I Wayan Niryana1,2 , Sri Maliawan1,2
, Sri Maliawan1,2 , I Made Bakta2
, I Made Bakta2 and I Nyoman Mantik Astawa2
and I Nyoman Mantik Astawa2
1Department of Neurosurgery, Faculty of Medicine, Udayana University-Sanglah General Hospital, Bali, Indonesia
2Doctoral Program, Faculty of Medicine, Udayana University, Bali, Indonesia
Corresponding Author E-mail: niryana_wayan@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1820
Abstract
One option to control intracranial pressure (ICP) is by performing decompressive craniectomy (DC) before definitive treatment. A common problem after DC is adhesion. Separated injured tissue surfaces by using bioabsorbable membranes over a critical/certain period are predicted to prevent fibrin bridge formation and adhesion. This study is conducted to prove the effects of DC with mesh on TGF-β levels, fibroblast cell count, fibrosis size and clinical adhesions in wistar rat with traumatic brain injury. The current research uses animal model with randomized post-test only control group design. A total of 20 samples that met the eligibility criteria were included in the study and randomized. A total of 10 samples were grouped as a control group (standard DC) and 10 samples were grouped as a study group (DC with mesh). On day 7, we performed euthanasia and took peridural tissue for examination of TGF-β levels, fibroblast cell count, and fibrosis size. The mean results of TGF-β in the study group were lower than the control group (81.28±17.48 pg/dl vs 93.83±9.51 pg/dl) although this difference was not statistically significant (p=0.062). For the mean number of fibroblasts cells, there was a significant difference where the study group was lower than the control group (56.8±20.59 cells/HPF vs 94.8±18.56 cells/HPF) with p<0.001. The mean size of peridural fibrosis also showed a significant difference where the mean size of peridural fibrosis in the study group was lower than the control group (1182.52±347.05 μm vs 1545.78±378.28 μm) with p=0.038. Only DC samples (without the use of mesh) had a relative risk 8 times greater for clinical adhesions compared to mesh (RR=8; 95% CI, 1,215-52,693; p=0,005). DC with mesh significantly resulted in low fibroblast cells count, size of peridural fibrosis, and the risk of clinical adhesions in wistar rat with traumatic brain injury.
Keywords
Traumatic brain injury; mesh, TGF-β; fibroblast cell counts; fibrosis size; clinical adhesions; peridural tissue
Download this article as:| Copy the following to cite this article: Niryana I. W, Maliawan S, Bakta I. M, Astawa I. N. M. Effects of Decompressive Craniectomy with Mesh in the Level of Transforming Growth Factor Beta, Fibroblast Cell Count, Size of Fibrosis, and Clinical Adhesion of Peridural Tissues in Wistar Rat with Traumatic Brain Injury. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Niryana I. W, Maliawan S, Bakta I. M, Astawa I. N. M. Effects of Decompressive Craniectomy with Mesh in the Level of Transforming Growth Factor Beta, Fibroblast Cell Count, Size of Fibrosis, and Clinical Adhesion of Peridural Tissues in Wistar Rat with Traumatic Brain Injury. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/361masB |
Introduction
Intracranial hypertension was a major cause of complications and death among many problems because of TBI. One attempt to control intracranial pressure (ICP) in patients with TBI is by performing Decompressive Craniectomy (DC) [2]. In the last few decades, DC methods for TBI management have become the focus of attention.
