Inas R. El- Alameey1, Hanaa H. Ahmed2 and Mones M. Abushady1
1Child Health Department, Medical division,National Research Centre, Egypt
2Hormones department, Medical division, National Research Centre, Egypt
Corresponding Author E-mail: inasno@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1799
Abstract
Dipeptidyl peptidase-IV (DPP-IV) is a circulating glycoprotein that reduces uptake of insulin-stimulated glucose and is related to obesity and metabolic syndrome. However, the influence of exercise and nutritional plan on serum DPP-IV in children and adolescents with metabolic syndrome remains unclear. To judge serum activity of DPP-IV in obese children and adolescents with and without metabolic syndrome, and to assess the impact of exercise, and nutritional regimen on serum DPP-IV activity, metabolic syndrome components, and insulin resistance issue in children and adolescents with obesity. This study included 80 Egyptian individuals; 40 obese subjects (group Ι), and 40 healthy non-obese subjects (group ΙΙ) with matched age and sex. Serum DPP-IV activity, lipid panel, glucose, and insulin levels were quantified. Serum DPP-IV enzyme activity of obese patients with MS revealed significant elevation than those who did not have MS, and control counterparts (P < 0.001). The serum DPP-IV enzyme activity, lipid panel except HDL, and HOMA-IR were significantly suppressed after weight loss due to exercise and nutritional regimen. In obese patients at baseline, serum High BMI Z-score, W/H ratio, BAI, and serum triglycerides are the main actors in stimulating DPP-IV enzyme activity in obese patients by linear regression analysis, and they were positively correlated with DPP-IV enzyme activity. BMI z-score, W/H ratio, BAI, and serum triglycerides are closely associated with high serum DPP-IV enzyme activity in obese patients. The reduced DPP-IV enzyme activity after weight loss is paralleled by a significant modulation of HOMA-IR.
Keywords
Dipeptidyl Peptidase-IV; childhood; adolescent; obesity; metabolic syndrome
Download this article as:| Copy the following to cite this article: El- Alameey I. R, Ahmed H. H, Abushady M. M. Dipeptidyl Peptidase IV: A Target for Improving Metabolic Syndrome Components in Obese Children and Adolescents. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: El- Alameey I. R, Ahmed H. H, Abushady M. M. Dipeptidyl Peptidase IV: A Target for Improving Metabolic Syndrome Components in Obese Children and Adolescents. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2tFyhgu |
Introduction
Dipeptidyl-peptidase IV (DPP-IV) is present as a soluble enzyme in plasma [1], and on the surface of T-cell lymphocytes as a membrane-bound antigen, on blood vessels endothelial layer, skeletal muscle, liver, and adipose tissue [2], and in the kidney [3]. It also known as CD26 and acts as a key player in controlling satiety by the hypothalamus, immune function by activating T-cells, and in monitoring release of insulin [4]. Also, it is accounted as a cytokine that has been involved in the onset of metabolic syndrome and type II diabetes. It impairs insulin signaling in the skeletal muscle thereby affecting glucose tolerance and raising cardiometabolic risk [5]. The development of pharmacological DPP-IV inhibitors enhances glycemic control and cardiometabolic health [6]. According to previous studies; it seems that DPP-IV activity has a certain link with body composition in obese people [7-8].
Exercise and nutritional plan has been found to reduce metabolic syndrome criteria and modulate glucose tolerance and insulin sensitivity in adults [9-13]. To our knowledge, assessment of DPP-IV activity in children after weight loss due to lifestyle changes is lacking, and the changes in serum DPP-IV activity in obese children and adolescents as regard to metabolic syndrome are still unclear. Therefore, the focus of the present approach was to assess DPP-IV enzyme activity in serum of obese children and adolescents with metabolic syndrome before and after weight loss and to investigate the association of serum DPP-IV enzyme activity with adiposity parameters and metabolic syndrome components as well as insulin resistance in a sample of Egyptian obese patients.
Subjects and Methods
Design and setting of the study
This descriptive comparative case–control study consisted of 80 children and adolescents aged 10–14 years. They were chosen from the Child Health Outpatients’ Clinic at the Center of Excellence, National Research Centre (NRC) in Egypt.
Sample size
The sample size was calculated to assess the risk factors probably affecting serum DPP-IV enzyme activity in obese children. The sample size was calculated by using Open Epi sample size calculator. The calculation based on a confidence interval of 95%, power 80%, ratio of control to cases is 1, and the least extreme Odds ratio to be detected is 2.
