Omnia Magdy Hendawy 1,2, Mona Anwar ELBana 3, Hassan A. Abdelmawlla4,5, Naseer Maliyakkal6 and Gomaa Mostafa-Hedeab2,7
1Pharmacology Department, Faculty of Pharmacy, Jouf University, Saudi Arabia
2Clinical Pharmacology Department, Faculty of Medicine, Beni-Suef University, Egypt
3Medical Biochemistry Department, National Research Centre, Giza, Egypt
4Department of anatomy, college of medicine, Jouf University, Saudi Arabia
5Anatomy and embryology department, College of Medicine, Beni-Suef University, Egypt
6Pharmacology Department of Basic Medical Sciences - College of Applied Medical Sciences - King Khalid University, KSA
7Pharmacology Department, Faculty of Medicine, Jouf University, Saudi Arabia
Corresponding Author E-mail: gomaa_hedeab@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1801
Abstract
Aluminum (Al) is present daily in our life, the long-term excessive Al intake induces neuroinflammation and cognition retardation. Annona squamosa leaves showed some medicinal activities as anti-inflammatory, antioxidant and antidiabetic drugs. This study was designed to examine the effect of ethanolic and aqueous extracts of annona squamosa leaves against aluminum chloride (AlCl3-induced neuroinflammation in rats. 40 male albino rats were randomly divided into 4 groups, 10 rats each. Group 1; (Control rats), Group 2; (rats received AlCl3 50mg/kg body weight orally (p.o), Group 3; (rats received AlCl3 and annona squamosa leave aqueous extracts (300mg/kg) and Group 4; (rats received AlCl3 and annona squamosa ethanolic extracts (300mg/kg). After two months; blood samples were collected for assessment of serum nuclear factor- ҡβ (NF-ҡβ) and Acetyl cholinesterase (Ach E). The brain of each rat was removed for assessment Brain nitric oxide, reduced glutathione (GSH), malondialdehyde (MDA), superoxide dismutase (SOD), caspase 3 and brain-derived neurotrophic factor (BDNF). AlCl3 increase brain MDA, NO, Ach E activity, NF-ҡβ and caspase 3, significant decreases in GSH, SOD activity and BDNF. Ethanolic or aqueous annona squamosa leaves extracts ameliorate MDA, NO, Ach. E activity, NF-ҡβ and caspase 3 and restore GSH, SOD activity and BDNF to near normal levels in AlCl3 treated rats. Conclusion: Both of ethanolic and aqueous annona squamosa leave extracts protect rat brain against oxidative stress and inflammation induced by AlCl3.
Keywords
Aluminum chloride; Annona Squamosa leaves; Neuroinflammation.
Download this article as:| Copy the following to cite this article: Hendawy O. M, ELBana M. A, Abdelmawlla H. A, Maliyakkal N, Hedeab G. M. Effect of Annona Squamosa Ethanolic and Aqueous Leave Extracts on Aluminum Chloride-Induced Neuroinflammation in Albino Rats. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Hendawy O. M, ELBana M. A, Abdelmawlla H. A, Maliyakkal N, Hedeab G. M. Effect of Annona Squamosa Ethanolic and Aqueous Leave Extracts on Aluminum Chloride-Induced Neuroinflammation in Albino Rats. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2MCLUUp |
Introduction
The brain controls most of the body activities, processing, integrating, and coordinating the information. Oxidative stress critically affect brain function due to its large oxygen consumption, generation of large amounts of the reactive oxygen species (ROS), polyunsaturated fatty acids and decrease of the protective antioxidant enzymes1.
Aluminum (Al) is a natural toxin that human may consume through food, drinking water or drugs containing Al2 causing toxicity in most of organ especially the liver, bone, spleen and brain which is the most affected organ by its toxicity 3. The toxic effect of Al on the brain tissue is due to its ability to cross the blood brain barrier 4, causing neurons apoptosis and neuro-inflammation that result in gradual amnesia and learning activities.
Previous studies were conducted to explore the mechanism of Al-induced neuroinflammation; among which oxidative stress-induced brain matter apoptosis and injury, as well as neuroinflammatory process, are highly accepted 5.
Consumption of diets or nutrients having antioxidant, anti-inflammatory, neuro-protective properties may provide valuable effects in alleviating or protecting from toxics mediated neuro-disorders problems6. Annona squamosa (AS) leaves contain several active materials as alkaloids flavonoids particularly quercetin and eugenol, glycoside, saponins, tannins, phytosterols and phenolic compounds that exhibited it’s therapeutic properties like anti-inflammatory, antioxidant, antidiabetic and antimicrobial activities7.