Decompressive craniectomy frequently performed by a neurosurgeon in the treatment of persistently high ICP and having a midline shift on a patient with traumatic brain injury. This surgical management also performed on ischemic stroke patients, hemorrhagic strokes and subarachnoid hemorrhage. The DC aims to reduce ICP by removing cranium bone so that there is more room for the swollen brain. It is an early action for life-saving which will be followed by a second operation of cranioplasty after the patient is stable [10].A common problem after DC is adhesion between soft tissues, especially between the dura mater, the temporal muscle, and the galea. The adhesion formation due to difficulties during tissue dissection resulting in increased operating time and increased risk of dural perforation [12]. At the time of cranioplasty, temporal muscle is separated from the dura mater, sometimes it can be very difficult and takes a long time, the risk of cerebrospinal fluid effusion (CSF) with potential postoperative complications can occur.By separating the injured tissue surfaces using bioabsorbable membranes over a critical/certain period, fibrin bridge formation and adhesion are predicted to be prevented. Seprafilm mesh contains Hyaluronic Acid carboxymethylcellulose (HA-CMC) is a bioabsorbable membrane that has been used for anti-adhesive. The combination of these 2 materials looks transparent, thin, adherent and absorbable membrane can be used as a mechanical separation device. In and randomized clinical trials of animal model, it has been demonstrated that HA-CMC decreases incidence and severity of postoperative adhesion [6].In 1996, HA-CMC was combined as an anti-adhesion membrane in clinical trials of abdominal surgery, where there was a significant decrease in adhesion/postoperative adhesion in animal models and clinical studies of adult and child patients. HA-CMC membranes serve as the physical barrier that can decrease the activity/proliferation of fibroblasts, prevent fibrin deposition on serous surfaces and cellular elements during peritoneal repair [6]. HA also has anti-inflammatory activity and acts as a synthetic membrane to inhibit invasion of inflammatory cell inflammation and vascularization [7].Research on the use of anti-adhesion materials during DC operation has not been done in Bali or Indonesia. For that reason, researchers wanted to conduct experimental research on Wistar rat whether the use of mesh as anti-adhesion in DC can reduce adhesion with low TGF-β levels, fibroblast cell count and peridural fibrosis.
Methods and materials
This current study is a laboratory experimental study using an animal model with randomized post-test control group design. We conducted this study in February-Agustus 2017 in the Pharmacologic Laboratory of Medical Faculty of Udayana University and Laboratory of Veterinary Medicine Faculty of Udayana University. The inclusion criteria of wistar rats are male, healthy, age 6-8 weeks, body weight 150-200 gram. Drop out criteria are severe illness or death which is determined by a veterinarian. Randomization was done to allocate samples to each group, 10 samples to study group and 10 samples to control group.
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted No:363/UN.14.2/KEP/2017. The study was done by trained staff and all animal model got comfort environment.
A traumatic brain injury of the animal model was made first by injection of an anesthetic agent to reach pain-free of animal model, using ketamine 44-100 mg/body weight intramuscularly. We used weight drop model technique to induce traumatic brain injury. The feathers on the rat’s head were shaved and cleaned with 70% alcohol. A linear incision was done on the rat head in the midline, then a 75-gram iron cylinder was dropped once from 10 cm height, 90-degree angle. The energy of the impact was 0.01 Joule.
The models were prepared to undergo the DC procedure, the dura mater was opened with micro scissor leaving the cortical intact. In control group, at the end of the procedure, the temporalis muscle, fascia, and skin were sutured without mesh, whereas, in the study group, the mesh was placed proportionately to the bone flap above the dura mater without suturing, and the temporalis muscle, fascia, and skin were sutured layer by layer.
The TGF-β level was measured using ELISA, fibroblast cells were counted using immunohistochemistry method, peridural fibrosis was evaluated based on its size (using immunohistochemistry method) and clinical adhesion (the adhesion between dura mater and temporalis muscle, facia or the skin).
The data for transforming growth factor beta (TGF-β), fibroblast cells count, and fibrosis size in the peridural area were tested for normality using Shapiro-Wilk. The results came out the data were normally distributed. Independent t-test was used to evaluate the level of transforming growth factor beta (TGF-β), fibroblast cells count, and fibrosis size in the peridural tissue between study and control group, confidence interval 95% (p<0,05). Fisher Exact was used to evaluate the difference in clinical adhesion between both groups.
Results
The mean of TGF-β level in study and control group were 81,28±17,48 pg/dl and 93,83±9,51 pg/dl respectively. Based on analysis results, an independent t-test showed p-value 0,062, therefore we concluded that the mean TGF-β level after day 7 in the study group was lower compared to control group and the difference was statistically not significant (Table 1).