Subjects
The study was carried out on 80 Egyptian children; 40 obese (group Ι), and 40 healthy non-obese subjects (group ΙΙ) age and sex matched. Group Ι was subdivided according to the criteria of MS into 2 groups (group Ιa) consisted of 20 obese patients who apply for the criteria of MS, and (group Ιb) enclosed 20 obese without MS. Metabolic syndrome was determined by as age-modified criteria with a least 3 of the 5 criteria: systolic or diastolic blood pressure, waist circumference, fasting blood glucose, HDL, and triglycerides [14]. For calculation of the metabolic syndrome risk score, we used Adult Treatment Panel-III definitions as follows: 1) blood pressure equals 135/85 mmHg, 2) abdominal obesity characterized by a waist circumference equals 98 cm for males and 88 cm for females, 3) fasting glucose, 110 mg/dl, 4) serum triglycerides, 150 mg/dl, and 5) HDL-cholesterol, 40 mg/dl. Patients with a risk score of ≥ 3 are determined as having metabolic syndrome [15].
Criteria of selection
Obese patients were selected according to the inclusion and exclusion criteria. Inclusion criteria include children and adolescents with simple obesity based on BMI for age percentile > 95 th percentile and BMI for age z-score ≥ 2 on the growth charts according to WHO growth curve [16]. Exclusion criteria enclosed endocrinal and genetic causes of obesity, children and adolescents with chronic debilitating diseases, and also the use of drugs that influence blood pressure, glucose level or lipid profile.
Standard protocol approvals and consents
Before the study enrollment, written consent was get from the parents after explaining the nature of the study, and according to the approval obtained from the Institutional Medical Research Ethics Committee of the NRC, Egypt with the Ethics Committee approval Number 18041.
Methods
A full history was obtained from parents with specific stress on hypertension, and diabetes mellitus. Clinical examination was done for all subjects. Puberty was determined by rating breast development in girls and genital development in boys and also pubic and axillary development for boys and girls [17].
Anthropometric parameters were measured for all subjects at baseline and 6 months after weight loss. Height was measured in centimeters by Harpenden stadiometer. Body weight was recorded in kilograms using an electronic weight scale [18]. The BMI was calculated as weight (in kilograms) divided by height (in meters) squared. A body mass index from 18.5 to 24.9 means that the subject falls in the normal range, 25.0 to 29.9 means a child is overweight and a score of 30.0 and higher means the patients is obese. BMI for age percentile and z-score was calculated based on the WHO growth standards [16] with the help of the Anthro-plus Program of PC.
Waist circumference (WC) was measured at the midpoint between the lower border of the ribcage and the iliac crest using a non-elastic tape measure. For measuring the hip circumference (cm), the subject stood erect with feet together and weight evenly distributed on both feet. The non-stretchable stain steal tape was held horizontally around the maximum extension of the buttocks, the reading was approximated to the nearest 0.1 cm. A waist: hip ratio over 0.96 for males and 0.83 for females are considered to be at risk for cardiovascular disease associated with obesity according to the Centers for Disease Control and Prevention (CDC) [19].
Body fat percentage (%), fat mass (kg), and body adiposity index (%) were determined by using Tanita Segmental Body Composition Analyzer. They provide the most accurate and detailed analysis of adiposity measures. The Body adiposity index is a good tool in measuring adiposity. It is considered to be high if it is >32 for obese females, and >25 for obese males [20].
At the baseline evaluation, dietary history was collected from parents including type of food and eating behaviors, physical activity daily. Food frequency questionnaire for children and adolescents was brief, easy to read, self-explanatory, and age appropriate. Questionnaires that were completed by parents at home are used to determine dietary intake. A personalized balanced normocaloric diet adjusted by age using the nutrient density method [21] was given to the parents of obese patients. After 6 months of follow-up, the same clinical examination, anthropometric measurements, and metabolic evaluations were repeated in all obese individuals.
Laboratory analyses
A venous blood sample was taken from every participant after overnight fasting, and the serum was separated within one hour after blood collection and preserved at – 20 C until assessment of serum DPP-IV enzyme activity with a commercially available ELISA kit (Glory Science Co., Ltd., USA), according to manufacturer’s instructions. The color change was measured at a wavelength of 450 nm. The serum DPP4 enzyme activity was then determined by comparing the O.D. of the samples to the standard curve.