Nevertheless, few studies were found providing detailed information on the in vivo studies about neuro-protective effect of ethanolic (alc.) and aqueous (aq.) extracts AS leaves. Based on the previous mentioned data, the present work was designed to assess the effect of alc. and aq. AS leaves extracts on AlCl3-induced neuroinflammation.
Material and method
Chemicals
Aluminum chloride – hydrated (AlCl3.6H2O) (Sigma Chemical Co.; St. Louis, MO, USA).
Fresh leaves of squamosa were collected locally during the month of October to December. These plant materials were identified and authenticated by our colleague, botany department, National Research Center, Egypt.
Animals
Forty adult albino male rats (150 to 200 g) were obtained from the Animal House; National Research Centre, Cairo, Egypt, kept on standard laboratory diet and water ad libitum, (23 ± 1ºC), (12 h dark/light cycle), they were left for one week before starting experiments for acclimatization. Our proposal was approved by our local institute Ethics Committee which adhere to the international animal ethical guidelines.
Preparation of the aluminum chloride
Aluminum chloride was dissolute in sterile water at pH 7.0. AlCl3 was administered p.o. by an oral feeding syringe at a dosage 50mg/kg/day for two months to induce neuroinflammation8
Experimental design
Following the acclimatization period, animals were randomly divided into 4 groups, 10 animals each:
Group I: rats received only saline as a control group.
Group II: rats received AlCl3 only to induce neuroinflammation.
Group III: rats received AlCl3 and A. squamosa aqueous extract (300mg/kg/day)9 for two months.
Group IV: rats received AlCl3 rats and A. squamosa ethanolic extract (300mg/kg/day)9 for two months.
Collection of blood samples
After two months experimental period, two blood samples were collected from retroorbital plexuses from each rat; one in heparinized tube (for plasma) and another one in non-heparinized tube (for serum), then centrifuged at 3000 rpm for ten minutes to obtain plasma and serum. The clear supernatant plasma and serum was then stored frozen at -20 ºC for further biochemical assessments.
Preparation of tissue
Whole-brain of each rat was manipulated and processed as previously described10 and the resulting supernatant was stored at –80 °C for biochemical analysis.
Biochemical analysis
Using commercially available-ready to use kits; the following parameters were determined:
Brain nitric oxide (NO) using a spectrophotometer.
Brain malondialdehyde (MDA) using a spectrophotometer.
Brain reduced glutathione (GSH) using a spectrophotometer.
Superoxide dismutase (SOD) activity.
Serum acetylcholinesterase (Ach E) using a spectrophotometer.
Brain-derived neurotrophic factor (BDNF) using ELISA.
Serum nuclear factor-κB (NF-κB) was determined using an enzyme amplified sensitivity immunoassay (EASIA) according to manufacture kit.
Brain caspase3 were determined using EASIA method according to manufacture kit.
Statistical analysis
Results were collected, tabulated and expressed as mean ± standard error of mean. Independent sample t test was used for data analysis using (SPSS) version 15. P value <0.05 was considered significant.
Results
Aluminum chloride (AlCl3) administration showed a significant increase in brain MDA and NO and a significant decreased SDO and GSH compared to control group.
Compared to the neuro-inflamed rats; Aqueous or alcoholic annona squamosa-treated rats showed a significant decrease in MDA and NO, while significant decrease in SDO and GSH Table (1).
Table 1: Levels of oxidant and antioxidant parameters among different studies groups.
| Parameters Groups |
MDA
(nmol/gm) |
NO
(μmol/ gm) |
SOD (U/gm) | GSH
(mg/gm) |
| Control | 53.4±4.0 | 6.9±0.5 | 7.0±0.3 | 4.4±0.2 |
| AlCl3 | 128.2±6.0a | 13.8±0.9 a | 3.9±0.2 a | 2.3±0.1 a |
| AlCl3+Aq.s ex. | 60.0±5.2b | 8.6±0.8 b | 5.6±0.3a b | 3.7±0.3 b |
| AlCl3+Eth. ex. | 67.0±5.8 b | 8.3±0.6 b | 6.0±0.1 ab | 4.1±0.28 b |
a = significant compared to control group, b = significant compared to AlCl3-treated group.
NF- ҡB was significantly increased in AlCl3– inflamed rats when compared to control group; meanwhile treatment with either aqueous or alcoholic extracts of annona squamosa leaves results in significant decrease in NF- ҡB compared to diseased group Figure (1).
