. Upon ligation of TRAIL and its receptors, Fas-associated death receptor (FADD) is recruited as an adaptor molecule which subsequently leads to activation of caspase 836. This ultimately results in apoptosis execution through activation of caspases 3, 6 and 7.37,38
Heat Shock Protein (HSP) is a chaperonin that provides protein protection through the facilitation of protein folding, repair, refolding of misfolded peptides, and possible degradation of irreparable proteins.39 The cytoprotective effects of HSPs are also found to be associated with inhibiting apoptosis and maintaining cytoskeletal integrity under stressful condition.40 HSPs can be found in all living cells. Its division is based on molecular weight ranging from 60 to 110 kDa. HSP60 is predominantly present in mitochondria apart of cytosol, cell surface, and extracellular space.41 Due to its natural ability to evade apoptosis, HSP60 has been found to play an essential role in promoting various types of cancers. These were observed through its overexpression in cancers like liver, bladder, prostate, breast, gastric, colorectal, ovarian, and cervical.42,43,44,45,46,47,48,49 Pieces of evidence show that suppression of HSP60 consequently results in two phenomena. First, it releases the mitochondrial survivin into the cytosol. The loss of mitochondrial pool of survivin specifically induces apoptosis.50 This is because the mitochondrial survivin is designated to inhibit apoptosis by establishing an association with XIAP to synergistically inhibit caspase 9. Second, it causes the upregulation of p53. This would subsequently activate the p53-dependent apoptosis cascade.51 The ability of S.asper to downregulate HSP60 as seen in cells and tumour tissues seems to hold promising hope in cervical cancer therapy.
Conclusion
Streblus asper possess the ability to induce apoptosis on cervical cancer. Based on our preliminary findings, we suggest that the apoptosis process triggered via TNF signalling pathway. The TNF signalling mediates the activation of SMAC signalling and blockage of NF-κB cascade. It is recommended that future study emphasizes on analysis of executor caspases pertaining to apoptosis as validation to our conclusion.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Acknowledgment
The authors would like to thank the Malaysian Ministry of Education and the Universiti Teknologi MARA for providing financial support under the National Research Acculturation Grant Scheme (RAGS) (Ref: RAGS/1/2015/SKK08/UITM/03/1). The authors are also thankful to Advanced Medical and Dental Institute (AMDI), Universiti Sains Malaysia (USM) and Universiti Teknologi MARA (UiTM) Penang Branch, for providing necessary resources and related facilities for completing the study.
References
- Bruni, L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, and others, ‘Human Papillomavirus and Related Diseases in Malaysia’, ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre), 2018
- Waggoner, Steven E., ‘Cervical Cancer’, Lancet, 2003; 361: 2217–2225
CrossRef - Smith, Jennifer S, Lisa Lindsay, Brooke Hoots, Jessica Keys, Silvia Franceschi, Rachel Winer, and others, ‘Human Papillomavirus Type Distribution in Invasive Cervical Cancer and High‐grade Cervical Lesions: A Meta‐analysis Update’, International Journal of Cancer, 2007; 121: 621–632
CrossRef - Crowe, Elizabeth, Nirmala Pandeya, Julia M L Brotherton, Annette J Dobson, Stephen Kisely, Stephen B Lambert, and others, ‘Effectiveness of Quadrivalent Human Papillomavirus Vaccine for the Prevention of Cervical Abnormalities: Case-Control Study Nested within a Population Based Screening Programme in Australia’, Bmj, 2014; 348: g1458