Serum glucose, and lipid profile were determined by colorimetric method using the Stanbio kit (USA). Insulin in serum was evaluated by an immune-enzymometric assay using the Monobind Inc. kit (USA).
Insulin resistance was calculated by Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) = fasting insulin (µU/ml) x fasting glucose (mg/dl)/405 [23]. The healthy range should be equal to 1.0 (0.5–1.4) but above 1.9 indicates early insulin resistance, while, above 2.9 indicates significant insulin resistance [24].
Statistics
Statistical analysis was performed using the statistical package for social sciences, version 23 for Windows (SPSS Inc., USA). Continuous data were expressed as mean ± SD and were compared using Student’s t-test. Non-parametric data were expressed as a median and range. Between groups, comparisons were done using the Mann Whitney test. Chi-square was carried out for comparison of qualitative data that were expressed as frequencies and percentages. Pearson Correlation was done for relations between variables. The analysis of variance (ANOVA) with the Turkey test was used to compare DPP-IV enzyme activity between obese children group (with and without metabolic syndrome) and control group.
As the concentration of DPP-IV enzyme activity has a normal distribution, linear regression analysis was used to estimate the effects of different risk factors as adiposity measures as BMI for age z-score, body adiposity index, as well as lipid profile, serum glucose, and insulin resistance in potentially changing the serum DPP-IV enzyme activity. A receiver operating characteristic (ROC) curve analysis was performed to estimate the cut off values of serum DPP-IV enzyme activity with the highest sensitivity, specificity and area under the curve (AUC) for early detection of cardiovascular and metabolic risk among obese children. Values of P < 0.05 were considered statistically significant, and p<0.01 was considered highly significant.
Results
Forty obese Egyptian patients; 20 boys, and 20 girls are consecutively enrolled in the study. The age of the subjects ranges from 10 to 14 years old. There is no significant difference between the ages in the obese group and the non-obese control group (P> 0.05). The mean waist and hip circumferences, waist: hip ratio, BMI, BMI for age percentile and Z-score, body fat percentage (%), fat mass (kg), and body adiposity index (%) in the obese group with MS shows a highly significantly increase relative to those of subjects who did not have MS, and the control ones (P < 0.001). Meanwhile muscle mass reveals a highly significant decrease in the obese group with MS in respect without MS, and control group (P < 0.001). Table (1) shows comparison by ANOVA between total subjects (obese with and without metabolic syndrome) and non- obese subjects with regards to anthropometric parameters.
Table 1: Comparison between total cases (obese with and without metabolic syndrome) and control group as regard to anthropometricparameters (ANOVA test).
|
Variables |
Control
group
|
Obese group
with metabolic syndrome |
Obese group without metabolic syndrome | F | P-value |
| Mean ± SD | Mean ± SD | Mean± SD | |||
| Age (yrs) | 14.80a±1.88 | 14.92b±0.91 | 14.89c±0.72 | 10.916 | 0.7 ca,cb |
| Waist circumference | 58.90a±4.74 | 108.73b±5.87 | 108.67c±8.96 | 644.283 | 0.000*ab-ac |
| Hip circumference | 78.45a±4.93 | 124.64b±7.75 | 120.00c±12.51 | 309.179 | 0.000*ab-ac |
| Waist : hip ratio | 0.75a±0.02 | 0.88b±0.02 | 0.91c±0.03 | 453.336 | 0.000*ab-ac-bc |
| BMI for age percentile | 47.70a±23.07 | 99.27b±0.46 | 99.00c±0.69 | 98.161 | 0.000*ab, ac |
| BMI for age Z score | -0.42a±0.71 | 2.39b±0.29 | 2.36c±0.31 | 259.584 | 0.000*ab, ac |
| Body fat percentage (%) | 11.25a±5.36 | 52.38b±11.37 | 37.00c±12.19 | 155.873 | 0.000* ab, ac, bc |
| Fat mass ( kg) | 5.07a±3.28 | 42.74b±11.00 | 33.24c±10.88 | 179.488 | 0.000* ab,ac,bc |
| Muscle mass (kg) | 54.64c±16.23 | 37. 72b±11.45 | 36.37a±4.50 | 21.314 | 0.000* ca,cb |
| Body adiposity index (%) | 25.28a±1.04 | 48.86b±5.18 | 41.93c±8.07 | 199.151 | 0.000* ab, ac, bc |
*Significant difference at p< 0.05 abc= significance between groups
Serum DPP-IV enzyme activity of the obese group with MS displays highly significant enhancement versus those who did not have MS, and the non-obese counterparts (P< 0.001) (Figure 1). The obese group with MS also had significantly higher serum cholesterol, triglycerides, LDL- cholesterol, glucose, insulin, and HOMA-IR values , while, they had significantly lower serum HDL- cholesterol in comparison with those who did not have MS, and the control group (𝑃< .001), as shown in Table (2).