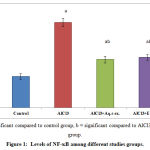 |
Figure 1: Levels of NF-ҡB among different studies groups.
|
Induction of neuroinflammation in rat via AlCl3 for 2 months results in a statistically significant increase in brain Caspase 3 activity compared to control group. Treatment with either aqueous or alcoholic extracts of annona squamosa leaves results in significant decrease in brain Caspase 3 level compared to diseased group. Figure (2).
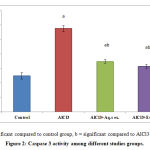 |
Figure 2: Caspase 3 activity among different studies groups.
|
AlCl3 administration results in significant decrease in the level of Brain-Derived Neutrophic Factor in comparison with control group; while administration of either aqueous or alcoholic extracts of annona squamosa leaves results in significant increase in BDNF Factor compared to diseased group Figure (3).
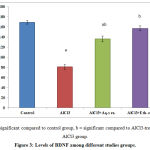 |
Figure 3: Levels of BDNF among different studies groups.
|
Acetyl choline esterase significantly increased following administration of AlCl3 for two months in AlCl3 treated rats, while treatment with either alcoholic or aqueous extracts of annona squamosa leaves results in significant decrease in Ach. E compared to diseased group Figure (4).
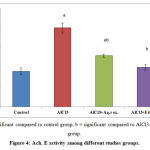 |
Figure 4: Ach. E activity among different studies groups.
|
Discussion
The present study was conducted to explore the possible neuro-protective properties of aqueous and alcoholic extract of A.squamosa on AlCl3 induced neuroinflammation in rat brain.
Previous studies reported that Al can induce neuroinflammation but the exact mechanism is still elusive, among which upregulation of oxidative stress and inflammatory biomarkers has been suggested 11which is responsible for intraneuronal metal homeostasis disruption.
Due to numerous drug related adverse effects, we seek for natural plant extract with more powerful therapeutic effects and less recorded side effects 12,13
In the present study, the AlCl3 inflamed rat brain showed disturbance of anti-oxidant protective mechanism; where oxidative stress biomarker MDA and NO are increased while the antioxidants SOD and GSH decreased.
Lipid peroxidation is considered as a corner stone of oxidative stress which is indicated by upregulation of the brain MDA level in AlCl3 treated rats14. Lipid peroxidation products results in dysfunction of the brain mitochondrial through disruption of the brain homeostasis between excitatory and inhibitory neuron in the brain matter 15.
The binding action of Al to iron regulatory protein, induces upregulation of iron-binding proteins, that stabilize the iron in the ferrous (Fe+2) state resulting into induction of Fenton reaction that end by lipid peroxidation 2.
The first defensive cellular line against free radical is SOD; it dismutase the superoxide anion to H2O2 and O216. GSH is another important defensive factor present in animal cell. It can, in addition to its detoxification action, protect against oxidative damage by the effect of its thiol group. Based on that GSH depletion increases lipid peroxidation and induce cellular oxidative damage14.
In the current work, AlCl3 administration showed a significant decrease in SOD activity and GSH content in the brain of AlCl3 treated rats. Our results are in accordance with study of Kuar et al.,17, he showed AlCl3 markedly increased lipid peroxide and nitrite associated with downregulation of SOD, GSH and glutathione.
On the other hand our investigation indicated that the aq.s and alc. extracts of AS leaves have strong antioxidant activity proved by increase SOD activity and GSH contents and to lower the MDA and NO levels in brain. In agreement with these results was 18 who reported that the aqueous and alcohol extracts of A. squamosa leaves counteract the effect of nitric oxide induced in vitro18.
Furthermore; Zhu et al., showed a significant decrease of the MDA and increase GSH levels in aqueous extract-treated diabetic rat 19 which was attributed to the presence of the flavonoids and phenols.
Acetyl Choline (Ach) is a biological membrane component essential in its integrity and play an important role in neuron function as learning and memory. Acetyl Choline Esrerase (Ach.E ) hydrolyses acetylcholine to Choline and Acetate in cholinergic brain synapses and at neuromuscular junctions 20. Upregulation of Ach. E activity enhance more degradation of Ach. and suppression of cholinergic receptors, leading to marked deterioration of related actions either at cholinergic neurons as learning and memory, or at non-cholinergic neuron as cell proliferation and neuron growth functions21.
Currently, AlCl3 induced significantly increase Ach. E activity. Our results run with Said and Abd Rabo 2017 2, he explained the cholino-toxic effects of Al which either by decreasing the Ach. E, blocking the provision of acetyl-CoA or decreasing the activity of membrane Ach. E 2.
Ach. E activity in the current study was significantly decreased in the aq.s and alc. extracts treated group compared with AlCl3 treated group. This may be due to presence of alkaloids which are naturally occurring compounds containing carbon, hydrogen, nitrogen, and usually oxygen and are primarily found in plants, especially in certain flowering plants; Alkaloids attenuate the development of neurodegenerative diseases through inhibiting the activity of Ach. E enzyme 22.