CrossRef - O’Leary, Sean T, Steven Lockhart, Juliana Barnard, Anna Furniss, Miriam Dickinson, Amanda F Dempsey, and others, ‘Exploring Facilitators and Barriers to Initiation and Completion of the Human Papillomavirus (HPV) Vaccine Series among Parents of Girls in a Safety Net System.’, International Journal of Environmental Research and Public Health, 2018; 15
CrossRef - Vicus, Danielle, Rinku Sutradhar, Yan Lu, Laurie Elit, Rachel Kupets, and Lawrence Paszat, ‘Gynecologic Oncology The Association between Cervical Cancer Screening and Mortality from Cervical Cancer : A Population Based Case – Control Study’, 2014; 133: 167–171
CrossRef - Ashtarian, Hossein, Elaheh Mirzabeigi, Elham Mahmoodi, and Mehdi Khezeli, ‘Knowledge about Cervical Cancer and Pap Smear and the Factors Influencing the Pap Test Screening among Women’, International Journal of Community Based Nursing and Midwifery, 2017; 5: 188
- Suba, Eric J., Sean K. Murphy, Amber D. Donnelly, Lisa M. Furia, Linh D. Huynh, and Stephen S. Raab, ‘Systems Analysis of Real-World Obstacles to Successful Cervical Cancer Prevention in Developing Countries’, American Journal of Public Health, 2006; 96: 480–487
CrossRef - Zhang, Zhaoxia, Thomas J. Knobloch, Leigh G. Seamon, Gary D. Stoner, David E. Cohn, Electra D. Paskett, and others, ‘A Black Raspberry Extract Inhibits Proliferation and Regulates Apoptosis in Cervical Cancer Cells’, Gynecologic Oncology, 2011; 123: 401–406
CrossRef - Tunjung, Woro Anindito Sri, Jindrich Cinatl, Martin Michaelis, and C. Mark Smales, ‘Anti-Cancer Effect of Kaffir Lime (Citrus Hystrix DC) Leaf Extract in Cervical Cancer and Neuroblastoma Cell Lines’, Procedia Chemistry, 2015; 14: 465–468
CrossRef - Jamil, Muhammad Mahadi Abdul, Suhassni Ganeson, Hassan Buhari Mammam, and Ridhwan Abdul Wahab, ‘Artocarpus Altilis Extract Effect on Cervical Cancer Cells’, Materials Today: Proceedings, 2018; 5: 15559–15566
CrossRef - Mahata, Sutapa, Saurabh Maru, Shirish Shukla, Arvind Pandey, G Mugesh, Bhudev C Das, and others, ‘Anticancer Property of Bryophyllum Pinnata (Lam.) Oken. Leaf on Human Cervical Cancer Cells’, BMC Complementary and Alternative Medicine, 2012; 12: 15
CrossRef - Maness, L, N Sneed, B Hardy, Jianmei Yu, M Ahmedna, and I Goktepe, ‘Anti-Proliferative Effect of Pleurotus Tuberregium against Colon and Cervical Cancer Cells’, Journal of Medicinal Plants Research, 2011; 5: 6650–6655
CrossRef - Doosti, Mohammad Hossein, Kazem Ahmadi, and Mahdi Fasihi-Ramandi, ‘The Effect of Ethanolic Extract of Thymus Kotschyanus on Cancer Cell Growth in Vitro and Depression-like Behavior in the Mouse’, Journal of Traditional and Complementary Medicine, 2018; 8: 89–94
CrossRef - Gallego, Ana, Isidoro Metón, Isabel V Baanante, Jamal Ouazzani, Emilie Adelin, Javier Palazon, and others, ‘ScienceDirect Viability-Reducing Activity of Coryllus Avellana L . Extracts against Human Cancer Cell Lines’, 2017; 89: 565–572
CrossRef - Oghenesuvwe, Erhirhie Earnest, E Nwoke, and Ajaghaku Daniel Lotanna, ‘Guidelines on Dosage Calculation and Stock Solution Preparation in Experimental Animal s ’ Studies’, 2014; 4: 100–106
- Bradford, M M, ‘A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding.’, Analytical Biochemistry, 1976; 72: 248–254
