Moushira Erfan Zaki1*, Eman Youness2, Mohamed Gadelhak3, Marwa Shehab4, Safinaz El-Toukhy2, Doaa Soliman5, Walaa Yousef1 and Hala El-Bassyouni3
1Biological Anthropology Department, Medical Research Division, Giza, Egypt
2Medical Biochemistry Department, Medical Research Division, National Research Centre, Giza, Egypt
3Clinical Genetics Department, National Research Centre, Cairo, Egypt
4Cytogenetic Department, National Research Centre, Cairo, Egypt
5Department of Pediatrics, Faculty of Medicine, Benha, Egypt
Corresponding Autjor E-mail: moushiraz@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1828
Abstract
Obesity is the most common cause of metabolic problems in Prader-Willi syndrome (PWS). Obesity has been joined to a low grade pro-inflammatory state, in which impairments in the oxidative stress and antioxidant mechanism could be involved. The aim of the work is to investigate the level of DNA damage and inflammatory marker neutrophil elastase in PWS patients. The study included 21 children with PWS detected by fluorescence in situ hybridization (FISH) method and 20 age and sex healthy matched obese controls. Their mean age was 6 ± 2.24 years. Leukocyte DNA damage was evaluated by comet assay and neutrophil elastase was assessed by ELISA. All patients presented with distinctive faces, hypotonia, obesity, short stature and various other criteria. FISH revealed deletion 15q11–13 in all PWS patients. The mean of DNA damage frequency was significantly higher in PWS than controls. The body fat%, body mass index (BMI) z score were elevated in PWS cases. Moreover, the neutrophil elastase was significantly higher in patients compared to controls. The present study highlights the existence of oxidative stress and inflammation in Prader Willi syndrome that may have a role in the management and treatment of these patients.
Keywords
Comet Assay Prader Willi syndrome; DNA damage; Neutrophil Elastase; BMI
Download this article as:| Copy the following to cite this article: Zaki M. E, Youness E, Gadelhak M, Shehab M, El-Toukhy S, Soliman D, Yousef W, El-Bassyouni H. DNA Damage and Neutrophil Elastase in Children with Prader-Willi Syndrome. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Zaki M. E, Youness E, Gadelhak M, Shehab M, El-Toukhy S, Soliman D, Yousef W, El-Bassyouni H. DNA Damage and Neutrophil Elastase in Children with Prader-Willi Syndrome. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2ZuhnNZ |
Introduction
Prader, Willi, and others described Prader-Willi syndrome (PWS) in 1956, and more than 1500 subjects have now been reported in the medical literature.
Prader-Willi syndrome (PWS; 176270) is a complex genetic disorder that affects many parts of the body. The absence of expression of the paternal genes from the imprinted chromosomal region 15q11.2—q13 is the cause of PWS syndrome 1,2. PWS is distinguished by hypotonia (94% of subjects), childhood obesity (94%), deficiency in mental status with average IQ of 65, ranged from 20 to 90 (97%), shortness in stature (76%), small hands and feet (83%), hypogenitalism/hypogonadism (95%), and a typical face with narrow bi-frontal diameter, almond-shaped eyes and a triangular mouth 3,4.The occurrence of PWS is expected to be about one in 10,000 to 25,000 live births and is considered the most frequent syndrome cause of marked human obesity. Hyperphagia, persistent hunger, decreased perception of satiety and an uncontrollable appetite with impaired emesis are thought to cause obesity 4.
Oxidative stress is considered one of several adverse cellular responses to obesity. It can then damage cellular structures and triggers a low grade pro-inflammatory state. Association between adipose tissue inflammation and insulin resistance is considered as an important risk factor in the etiology. Immune cells such as macrophages, T–cells, B–cells, mast cells and eosinophils have all been involved in this process5. Neutrophils are the first immune cells which respond to inflammation and can promote a more chronic inflammatory state by helping to recruit macrophages and interacting with antigen presenting cells. Neutrophil elastase (NE) is one of several proteases which is secreted from neutrophils and it canendorse inflammatory responses in several diseases. Recent studies have suggested that obesity is associated with chronic adipose tissue inflammation, which results in increased levels of proinflammatory factors, such as neutrophil elastase6–9
This study aimed to investigate whether PWS is associated with oxidative stress and inflammatory marker neutrophil elastase.