 |
Figure 1: Comparison between total cases (obese with and without metabolic syndrome) and control group as regard to serum DPP -IV enzyme activity. |
Table 2: Comparison between total cases (obese with and without metabolic syndrome) and controls as regard to laboratory analyses (ANOVA test).
|
Variables |
Control group
|
Obese group with metabolic syndrome | Obese group without metabolic syndrome | F | P-value |
| Mean ± SD | Mean ± SD | Mean± SD | |||
|
Serum DPP-IV(IU/L) |
789a±52.32 | 903.64b±47.46 | 882.22c±46.22 | 45.509 | 0.000*Ab, ac |
| Serum cholesterol | 128a±6.06 | 174.1b±23.33 | 141.56c±1.45 | 86.287 | 0.000* ab, ac, bc |
| Serum triglycerides | 67.6a±14.68 | 94.73b±22.40 | 77.44c±15.65 | 17.410 | 0.000*Ab, ac,bc |
| Serum HDL | 57.3a±4.13 | 41.55b±10.75 | 44.56c±8.84 | 36.919 | 0.000* Ab, ac |
| Serum LDL | 54.1a±8.83 | 91.18b±19.47 | 70.78c±20.78 | 41.329 | 0.000* Ab, ac, bc |
| serum fasting glucose | 85a±4.69 | 91.82b±14.83 | 81.44c±10.06 | 6.198 | 0.003* ab, bc |
| serum insulin | 15.48a±0.69 | 28.28b±3.81 | 30.02c±4.79 | 200.855 | 0.000* ab, ac |
| HOMA-IR | 3.48a±0.96 | 6.86b±0.77 | 6.19c±1.39 | 92.399 | 0.000* ab, ac, bc |
*Significant difference at p< 0.05, **highly significant difference at p≤ 0.01. abc = significance between groups
No sex differences are recorded on comparing serum DPP-IV enzyme activity between males and females in the obese group and Table (3) represents a comparison between obese cases as regard to sex differences.
Table 3: Comparison between obese cases as regard to sex differences.
|
Variables |
Obese males | Obese females |
t-test |
P value |
| Mean ± SD | Mean ± SD | |||
| Serum DPP-IV enzyme activity
|
895±36.71 | 892.5±61.70 | 0.161 | 0.873 |
In obese patients after the one-year lifestyle intervention program, the mean BMI, BMI for age percentile and z-score, waist, and hip circumferences and the waist: hip ratios show significant drop relative to those recorded before the intervention, and those of the non-obese counterparts. Likewise, adiposity measures as body fat percentage (%), fat mass (kg), and body adiposity index (%) reveal significant blunting in the obese patients after a one-year lifestyle intervention program in comparison with those before the intervention and those of the non-obese ones. Concerning the laboratory determinations, serum DPP-IV enzyme activity and serum levels of cholesterol, triglycerides, LDL cholesterol, glucose, insulin, and HOMA-IR are significantly lessened whereas serum level of HDL-cholesterol is significantly higher compared to baseline, and the non- obese group, as shown in Table (4), and figure (2).
 |
Figure 2: Comparison between obese group (at baseline and after weight loss), and non-obese group as regard to serum DPP IV enzyme activity. |
Table 4: Comparison between total cases group (at baseline and after weight loss), and control groups as regard to anthropometric and laboratory measures (ANOVA test).