CrossRef - Miao, Dan, Tengqian Zhang, Jian Xu, Congyu Ma, Wenyuan Liu, Takashi Kikuchi, and others, ‘Three New Cardiac Glycosides Obtained from the Roots of Streblus Asper Lour. and Their Cytotoxic and Melanogenesis-Inhibitory Activities’, RSC Advances, 2018; 8: 19570–19579
CrossRef - Rastogi, Subha, Dinesh K. Kulshreshtha, and Ajay Kumar Singh Rawat, ‘Streblus Asper Lour. (Shakhotaka): A Review of Its Chemical, Pharmacological and Ethnomedicinal Properties’, Evidence-Based Complementary and Alternative Medicine, 2006; 3:217–222
CrossRef - Seeni, Azman, Nur Ayunie Zulkepli, and Ridhwan Abdul Wahab, ‘Apoptosis Inducer from Streblus Asper Extracts for Cancer Chemoprevention’, Novel Apoptotic Regulators in Carcinogenesis, 2012; 9789400749: 1–25
CrossRef - Mosmann, Tim, ‘Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays’, Journal of Immunological Methods, 1983; 65: 55–63
CrossRef - Kerr, J Wyllie, A H Wyllie, and A R Currie, ‘A. 1972. Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue Kinetics’, British Journal of Cancer, 1972; 26: 239–257
CrossRef - Doonan, Francesca, and Thomas G Cotter, ‘Morphological Assessment of Apoptosis’, Methods, 2008; 44: 200–204
CrossRef - Brentnall, Matthew, Luis Rodriguez-Menocal, Rebeka Ladron De Guevara, Enrique Cepero, and Lawrence H Boise, ‘Caspase-9, Caspase-3 and Caspase-7 Have Distinct Roles during Intrinsic Apoptosis’, BMC Cell Biology, 2013; 14: 32
CrossRef - Lu, Jianfei, Yan Li, Zhaoyuan Shen, Cuiyu Lu, and Liqun Lu, ‘TNF-α Is Involved in Apoptosis Triggered by Grass Carp Reovirus Infection in Vitro’, Fish & Shellfish Immunology, 2016; 55: 559–567
CrossRef - Aggarwal, Bharat B, ‘Signalling Pathways of the TNF Superfamily: A Double-Edged Sword.’, Nature Reviews. Immunology, 2003; 3: 745–756
CrossRef - Wajant, H, K Pfizenmaier, and P Scheurich, ‘Tumor Necrosis Factor Signaling.’, Cell Death and Differentiation, 2003; 10: 45–65
CrossRef - Catz, S D, and J L Johnson, ‘Transcriptional Regulation of Bcl-2 by Nuclear Factor Kappa B and Its Significance in Prostate Cancer.’, Oncogene, 2001; 20: 7342–7351
CrossRef - Salvesen, Guy S, and Colin S Duckett, ‘IAP Proteins: Blocking the Road to Death’s Door.’, Nature Reviews. Molecular Cell Biology, 2002; 3: 401–410
CrossRef - Baud, V, and M Karin, ‘Signal Transduction by Tumor Necrosis Factor and Its Relatives.’, Trends in Cell Biology, 2001; 11: 372–377
CrossRef - Shearwin-Whyatt, L M, N L Harvey, and S Kumar, ‘Subcellular Localization and CARD-Dependent Oligomerization of the Death Adaptor RAIDD.’, Cell Death and Differentiation, 2000; 7: 155–165
CrossRef - El-Deiry, W S, ‘Insights into Cancer Therapeutic Design Based on P53 and TRAIL Receptor Signaling’, Cell Death and Differentiation, 2001; 8: 1066
CrossRef - Seki, Naoko, Yoshihiro Hayakawa, Alan D Brooks, John Wine, Robert H Wiltrout, Hideo Yagita, and others, ‘Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Mediated Apoptosis Is an Important Endogenous Mechanism for Resistance to Liver Metastases in Murine Renal Cancer’, Cancer Research, 2003; 63: 207–213
- Pan, G, J Ni, Y F Wei, G Yu, R Gentz, and V M Dixit, ‘An Antagonist Decoy Receptor and a Death Domain-Containing Receptor for TRAIL.’, Science (New York, N.Y.), 1997; 277: 815–818
CrossRef - Pan, G, K O’Rourke, A M Chinnaiyan, R Gentz, R Ebner, J Ni, and others, ‘The Receptor for the Cytotoxic Ligand TRAIL.’, Science (New York, N.Y.), 1997; 276: 111–113
CrossRef - Wang, Shulin, and Wafik S El-Deiry, ‘TRAIL and Apoptosis Induction by TNF-Family Death Receptors.’, Oncogene, 2003; 22: 8628–8633