Materials and methods
We studied 82 obese patients, 42 revealed the clinical picture of PWS and 21 were confirmed as deleted by FISH.
The mean age of PWS cases with genetic confirmation was 6 ± 2.24 years and age ranged from 4 to 17 years, 10 males and 11 females. For comparison, 20 healthy age and sex matched obese controls were recruited. PWS were referred to the outpatient clinic of the Pediatric Department, Faculty of Medicine, BenhaUniversity. Written informed consent was obtained from all the patients.
Exclusion criteria for all participants included type 1 diabetes , presence of metabolic disease (confirmed by use of drugs), pulmonary diseases and sepsis.
All participants were subjected to thorough medical history and clinical examination, and anthropometric measurements were performed as follows: height was measured to the nearest 0.5 cm; body weight was measured to the nearest 0.1 kg; body mass index (BMI) was calculated as weight/height2 (kg/m2). Z weight and ZBMI wascalculated according to WHO Anthro Plus program10. Body composition was carried out using a body composition analyzer TANITA SC–330 (Tanita Corporation, Tokyo, Japan). BF% was estimated to the nearest 0.1%.
Laboratory diagnosis of deleted type of PWS is achieved by FISH performance according to the modification of 11 and manufacturer instructions using locus-specific probe (LSI) Prader-Willi SNRPN (15q11) supplied by Kreatech Diagnostics (United Kingdom). Twenty one patients diagnosed by FISH, that had deletion as the ethiology of the disease were included in this study.
The level of serum neutrophil elastase was assessed by ELISA, supplied by Immunodiagnostic AG Stubenwald-Allee 8 Ad-64625 Bensheims12,13. Sample size of 21and 20 achieve 100% power to detect a difference of neutrophil elastase 6.5 between the null hypothesis that both groups means are 15.9 and the alternative hypothesis that means of group 2 is 9.4 with estimated group standard deviations of 5.0and 2.4 and with a significance level(alpha)of 0.05using a two-sided two-sample t-test14. DNA damage in leukocytes was performed and estimated according to Palus et al., 1999 15.
Measurement of Comet Assay
Cell preparation
Peripheral blood leukocytes were isolated by centrifugation (30 min at 1300g) in Ficoll-Paque density gradient (Pharmacia LKB Biotechnology, Piscataway, NJ, USA). After centrifugation, leukocytes in the buffy coat; were aspirated and washed twice by phosphate-buffered saline at pH 7.4 (PBS).
Preparation of cell microgels on slides
The comet assay was performed according to 15with modifications according to 16. Cell microgels were prepared as layers. The first layer of gel was made by applying 100 μl of normal melting point agarose (0.7%) onto a precleaned microscope charged slides and cover sliped gently. The coverslip was removed after the agarose solidified at 4ᵒC .Low melting-point agarose (0.5%) was prepared in 100 mmol/L PBS and kept at 37 ᵒC. Mononuclear cells were mixed with the low melting-point agarose and 100 μl of the mixture was applied to the first gel layer. The slides were then covered with a covers lip and placed at 4ᵒC for solidification. After the second layer solidified, the covers lips were removed from the cell microgels. A final layer of low-melting agarose was added followed by coverslips, left to solidify for 10 minutes then the coverslips were removed.