|
Variables |
Control
Group
(a) |
Cases
group at baseline (b) |
Cases
Group after weight loss (c) |
F | P-value |
| Mean± SD | Mean± SD | Mean± SD | |||
| BMI for age percentile | 47.70a±23.07 | 99.27b±0.46 | 99.00c±0.69 | 98.161 | 0.000*ab, ac |
| BMI for age Z score | -0.42±0.71 | 2.37±0.29 | 1.44±0.70 | 223.543 | 0.000**ab,ac,bc |
| Waist circumference | 58.90±4.74 | 108.70±7.32 | 100.20±6.19 | 744.936 | 0.000**ab,ac,bc |
| Hip circumference | 78.45±4.93 | 122.55±10.30 | 112.65±10.16 | 274.994 | 0.000**ab,ac,bc |
| Waist : hip ratio | 0.75± 0.02 | 0.89±0.027 | 0.89±0.034 | 337.484 | 0.000**ab,ac |
| Body fat percentage (%) | 11.25±5.36 | 45.46±13.95 | 31.65±3.85 | 149.385 | 0.000**ab,ac,bc |
| Fat mass ( kg) | 5.07±3.28 | 38.47±11.82 | 23.39±5.74 | 183 | 0.000**ab,ac,bc |
| Lean mass (kg) | 36..37±4.50 | 45.34±16.07 | 50.38±10.84 | 15.256 | 0.000**ab,ac |
| Body Adiposity index (%) | 25.28±1.04 | 45.75±7.42 | 37.22±6.26 | 133.135 | 0.000**ab,ac,bc |
| Serum cholesterol | 128±6.05 | 159.45±24 .05 | 152.75±17.31 | 36.010 | 0.000**ab,ac |
| Serum triglycerides | 67.6± 14.67 | 86.95± 21.28 | 83.20±17.05 | 13.180 | 0.000**ab,ac |
| Serum HDL | 57.3± 4.13 | 42.9± 9.93 | 45.40±7.64 | 40.856 | 0.000**ab,ac |
| Serum LDL | 54.1± 8.83 | 82± 22.32 | 79.40±19.47 | 29.848 | 0.000**ab,ac |
| Serum fasting glucose | 85.0± 4.69 | 90.15± 13.78 | 83.10±9.22 | 1.660 | 0.019*ab |
| Serum insulin | 15.48± 0.69 | 29.07±4.31 | 24.27±3.65 | 175.771 | 0.000**ab,ac,bc |
| HOMA-IR | 3.48±0.96 | 6.56±1.13 | 4.44±0.75 | 108.263 | 0.000**ab,ac,bc |
| Serum DPP-IV (IU/L) | 789±52.32 | 894±47.55 | 812±36.18 | 57.964 | 0.000**ab, ac,bc |
*Significant difference at p< 0.05, **highly significant difference at p≤ 0.01
At baseline, serum DPP-IV enzyme activity in obese children was positively correlated with BMI for age percentile, and z-score, waist: hip ratio, in Table (5).
Table 5: Correlation between serum DPP-IV enzyme activity at baseline and anthropometric measures and blood pressure in the studied cases.
|
Variables |
Age | BMI | BMI
z- score |
BMI
percentile |
Waist: hip
ratio |
Body fat percentage | Fat mass | Muscle mass | body adiposity index | |
| Serum DPP-IV
(IU/L) |
Pearson Correlation | -.186 | .282 | .500(**) | .318(*) | .144(*) | -0.011 | 0.208 | 0.148 | -0.286 |
| Sig. (2-tailed) | .252 | .078 | .001 | .046 | .375 | 0.948 | 0.197 | 0.361 | 0.074 | |
*Significant difference at p< 0.05, **highly significant difference at p≤ 0.01
In obese patients at baseline, serum DPP-IV enzyme activity is positively correlated with serum levels of triglycerides, and HOMA-IR as shown in Table (6).
Table 6: Correlation between serum DPP-IV enzyme activity and other laboratory measures in the studied cases.
| Variables | Serum cholesterol | Serum
TGS |
Serum HDL | Serum
LDL |
glucose | Fasting
insulin |
HOMA-IR | |
| Serum
DPP-IV |
Pearson Correlation | .265 | .432(**) | .257 | .044 | -.240 | -.035 | .392(*) |
| Sig. (2-tailed) | .099 | .005 | .109 | .790 | .136 | .832 | .012 | |
*Significant difference at p< 0.05, **highly significant difference at p≤ 0.01
The linear regression analysis of the data for the obese patients at baseline showed high BMI z-score, waist: hip ratio, body adiposity index, and serum triglycerides. The obtained data indicate that these measures are good indicators for high serum DPP-IV enzyme activity in obese patients (Table 7).