CrossRef - Du, C, M Fang, Y Li, L Li, and X Wang, ‘Smac, a Mitochondrial Protein That Promotes Cytochrome c-Dependent Caspase Activation by Eliminating IAP Inhibition.’, Cell, 2000; 102: 33–42
CrossRef - Verhagen, A M, P G Ekert, M Pakusch, J Silke, L M Connolly, G E Reid, and others, ‘Identification of DIABLO, a Mammalian Protein That Promotes Apoptosis by Binding to and Antagonizing IAP Proteins.’, Cell, 2000; 102: 43–53
CrossRef - Ikwegbue, Paul Chukwudi, Priscilla Masamba, Babatunji Emmanuel Oyinloye, and Abidemi Paul Kappo, ‘Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer.’, Pharmaceuticals (Basel, Switzerland), 2017; 11
CrossRef - Hartl, F Ulrich, Andreas Bracher, and Manajit Hayer-Hartl, ‘Molecular Chaperones in Protein Folding and Proteostasis.’, Nature, 2011; 475: 324–332
CrossRef - Cappello, Francesco, Everly Conway de Macario, Lorenzo Marasà, Giovanni Zummo, and Alberto J L Macario, ‘Hsp60 Expression, New Locations, Functions, and Perspectives for Cancer Diagnosis and Therapy’, Cancer Biology & Therapy, 2008; 7: 801–809
CrossRef - Abdalla, Moemen Ak, and Yousef Haj-Ahmad, ‘Promising Urinary Protein Biomarkers for the Early Detection of Hepatocellular Carcinoma among High-Risk Hepatitis C Virus Egyptian Patients.’, Journal of Cancer, 2012; 3: 390–403
CrossRef - Abu-Hadid, M, J D Wilkes, Z Elakawi, L Pendyala, and R P Perez, ‘Relationship between Heat Shock Protein 60 (HSP60) MRNA Expression and Resistance to Platinum Analogues in Human Ovarian and Bladder Carcinoma Cell Lines.’, Cancer Letters, 1997; 119: 63–70
CrossRef - Castilla, Carolina, Belen Congregado, Jose M Conde, Rafael Medina, Francisco J Torrubia, Miguel A Japon, and others, ‘Immunohistochemical Expression of Hsp60 Correlates with Tumor Progression and Hormone Resistance in Prostate Cancer.’, Urology, 2010; 76: 1017.e1-6
CrossRef - Desmetz, C, F Bibeau, F Boissiere, V Bellet, P Rouanet, T Maudelonde, and others, ‘Proteomics-Based Identification of HSP60 as a Tumor-Associated Antigen in Early Stage Breast Cancer and Ductal Carcinoma in Situ.’, Journal of Proteome Research, 2008; 7: 3830–3837
CrossRef - Giaginis, Constantinos, Stella S Daskalopoulou, Stephanie Vgenopoulou, Ioannis Sfiniadakis, Gregorios Kouraklis, and Stamatios E Theocharis, ‘Heat Shock Protein-27, -60 and -90 Expression in Gastric Cancer: Association with Clinicopathological Variables and Patient Survival.’, BMC Gastroenterology, 2009; 9: 14
CrossRef - Hamelin, Celine, Emilie Cornut, Florence Poirier, Sylvie Pons, Corinne Beaulieu, Jean-Philippe Charrier, and others, ‘Identification and Verification of Heat Shock Protein 60 as a Potential Serum Marker for Colorectal Cancer.’, The FEBS Journal, 2011; 278: 4845–4859
CrossRef - Hjerpe, Elisabet, Suzanne Egyhazi, Joseph Carlson, Marianne Frostvik Stolt, Kjell Schedvins, Hemming Johansson, and others, ‘HSP60 Predicts Survival in Advanced Serous Ovarian Cancer.’, International Journal of Gynecological Cancer : Official Journal of the International Gynecological Cancer Society, 2013; 23: 448–455
CrossRef - Hwang, You Jin, Soon Pyo Lee, Suk Young Kim, Young Hwan Choi, Min Ji Kim, Choong Ho Lee, and others, ‘Expression of Heat Shock Protein 60 KDa Is Upregulated in Cervical Cancer.’, Yonsei Medical Journal, 2009; 50: 399–406
CrossRef - Dohi, Takehiko, Fang Xia, and Dario C Altieri, ‘Article Compartmentalized Phosphorylation of IAP by Protein Kinase A Regulates Cytoprotection’, 2007; 4:17–28
CrossRef - Ghosh, Jagadish C, Takehiko Dohi, Byoung Heon Kang, and Dario C Altieri, ‘Hsp60 Regulation of Tumor Cell Apoptosis *’, 2008; 283: 5188–5194
CrossRef