Table 1: Mean difference of TGF-β level in both group
| Subject | n | Mean ± SD
(pg/dl) |
Mean difference | 95% CI | p |
| Control group | 10 | 93,83 ± 9,51 | 12,55 | (-0,67-25,77) | 0,062 |
| Study group | 10 | 81,28 ± 17,48 |
SD= standard deviation; p= p-value; n= sample number
We obtained an average fibroblast cells count in the control group that is 94.8 ± 18.56 cells/HPF while in the study group was 56.8 ± 20.59 cells/HPF. The significance value of the independent t-test showed the p-value <0.001. It can be concluded that there is a significant difference of mean fibroblast cells counts between the control group and the study group where the average fibroblast cells counts in the study group are lower than the control group on day 7 (Table 2). Fig. 1 showed the microscopic view of fibroblast cells.
Table 2: Mean difference of fibroblast cells count in both group
| Subject | n | Mean ± SD
(cells/HPF) |
Mean difference | 95% CI | p |
| Control group | 10 | 94,8 ± 18,56 | 38 | (19,58-56,42) | <0,001 |
| Study group | 10 | 56,8 ± 20,59 |
SD= standard deviation; p= p-value; n= sample number
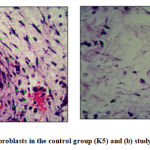 |
Figure 1: (a) Number of fibroblasts in the control group (K5) and (B) study group (P4). (H-E 400X)
|
The mean size of fibrosis on the samples peridural tissue 7 days after DC with mesh was 1182,52 ± 347,05μm and after standard DC was 1545,78 ± 378,28μm. Statistical analysis showed was p<0.05. This p-value means that there was a significant difference in the size of fibrosis between the control group and the study group, where the mean fibrosis size in the study group was lower than the control group (Table 3). The size of fibroblast cells in both groups was shown in fig. 2. [Table 3. The difference in mean size of fibrosis in both groups]
Table 3: The difference in mean size of fibrosis in both groups
| Subject | n | Mean ± SD
(μm) |
Mean Difference | 95% CI | p |
| Control group | 10 | 1545,78 ± 378,28 | 363,26 | (22,2-704,32) | 0,038 |
| Study group | 10 | 1182,52 ± 347,05 |
SD= standard deviation; p= p-value; n= sample number
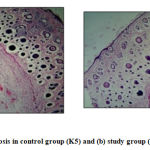 |
Figure 2: (A) Size of fibrosis in control group (K5) and (B) study group (P4). (H-E 40X)
|
Table 4 showed the incidence of clinical adhesions in the study group was lower than the control group with RR = 8. The results of Fisher’s test analysis showed that p <0.05, it can be concluded that the standard DC samples (without mesh placement) had a relative risk 8 times greater for clinical adhesion compared to mesh (RR=8; 95% CI, 215-52,693; p=0.005). Analysis with 2×2 tables was done based on the division of each variable (group samples and clinical adhesions). Based on the analysis, there were 50% cells having an expected count value of less than five, therefore the 2×2 table was not feasible to be tested by the Chi-Square test. We decided to use the Fisher test, instead.
Table 4: The differences in clinical adhesion risk in both groups
| Clinical Adhesion | p | RR | 95% CI | ||||||||
| Positive | Negative | ||||||||||
| n | % | n | % | Lower | Upper | ||||||
| Group | Control | 8a | 80 | 2 | 20 | 0,005*b | 8 | 1,215 | 52,693 | ||
| Study | 1a | 10 | 9 | 90 | |||||||
| Total | 9 | 45 | 11 | 55 | |||||||
RR: Relative risk; p= p-value; n= sample number; CI= confidence interval; a= expected count <5; b= Fisher test; *significant p-value<0,05
The new findings (research novelty) of this study are:1. This study was the first study which examines DC with mesh to reduce peridural fibrosis in wistar rat with traumatic brain injury.