Table 7: Linear regression analysis for the predictors of serum DPP-IV enzyme activity in obese patients.
|
Variables
|
Un-standardized Coefficients | Standardized Coefficients | t | Sig. | 95% Confidence Interval for B | ||
| B | Std. Error | Beta | Lower
Bound |
Upper Bound | |||
| (Constant) | -6134.488 | 1420.890 | -4.317 | .000* | -9032.412 | -3236.563 | |
| BMI z-score | 144.296 | 81.012 | .887 | 1.781 | .085 | -20.929 | 309.521 |
| Waist: hip ratio | 5652.942 | 1167.551 | 3.215 | 4.842 | .000* | 3271.706 | 8034.178 |
| Body adiposity index | 23.064 | 5.037 | 3.598 | 4.579 | .000* | 12.791 | 33.337 |
| Serum cholesterol | -.766 | 1.004 | -.387 | -.763 | .451 | -2.814 | 1.282 |
| Serum TGS | 3.970 | 1.540 | 1.777 | 2.577 | .015* | .828 | 7.112 |
| HOMA-IR | 6.839 | 11.330 | .162 | .604 | .550 | -16.270 | 29.948 |
| Serum HDL | 5.812 | 1.491 | 1.631 | 5.239 | .056 | 4.771 | 10.854 |
| Serum LDL | 6.383E-02 | .445 | .030 | .144 | .887 | -.843 | .971 |
*Significant difference at p< 0.05.
Evaluating the diagnostic performance of serum DPP-IV enzyme activity as a biomarker for obesity by calculating the area under the ROC curve is shown in Table (8) and Figure (3). This analysis demonstrates that serum DDP-IV enzyme activity may detect obesity metabolic complications with the AUC of 0.935 (95% confidence interval [CI] 0.872- 0.998, with a sensitivity of 90% and a specificity of 90% at a cutoff value of 835IU/L).
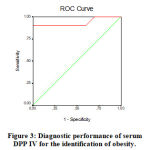 |
Figure 3: Diagnostic performance of serum DPP IV for the identification of obesity. |
Table 8: The area under the ROC curve and the cutoff value of serum DPP- IV enzyme activity in obese patients.
| AUC | 95% CI | p | Cutoff value (IU/L) | Sensitivity | Specificity | |
| Serum DPP-IV
(IU/L) |
0.935 | 0.872- 0.998 | 0.000* | 835 | 90% | 90% |
*P < 0.05 is significant
Discussion
Metabolic syndrome (MS) describes a collection of factors with a metabolic origin, such as obesity, hypertriglyceridemia, low HDL-cholesterol, arterial hypertension and glucose metabolism disorders, which are associated with cardiovascular diseases and type II Diabetes mellitus [25].
Excess body fat promotes insulin resistance which triggers the metabolic disorders that include MS. The subjects were diagnosed as having MS if they met three of the subsequent five WHO criteria [15&26].
Our findings indicate that the metabolic syndrome is more common in our sample of obese participants. Twenty-two (51%) out of forty in our cases with obesity was diagnosed with MS. This comes in line with Weiss et al. [27], who observed that approximately 50% of severely obese patients have MS. In contrast to our study, Brazilian studies reported prevalence rates of MS varying from 17.3% to 26.1% in obese children and adolescents [28-29].
The accumulation of visceral fat has been demonstrated to be strongly associated with MS in childhood and waist circumference has been identified as the best clinical indirect indicator of visceral fat accumulation [30-31]. The usefulness of waist circumference measurement is confirmed by previous pediatric studies demonstrating that, within a given BMI category, a higher cardiovascular and metabolic risk with the development of insulin resistance has been found in subjects with a large waist circumference compared to those with a low waist circumference [31]. Although reference values for waist circumference in children still used for a few countries like Canada [32] UK [33] and USA [34], the clinical use of waist circumference in children and adolescents is restricted by the shortage of an internationally approved classification which provides cut-offs of age-specific waist circumference and by the shortage of reference values of population in most nations. Using the waist/ hip ratio has recently been proposed to overcome these limitations, [35–37]. Santoro et al.[38] mentioned that a statistically significant increased risk of metabolic syndrome, high blood pressure, prediabetes, and lipidemia is correlated with waist/ hip ratio> 0.80
In the current study, the mean waist, and hip circumferences, waist: hip ratio, BMI, BMI for age percentile, and z-score in the obese group with MS were highly significantly greater than those of subjects who did not have MS, and control group (P < 0.001).These results echo those of the previous studies [39-40] which registered higher waist, and hip circumferences, waist: hip ratio, BMI, BMI for age percentile, and z-score in the obese children with MS than those who did not have MS and control counterparts (P< 0.001).