Lysis of cells, DNA unwinding, gel electrophoresis, DNA staining
The slides were covered with 100 ml of fresh lysis buffer at pH 10 at 4ᵒC for 1h. Buffer contains; 2.5 mol/L NaCl, 100 mmol/L EDTA, 1% sodium hydroxide, 10 mmol/L Tris, 1% Triton X-100, 10% (Dimethylsulfoxide) DMSO. After draining, microgels slides were treated with DNA unwinding solution (300 mmol/L NaOH, 1 mmol/L EDTA, pH 13) for 30 min at 4ᵒC, and placed directly into a horizontal gel electrophoresis chamber filled with DNA-unwinding solution. Gels were run with constant current (300 mA at 4ᵒC) for 30 min. After electrophoresis, the microgels were neutralized with 0.4 M Trisma base at pH 7.5 for 10 min. The slides were stained with 20 μlethidium bromide (10 μg/ml).
Visualization and analysis of Comet Slides
The slides were examined at 400× magnification using a fluorescence microscope (Leica Microsystems, CMS GM b H, Wetzlar, Germany. Model DM 2500), equipped with an excitation filter of 549 nm and a barrier filter of 590 nm. A damaged cell is visualized as each cell had the appearance of a comet, with a brightly fluorescent head and a tail to one side formed by the DNA containing strand breaks that were drawn away during electrophoresis. Samples were analyzed by counting the damaged cell out of 100 cells per slide to calculate the percent of damage.
Written informed consent was obtained from the parents afterthe explanation of the aim of the study. All the patients’ data were confidential, neither the data nor the collected samples were used in any other research.
Statistical analysis
The statistical analysis was conducted with the Statistical Package for Social Sciences (SPSS version 17. Initially, the normality of the data using the Kolmogorov-Smirnov test was tested. Data are presented as mean ± standard deviation.
Mann–Whitney U test has been used to compare comet assay results between the PWS cases and controls. While, Student t-test was applied for comparisons of continuous variables as the anthropometric and biochemical parameters. P values less than 0.05 were considered to be statistically significant for each test.
Results
Out of the 21 diagnosed patients with Prader Willi syndrome, fourteen patients (66.6%) were the offspring of consanguineous marriage. Dysmorphic features were present in 10 patients including round face, broad bossing forehead, almond eye. Hypotonia was present in all patients. Abdominal and pelvic ultrasonography showed hepatosplenomegaly in 4 patients. Genitalia showed absent labia minora and or hypoplastic clitoris in 7 females and micropenis or hypospadias in 6 males. Intellectual disability was present in 13 patients ranging from mild to moderate ID (Table 1).
Table 1: Clinical and demographic data of PWS patients.
| Clinical Picture | DM | FH | Cons | Age/sex | Case |
| Round face, micropenis, hypotonia | + | – | + | 7/M | 1 |
| Broad bossing forehead, full cheeks, absent labia minora, mild ID,hypotonia | + | + | + | 13/F | 2 |
| Broad bossing forehead, full cheeks, hypoplastic clitoris, hypotonia | + | – | + | 17/F | 3 |
| Almond eye, broad bossing forehead, full cheeks, hyperpigmentation, hypotonia, absent labia minora and clitoris | – | – | + | 9/F | 4 |
| Broad bossing forehead, large ears, short fingers,hypotonia,clinodactly, café au lait patches | – | – | + | 11/M | 5 |