Currently; AlCl3 induced apoptotic activity in the brain cells as evidenced by increased brain caspase-3 and increased expression of inflammatory cytokines demonstrated by increased NF-κB.
AlCl3 exposure promotes IkB kinases activation, then phosphorylates and degrades IkB, leading to the activation of NF-kB and subsequently translocate into nucleus23. Furthermore, activated NF-κB prevents signal transducer and activator of transcription-3 activation and trigger neuronal apoptotic death through caspase-3 expression24.
The current study revealed that aq.s and alc. extracts of AS leaves decreased NF-κB.The anti-inflammatory effect of these extracts attributed to their phytochemical’s contents such as alkaloid, saponin, flavonoids as eugenol and quercetin which could pool to this assumption. Is quinoline alkaloid treatment inhibited the activation of NF-κB via inhibiting the degradation of IҡBα and down regulation of other NF-κB target genes in A375 melanoma cells 20.Also eugenol has modulatory effects on NF-κB signaling in a rat model of gastric carcinogenesis where it inhibit IκBα phosphorylation and degradation as well as down regulate IκB kinase β (IKKβ) that promote NF-κB activation 25.
Furthermore; it was found strong inverse correlation between BDNF expression and occurrence of Alzheimer’s disease26. The reduction of BDNF following AlCl3 administration in the current study was previously reported, leading to hippocampus shrinkage 2.
Aq.s or alc. extracts treated rats significantly increase brain BDNF levels and decrease caspase 3 activities restoring them to near their normal level.
The role of BDNF signaling pathway in decreasing caspase 3 expression is previously proved 27, that help in maintaining cell survival and decreasing apoptosis through increasing growth factor signaling transduction and maintenance of neuronal integrity 28.
In addition flavonoids can suppress apoptosis via its ability to inhibit the NF-ҡB signaling pathway and inhibit staurosporine-induced caspase3 activity as well act as competitive inhibitors of caspase-3 29.
In conclusion, this study provides evidence that the extracts of aq. and alc. Annona squamosa leaves extract have anti-inflammatory effect which is mediated through reduction NF-ҡβ and attenuated apoptotic effect of AlCl3 on neural cells through reduction of caspase 3. Furthermore, they exert beneficial effects in the development, survival, and maintenance of neurons in the central nervous system which is mediated through increased BDNF. Therefore, it is worthwhile to explore that extracts of aq. and alc. Annona squamosa leaves in managing neuroinflammatory effect of AlCl3.
Conflict of Interest
There is no Conflict of interest
Funding Source
Not funded
References
- Chen J, Venkat P, Zacharek A, Chopp M. Neurorestorative therapy for stroke. Front Hum Neurosci. 2014; 8: 3822014.
- Said MM, Abd Rabo MM. Neuroprotective effects of eugenol against aluminiuminduced toxicity in the rat brain. Arhiv za higijenu rada i toksikologiju. 2017;68:27-36.
- Willhite CC, Karyakina NA, Yokel RA, et al. Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Critical reviews in toxicology. 2014;44:1-80.
- Zatta P, Ibn-Lkhayat-Idrissi M, Zambenedetti P, Kilyen M, Kiss T. In vivo and in vitro effects of aluminum on the activity of mouse brain acetylcholinesterase. Brain research bulletin. 2002;59:41-45.
- Kawahara M, Kato-Negishi M. Link between aluminum and the pathogenesis of Alzheimer’s disease: the integration of the aluminum and amyloid cascade hypotheses. International journal of Alzheimer’s disease. 2011;2011.
- Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Report. 2003;8:365-370.
- Pandey N, Barve D. Phytochemical and pharmacological review on Annona squamosa Linn. International Journal of research in pharmaceutical and biomedical sciences. 2011;2:1404-1412.
- Singh T, Goel RK. Neuroprotective effect of Allium cepa L. in aluminium chloride induced neurotoxicity. Neurotoxicology. 2015;49:1-7.
- Kaleem M, Medha P, Ahmed Q, Asif M, Bano B. Beneficial effects of Annona squamosa extract in streptozotocin-induced diabetic rats. Singapore medical journal. 2008;49:800.
- Mannaa F, Ahmed H, Estefan S, Sharaf H, Eskander E. Saccharomyces cerevisiae intervention for relieving flutamide-induced hepatotoxicity in male rats. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 2005;60:689-695.
- Kumar A, Dogra S, Prakash A. Protective effect of curcumin (Curcuma longa), against aluminium toxicity: Possible behavioral and biochemical alterations in rats. Behavioural brain research. 2009;205:384-390.