It was also found that DC with mesh can reduce adhesion of peridural tissue as evidenced by lower of fibroblast cells counts, the size of fibrosis, and clinical adhesions in the peridural tissues in rat with the traumatic brain injury. Mesh as an anti-adhesion membrane in DC serves as a physical or mechanical barrier between the dura mater and the tissue above it. Mesh acts as a barrier to the migration of inflammatory cells and fibroblast cells and indirectly reduce TGF-β levels, decreasing fibroblast/proliferation activity, reducing fibrin and collagen deposition on the surface of peridural tissue resulting in lower fibrosis and reduced the risk of adhesion.
Discussions
The TGF-β induces proliferation, differentiation, and cell angiogenesis. It has chemotactic property to macrophages and fibroblasts. This molecule is the most potent molecule for macrophages. In addition to macrophages signaling to the injury site, it also stimulates the synthesis of various growth factors and inhibits the production of hydrogen peroxide which is cytotoxic to the fibroblasts proliferation. The results of fibroblasts stimulation will cause chemotaxis, proliferation, and stimulation of the extracellular matrix molecules such as type 1 collagen, and inhibition of proteolytic enzymes that function to degrade the newly formed connective tissues [3]. Specifically, TGF-β inhibits matrix degradation by decreasing metalloprotease secretion. Furthermore, TGF-β increases the secretion of protease inhibitors, such as plasminogen activator inhibitor-1. As the final results, these mechanisms will lead to the formation of adhesion as a result of increased production of extracellular matrix and decrease of matrix degradation, combined with decreased fibrinolytic activity [5].Luo et al. (2015), conducted research on the effects of rapamycin (RPM) in preventing the proliferation of fibroblasts, the formation of peridural fibrosis, and inflammatory activity by default in vitro and in vivo in mice that was performed laminectomy by calculating fibroblasts, Rydell’s assessment, histological analysis, measurement of hydroxyproline levels, interleukin-6 (IL-6) and expression of TGF-β mRNA. The expression of TGF-β and IL-6 in the RPM group was significantly lower than control (p=0.002). TGF-β and IL-6 mRNA analyses were performed 4 weeks after laminectomy with a real-time PCR (RT-PCR) Quantitative technique in BioRad MYIQ2 (USA). In this study, the decrease in IL-6 and TGF-β regulation shows the efficacy of RMP in preventing inflammation [9].Fibroblasts are cells that produce collagen fibers, reticulum, elastin, glycosaminoglycans, and glycoproteins from intercellular substances. In adults, fibroblasts in the tissues experiencing changes. Mitosis is only visible if a person needs additional fibroblasts, i.e. if the connective tissue is injured. Fibroblasts are more active in synthesizing matrix components in response to wounds by proliferating and enhancing fibrinogenesis. Fibroblasts become the main agents in the wound healing process [11].Research by Chen et al. (2014), focusing on the effect of HA preventing fibrosis formation and post-laminectomy adhesion using rabbits as a model, showing fibroblast cells count was significantly lower in the study group compared to the control group (3078±313.68 vs 3742±455.65, respectively p=0.042). Fibroblasts may play an important role in the formation of peridural fibrosis derived from the perivertebral muscles and blood flow. Blocking the migration of fibroblasts into the surgical field is a measurable measure theoretically to prevent peridural fibrosis [4].Kelten et al. (2016) reported the use of pentoxifylline for inhibition of peridural fibrosis in Post-Laminectomy mice obtained similar results with this study. Evaluation of epidural fibrosis in 2 groups according to the macroscopic assessment and light microscope revealed that the formation of epidural scarring was lower in the study group compared with the control group (p<0.001) and the fibroblasts counts also decreased significantly in the pentoxifylline group study (p<0.05) [8].Oxidized regenerated cellulose is a degraded barrier which first used in clinical practice. It was a modification of its surgical precursor, which has long been used as a hemostasis agent. Mesh is designed to be placed above or between traumatized surfaces. Various studies have been conducted to evaluate the efficacy of ORC in preventing the formation of postoperative adhesion. A meta-analysis of 11 randomized control trial (RCT) trials with laparoscopy showed that ORC barrier proved safe and significantly reduced the incidence of de-novo adhesion compared with no use of the ORC barrier. Whereas in the laparotomy procedure, the study of a meta-analysis of several RCTs also showed a significant decrease in adhesion formation with the use of ORC compared to no barrier [1].Examination of TGF-β levels, fibroblast cell count, fibrosis size and clinical adhesion in this study were limited only 7 days postoperative DC with mesh, so it is necessary to check also on day 3, day 7, and day 28 post-operative DC with mesh to know the difference at all stages of wound healing.