| Microcephaly, undescended testis, micropenis, moderate ID,hypotonia | – | – | – | 7/M | 6 |
| Broad bossing forehead, hypospadias, mild ID, hypotonia | – | + | + | 10/M | 7 |
| Broad bossing forehead, micropenis, moderate ID,hypotonia | – | – | + | 12/M | 8 |
| Hypospadias, hyperpigmentation, nocturnal enuresis, mild ID, hypotonia | – | – | + | 7/M | 9 |
| Broad bossing forehead, hypoplastic clitoris, mild ID,hypotonia | + | + | + | 15/F | 10 |
| Broad bossing forehead, short fingers, absent labia minora, moderate ID,hypotonia | + | + | + | 10/F | 11 |
| Full cheeks, nystagmus, moderate ID,hypotonia | + | – | + | 7/F | 12 |
| Broad bossing forehead, micropenis, hepatosplenomegaly, mild ID,hypotonia | – | – | – | 11/M | 13 |
| Almond eyes, broad bossing forehead, full cheeks, epicanthal folds, syndactly, simian crease, acanthosisnegricans, hypotonia,hypoplastic labia minora, absent clitoris | + | + | + | 9/F | 14 |
| Broad bossing forehead, absent labia minora, hepatosplenomegaly, nystagmus,hypotonia | – | – | + | 7/F | 15 |
| Broad bossing forehead, bulbus nose, hypospadias, mild ID,hypotonia | + | – | – | 8/M | 16 |
| Broad bossing forehead, moderate ID,hypotonia | + | – | – | 10/M | 17 |
| Full cheeks, hepatosplenomegaly,hypotonia | – | – | – | 5/F | 18 |
| Broad bossing forehead, hypospadias, mild ID, hypotonia | – | – | + | 6/M | 19 |
| Almond eyes, broad bossing forehead, full cheeks, epicanthal folds, hypotonia,hypoplastic labia minora, moderate ID | + | + | + | 9/F | 20 |
| Full cheeks, hepatosplenomegaly, hypotonia | – | + | – | 4/F | 21 |
Cons=ConsanguinityFH=Family history DM=dysmorphism
FISH revealed deletion 15q11–q13 in 21 patients. Z weight was 3.7±2.1, BMI z score was 5.3±1.9 and body fat % was 44.5±15.9 (Table 2).
Table 2: Mean age, anthropometric indices and body fat % in PWS and obese healthy controls
| Anthropometric indices | PWS
Mean ± SD |
Obese healthy controls |
| Age (year) | 6.7 ± 2.2 | 7.1 ± 2.3 |
| Z weight | 3.7 ± 2.1 | 3.2 ± 1.9 |
| Z BMI | 5.3 ± 1.9 | 4.9 ± 1.9 |
| Body Fat % | 44.5 ± 15.9 | 41.5 ± 12.9 |
Levels of neutrophil elastase and DNA damage in Prader Willi patients were higher compared to controls (Table 3).
Table 3: Levels of neutrophil elastase and DNA damage in PWS and controls
| Neutrophil elastase | Patients
Mean ± SD |
Obese healthy controls
Mean ± SD |
| 15.9 ± 5* | 9.4±2.4 | |
| DNA damage | 74.0 ±10.5** | 13.5± 2.6 |
The DNA damage in the leukocytes of the patients (Fig. 1 and Fig. 2) was higher in patients than that of obese healthy controls (Fig. 3). The DNA was slightly damaged in some cases (Fig.1), while it was more severe in others (Fig.2). Fig. 4 shows image of FISH, 46,XX,del(15)(q11.2).ish del(15)(q11.2)(SNRPN-).
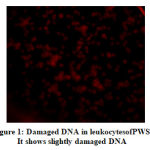 |
Figure 1:Damaged DNA in leukocytesofPWS. It shows slightly damaged DNA |
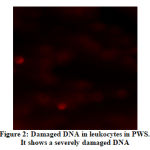 |
Figure 2: Damaged DNA in leukocytes in PWS. It shows a severely damaged DNA |
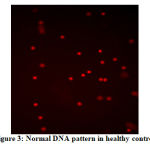 |
Figure 3: Normal DNA pattern in healthy control |
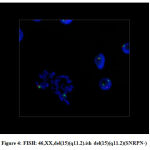 |
Figure 4: FISH: 46,XX,del(15)(q11.2).ish del(15)(q11.2)(SNRPN-) |
Discussion
PWS occurs equally in males and females and in all ethnic group salthough disproportionately more Caucasians are reported17–19. This is consistent with our study as we had 10 males and 11 females.