- Mostafa-Hedeab G, Sati LM, Elnaggar HM, et al. Ameliorating Effect of Olive Leaf Extract on Cyclosporineinduced Nephrotoxicity in Rats. Iranian journal of kidney diseases. 2015;9:361.
- Helal EG, El-Sayed RA, Mostafa-Hedeab G, El-Gamal MS. The Therapeutic Effects of Stem Cell Enhancer on Changes of Some Physiological Parameters in Male Albino Rats Treated With Mixture of Food Additives) Food Preservative, Food Coloring Agent, and Flavor Enhancer). Egyptian Journal of Hospital Medicine. 2017;67.
- Özkaya A, Celik S, Yüce A, Şahin Z, YILMAZ Ö. The effects of ellagic acid on some biochemical parameters in the liver of rats against oxidative stress induced by aluminum. Kafkas Univ Vet Fak Derg. 2010;16:263-268.
- Ritter L, Kleemann D, Hickmann FH, et al. Disturbance of energy and redox homeostasis and reduction of Na+, K+-ATPase activity provoked by in vivo intracerebral administration of ethylmalonic acid to young rats. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2015;1852:759-767.
- Al-Otaibi SS, Arafah MM, Sharma B, Alhomida AS, Siddiqi NJ. Synergistic Effect of Quercetin and α-Lipoic Acid on Aluminium Chloride Induced Neurotoxicity in Rats. Journal of toxicology. 2018;2018.
- Kaur R, Afzal M, Kazmi I, et al. Polypharmacy (herbal and synthetic drug combination): a novel approach in the treatment of type-2 diabetes and its complications in rats. Journal of natural medicines. 2013;67:662-671.
- Nandhakumar E, Indumathi P. In vitro antioxidant activities of methanol and aqueous extract of Annona squamosa (L.) fruit pulp. Journal of acupuncture and meridian studies. 2013;6:142-148.
- Zhu L-q, Fan J-P, Huang Q-F, et al. Study on the anti-apopotosis induced by hypoxia/hypoglycemia and reoxygenation of panax notoginseng saponins in cultured rat hippocampal neurons. Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China journal of Chinese materia medica. 2003;28:52-55.
- Singh V, Panwar R. In vivo antioxidative and neuroprotective effect of 4-Allyl-2-methoxyphenol against chlorpyrifos-induced neurotoxicity in rat brain. Molecular and cellular biochemistry. 2014;388:61-74.
- Prasad SN. Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: behavioral and biochemical evidence. Neurochemical research. 2013;38:330-345.
- Dey A, Mukherjee A. Plant-Derived Alkaloids: A Promising Window for Neuroprotective Drug Discovery. Discovery and Development of Neuroprotective Agents from Natural Products: Elsevier; 2018:237-320.
- Hayden MS, Ghosh S. NF-κB in immunobiology. Cell research. 2011;21:223.
- McFarland BC, Gray GK, Nozell SE, Hong SW, Benveniste EN. Activation of the NF-κB pathway by the STAT3 inhibitor JSI-124 in human glioblastoma cells. Molecular Cancer Research. 2013;11:494-505.
- Manikandan P, Vinothini G, Priyadarsini RV, Prathiba D, Nagini S. Eugenol inhibits cell proliferation via NF-κB suppression in a rat model of gastric carcinogenesis induced by MNNG. Investigational new drugs. 2011;29:110-117.
- Lin W-T, Chen R-C, Lu W-W, Liu S-H, Yang F-Y. Protective effects of low-intensity pulsed ultrasound on aluminum-induced cerebral damage in Alzheimer’s disease rat model. Scientific reports. 2015;5:9671.
- Yao R-Q, Qi D-S, Yu H-L, Liu J, Yang L-H, Wu X-X. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF–TrkB–PI3K/Akt signaling pathway. Neurochemical research. 2012;37:2777-2786.
- Lei X, Chao H, Zhang Z, et al. Neuroprotective effects of quercetin in a mouse model of brain ischemic/reperfusion injury via anti‑apoptotic mechanisms based on the Akt pathway. Molecular medicine reports. 2015;12:3688-3696.
- White JB, Beckford J, Yadegarynia S, Ngo N, Lialiutska T, d’Alarcao M. Some natural flavonoids are competitive inhibitors of caspase-1,-3, and-7 despite their cellular toxicity. Food chemistry. 2012;131:1453-1459.
- Saha R. Pharmacognosy and pharmacology of Annona squamosa. International Journal of Pharmacy & Life Sciences. 2011;2:1183-1189.