Conclusions
The TGF-β levels in the rat peridural tissue on the 7th day postoperative DC with mesh was lower than standard DC postoperatively, but not statistically significant. The number of fibroblast cells counts in mice peridural tissue on the 7th day postoperative DC with mesh was lower than standard DC postoperatively. The size of fibrosis in the rat peridural tissue on the 7th day postoperative DC with mesh was lower than standard DC postoperatively. The incidence of clinical adhesion in the rats peridural tissue on day 7 postoperative DC with mesh lower than standard DC postoperatively.
Acknowledgments
Authors would like to thank colleges in Faculty of Medicine, Udayana University for their advice and supports, I.B. Putra Manuaba, M. Zafrullah Arifin, I Dewa Made Sukrama, I Wayan Putu Sutirtayasa, Ni Putu Sriwidyani, I Putu Eka WIdyadharma.
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Funding Source
No funding was received for this research.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted No:363/UN.14.2/KEP/2017
References
- Ahmad G, Duffy JM, Farquhar C, Vail A, Vanderkerchove P, Watson A, Wiseman D (2008) Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst. Rev.
- Alvis-Miranda H, Castellar-Leones SM, Moscote-Salazar LR (2013) Decompressive craniectomy and traumatic brain injury: a review. Bull Emerg Trauma 1(2):60
- Chegini N (1997) The role of growth factors in peritoneal healing: transforming growth factor beta (TGF-beta). Eur J Surg Suppl Acta Chir Suppl (577):17–23
- Chen J-M, Lee S-H, Tsai T-T, Niu C-C, Chen L-H, Chen W-J (2014) Anti-adhesive effect of hyaluronate in a rabbit laminectomy model. Biomed J 37(4):218–224
- Gago LA, Saed GM, Wang RX, Kruger M, Diamond MP (2003) Effects of oxidized regenerated cellulose on the expression of extracellular matrix and transforming growth factor-β1 in human peritoneal fibroblasts and mesothelial cells. Am J Obstet Gynecol 189(6):1620–1625
- Kamer E, Unalp H, Tarcan E, Diniz G, Atahan K, Ortac R, Onal MA (2008) Effect of Hyaluronic Acid-carboxymethylcellulose Adhesion Barrier on Wound Healing: An Experimental Study. Wounds Compend Clin Res Pract 20(10):265–272
- Kato T, Haro H, Komori H, Shinomiya K (2005) Evaluation of hyaluronic acid sheet for the prevention of postlaminectomy adhesions. Spine J 5(5):479–488
- Kelten B, Erdogan H, Antar V, Sanel S, Tuncdemir M, Kutnu M, Karaoglan A, Orki T (2016) Pentoxifylline inhibits epidural fibrosis in post-laminectomy rats. Med Sci Monit Int Med J Exp Clin Res 22:840
- Luo L, Zhang C, Zhao J, Wei Q, Li X (2015) Effects of rapamycin on reduction of peridural fibrosis: an experimental study. Med Sci Monit Int Med J Exp Clin Res 21:482
- Oladunjoye AO, Schrot RJ, Zwienenberg-Lee M, Muizelaar JP, Shahlaie K (2013) Decompressive craniectomy using gelatin film and future bone flap replacement. J Neurosurg 118(4):776–782
- Tamariz E, Grinnell F (2002) Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Mol Biol Cell 13(11):3915–3929
- Vakis A, Koutentakis D, Karabetsos D, Kalostos G (2006) Use of polytetrafluoroethylene dural substitute as adhesion preventive material during craniectomies. Clin Neurol Neurosurg 108(8):798–802