Fourteen patients were the off springs of consanguineous marriage. High incidence of consanguinity was noticed in this study (66.6%) as in our population consanguinity is high about 60% 20–22. Dysmorphic features were present in 10 patients including round face, broad bossing forehead, almond eye. All patients in our study showed hypotonia which is a basic diagnostic criterion for PWS patients that might be due to the decrease of physical activity and depression 23. In meta-analysis of PWS the behavioral features associated with PWS has not been fully investigated. Significance difference in clinical features of cognitive development and psychiatric illness are associated with different molecular defects of PWS24.
Abdominal and pelvic ultrasonography showed hepatosplenomegaly in 4 patients may be due to the extreme obesity and associated liver enzyme dysfunctions. Genitalia showed absent labia minora and or hypoplastic clitoris in 7 females and micropenis or hypospadias in 6 males. Intellectual disability was present in 13 patients ranging from mild to moderate ID. These findings are consistent with previous reports who reported that clinical features include hypotonia, hypogonadism, short stature, intellectual disability, and obesity3,25. Our results show that Z weight and Z BMI were high in PWS patients. Also, body fat % was elevated in PWS cases. This findings in agreement with previous study26noted that the main cause of morbidity and mortality in PWS patients is obesity .
Excessive appetite and overeating and lack of sexual development are some of the symptoms of PWS 27. In comparison with obese subjects, those with PWS had lower energy expenditure and decreased lean body mass. Previous study reported that the energy expenditure of at rest in PWS was significantly suppressed by 16% compared to the obese subjects28 .
PWS is a complex phenomenon caused by disturbance in the hypothalamic satiety regulatory mechanisms contributed by several hormones, body composition differences, low physical activity, altered feeding behavior and increased dietary intake 26.
PWS represents the most common form of genetic obesity; one of the most concerning symptoms is hyperphagia. Obesity is a major cause of increased morbidity and mortality in patients with PWS29,30. The present study is the first one which examined the relationship between serum elastase and DNA damage in Prader Willi patients. This study found that serum elastase level and DNA damage were significantly higher in Prader Willi patients as compared to age and sex matched healthy controls.
Huang et al. 2017 highlighted that inflammation plays an important role in the development of obesity-related complications. Extensive investigation has demonstrated the relationship between obesity and increased systemic inflammation7,9,32. Subsequently, neutrophils are the most abundant leukocytes that are critical for innate immunity and acute inflammation and neutrophil elastase is considered a secretagouge for cytokines and a modulator of inflammation5,7.
Neutrophils play a critical role in protecting the host against microbial pathogens, but they produce proteolytic enzymes that can destroy tissue and lead to organ failure such as NE that cause lung injury and respiratory failure via pulmonary vascular and alveolar damage33.
El-Eshmawy et al. 2011 suggested that neutrophils play an essential role in obesity-related inflammation. Furthermore, other study34 elucidated that elastase may be used as a non-specific indicator to screen inflammation and infection. Obesity is also associated with metabolic dysfunction that contributes to higher incidence of inflammation-associated chronic disease and greater susceptibility to infection 35.
Currently, it is widely accepted that oxidative stress (OS) is involved in the pathogenesis of PWS36 . We verified the presence of OS by estimating the DNA damage in the leukocytes of the patients which was higher than that of controls. Previous studies have already exhibited a relationship between obesity and oxidative stress 37. Antioxidants such as vitamin A, E and C could improve oxidative stress and consequently the low-grade inflammation. Our study proposed that obesity in PWS is associated with chronic low-grade inflammation, this coincides with the findings of Khan et al., 2018. Some studies 38 used medications such as Beloranib that improved inflammatory biomarkers. Antioxidants and anti-inflammatory drugs could improve the condition in these patients. Moreover, epigenetic-based therapy for the PWS has been reported recently39.
Conclusion
The current study draws attention to the need of investigating the interplay between oxidative stress and inflammation with obesity in PWS.
Competing interests
The authors declare that they have no competing interests.
References
- Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. genetest review Prader-Willi syndrome GeneTest review. 2012;14(1). doi:10.1038/gim.0b013e31822bead0.
- Hashemi B, Bassett A, Chitayat D, et al. Deletion of 15q11.2(BP1-BP2) region: Further evidence for lack of phenotypic specificity in a pediatric population. Am J Med Genet Part A. 2015;167(9):2098-2102. doi:10.1002/ajmg.a.37134.
- Khedr AA, Meguid NA, Mohamed AM, et al. Genetic diagnosis of Prader–Willi syndrome. Middle East J Med Genet. 2016;5(2):45-53. doi:10.1097/01.MXE.0000484369.78928.9e.
- Findings G. HHS Public Access. 2016;10:1-25.
- Talukdar S, Oh DY, Bandyopadhyay G, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18(9):1407-1412. doi:10.1038/nm.2885.
- Lumeng CN, Saltiel AR. Review series Inflammatory links between obesity and metabolic disease. Life Sci. 2011;121(6):2111-2117. doi:10.1172/JCI57132.In.
- Mansuy-Aubert V, Zhou QL, Xie X, et al. Imbalance between neutrophil elastase and its inhibitor α1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 2013;17(4):534-548. doi:10.1016/j.cmet.2013.03.005.
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85-97. doi:10.1038/nri2921.
- Xu X, Su S, Wang X, et al. Obesity is associated with more activated neutrophils in African American male youth. Int J Obes. 2015;39(1):26-32. doi:10.1038/ijo.2014.194.
- Organization WH. Software for assessing growth of the worlds children and adolescents. Who Antro Plus Pers Comput Man. 2009.
- Kuo WL, Tenjin H, Segraves R, Pinkel D, Golbus MS, Gray J. Detection of aneuploidy involving chromosomes 13, 18, or 21, by fluorescence in situ hybridization (FISH) to interphase and metaphase amniocytes. Am J Hum Genet. 1991;49(1):112-119.
- Kit PE, Elastase PMN, Kit E. PMN-Elastase ELISA Kit. 2013.
- El-Eshmawy MM, El-Adawy EH, Mousa AA, et al. Elevated serum neutrophil elastase is related to prehypertension and airflow limitation in obese women. BMC Womens Health. 2011;11:1-7. doi:10.1186/1472-6874-11-1.
- Hintze J. PASS 11. Kaysville, Utah, USA NCSS, LLC. 2011.
- Palus J, Dziubałtowska E, Rydzyński K. DNA damage detected by the comet assay in the white blood cells of workers in a wooden furniture plant. Mutat Res Toxicol Environ Mutagen. 1999;444(1):61-74. doi:10.1016/S1383-5718(99)00089-3.
- Blasiak J, Gloc E, Drzewoski J, et al. Free radical scavengers can differentially modulate the genotoxicity of amsacrine in normal and cancer cells. Mutat Res Toxicol Environ Mutagen. 2003;535(1):25-34.
- Hudgins L, Geer JS, Cassidy SB. Phenotypic differences in African Americans with Prader- Willi syndrome. 1998;1(1):0-2.
- Smith A, Egan J, Ridley G, et al. Birth prevalence of Prader-Willi syndrome in Australia. Arch Dis Child. 2003;88(3):263-264. doi:10.1136/adc.88.3.263.
- Smith A, Hung D. The dilemma of diagnostic testing for Prader-Willi syndrome. 2017;6(1):46-56. doi:10.21037/tp.2016.07.04.
- Afifi HH, El-Ruby MO, El-Bassyouni HT, et al. The most encountered groups of genetic disorders in Giza Governorate, Egypt. Bratisl Lek List. 2010;111(2):62-69.
- Shawky RM, El-Awady MY, Elsayed SM, Hamadan GE. Consanguineous matings among Egyptian population. Egypt J Med Hum Genet. 2011;12(2):157-163.
- Shawky RM, Elsayed SM, Zaki ME, Nour El-Din SM, Kamal FM. Consanguinity and its relevance to clinical genetics. Egypt J Med Hum Genet. 2013;14(2). doi:10.1016/j.ejmhg.2013.01.002.
- Elena G, Bruna C, Benedetta M, Stefania DC, Giuseppe C. Prader-Willi Syndrome: Clinical aspects. J Obes. 2012;2012(October 2012). doi:10.1155/2012/473941.
- Yang L, Zhan G, Ding J, et al. Psychiatric illness and intellectual disability in the Prader–Willi syndrome with different molecular defects-a meta analysis. PLoS One. 2013;8(8):e72640.
- Cassidy SB, Driscoll DJ. Prader-Willi syndrome. Eur J Hum Genet. 2009;17(1):3-13. doi:10.1038/ejhg.2008.165.
- Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Mechanisms of obesity in Prader–Willi syndrome. Pediatr Obes. 2018;13(1):3-13. doi:10.1111/ijpo.12177.
- Rao VRBV, Rao MV, Bhattamisra SK. Obesity an overview: Genetic conditions and recent developments in therapeutic interventions. Diabetes Metab Syndr Clin Res Rev. 2019.
- Butler MG, Theodoro MF, Bittel DC, Donnelly JE. Energy expenditure and physical activity in Prader–Willi syndrome: comparison with obese subjects. Am J Med Genet Part A. 2007;143(5):449-459.
- East N, Maroney M. Topiramate in the treatment of {Prader}-{Willi} syndrome: {A} case report. Ment Heal Clin. 2017;7(1):7-9. doi:10.9740/mhc.2017.01.007.
- Salah EM, El-Bassyouni HT, Kholoussi S, Shehab M, Kandeel WA. Behavioral problems, biochemical, and anthropometric characteristics of patients with Prader–Willi syndrome. Middle East J Med Genet. 2015;4(2):63-69. doi:10.1097/01.MXE.0000465681.35933.7a.
- Huang JY, Zhou QL, Huang CH, et al. Neutrophil elastase regulates emergency myelopoiesis preceding systemic inflammation in diet-induced obesity. J Biol Chem. 2017;292(12):4770-4776. doi:10.1074/jbc.C116.758748.
- Ferretti G, Bacchetti T, Masciangelo S, Grugni G, Bicchiega V. Altered inflammation, paraoxonase-1 activity and HDL physicochemical properties in obese humans with and without Prader-Willi syndrome. Dis Model Mech. 2012;5(5):698-705. doi:10.1242/dmm.009209.
- Potey PMD, Rossi AG, Lucas CD, Dorward DA. Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential. J Pathol. 2019;247(5):672-685.
- Mania-Pramanik J, Potdar SS, Vadigoppula A, Sawant S. Elastase: A predictive marker of inflammation and/or infection. J Clin Lab Anal. 2004;18(3):153-158. doi:10.1002/jcla.20015.
- Singer K, Lumeng CN. The initiation of metabolic inflammation in childhood obesity. J Clin Invest. 2017;127(1):65-73.
- Anichini C, Lotti F, Longini M, et al. Antioxidant effects of potassium ascorbate with ribose therapy in a case with Prader Willi Syndrome. Dis Markers. 2012;33(4):179-183. doi:10.3233/DMA-2012-0922.
- Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem. 2012;68(4):701-711. doi:10.1007/s13105-012-0154-2.
- Miller J, Strong T, Heinemann J. Medication trials for hyperphagia and food-related behaviors in Prader–Willi syndrome. Diseases. 2015;3(2):78-85.
- Kim Y, Wang SE, Jiang Y. Epigenetic Therapy of Prader-Willi syndrome. Transl Res. 2019.