In our study, adiposity measures, as body fat percentage (%), fat mass (kg), and body adiposity index (%) in the obese group with MS showed highly significant increase relative to those who did not have MS, and the control group (P< 0.001), while muscle mass revealed a significant reduction as compared to that of subjects without MS, and the control group ( P < 0.001). These results match those in adults as reported by Sangun et al. [41]. These investigators observed higher adiposity measures in the obese group with MS, compared to those who did not have MS, and the controls.
Serum DPP-IV enzyme activity in the obese group with MS in the present study was statistically significantly higher than that of subjects who did not have MS and the non-obese participates (P < 0.001). Up to our knowledge, no studies till now compare the serum DPP-IV enzyme activity of the subjects with and without MS and non-obese ones. The obese group with MS also experienced highly significant elevation in serum cholesterol, triglycerides, LDL, glucose, insulin, and HOMA-IR values versus those of subjects without MS, and control group (𝑃<0.001). These findings fit those of Sangun et al. [41] who stated that obese children have a significantly higher MS prevalence with higher lipid profile and insulin resistance than the non-obese cases. The increased fasting insulin level in MS children and adolescents just reflects the increased insulin resistance in these cases and this supports the critical role of high insulin resistance in metabolic syndrome pathogenesis.
In the present study, no sex differences were observed in comparing serum DPP-IV enzyme activity between males and females in the obese group. This result is in conflict with that of Neidert et al. [42] in adults as this research group reported that women have lower average DPP-IV activity with respect to men, so it is possible to suggest that gynoid fat is a sex impact instead of a fat mass impact. However, there is very little data concerning sex and DPP-IV activity.
In general, serum DPP-IV activity of children is higher than in adults [43], and a decreased serum DPP-IV activity has been reported in the adults [44]. The finding of the present study showed no significant correlation between age and DPP-IV activity. This result is in harmony with that in the adult study done by Neidert et al. [42] who recorded no significant association between DPP-IV activity and age. However, this finding is in distinction with the previous study showing down-regulation of DPP-IV activity in older adults [44].
In the current study, serum DPP-IV enzyme activity in obese cases at baseline was positively correlated with BMIZ-score, waist: hip ratios, body adiposity index. This result converges with the previous report of Kirino et al. [8] who documented a relationship between DPP-IV activity and BMI in Japanese students. Malin et al. [45] observed increased serum DPP-IV enzyme activity with the increased fat mass. Lamers et al. [5] explained this phenomenon as more serum DPP-IV enzyme activity is liberated from adipose tissue, and particularly when adipocytes of obese patients stimulated by inflammatory mediators. However, this finding is not in agreement with Neidert et al. [42] who stated that enzyme activity of DPP- IV has a negative correlation with fat mass, suggesting that less DPP-IV activity would be found in people with more fat.
The linear regression analysis of our data for obese cases at baseline showed great BMI z-score, waist: hip ratio, body adiposity index, and serum triglycerides levels. This points to that these measures are good indicators of high serum DPP-IV enzyme activity in obese cases. Up to our knowledge, no studies until now assess the predictors for high serum DPP-IV enzyme activity in obese children or adolescents.
To the best of our knowledge, this is the first longitudinal study in obese children and adolescents demonstrating the decreased serum DPP-IV enzyme activity with weight loss. Moreover, our study proved that decreased serum DPP-IV enzyme activity in weight loss is associated with a significant improvement in insulin resistance. DPP-IV enzyme is wide expressed in several organs, however, fat mass reduction may be the possible cause of the regression of DPP-IV enzyme activity. The explanation of the retraction of DPP-IV enzyme activity after the reduction of fat mass is that the increase in adipose tissue is directly proportional to the elevated levels of inflammatory cytokines inducing the lymphocytes activation with an increased release of DPP-IV enzyme activity. Hence, the reduction of fat mass is paralleled by the suppression of the inflammatory mediators, regression of lymphocytes activity, and finally down-regulation of DPP-IV enzyme activity. This explanation stems from Malin et al. [45] who emphasized that following weight loss induced by surgery, the increased enzyme activity of DPP-IV is restored to the normal value of the lean control subjects.
In the present study, after 6 month lifestyle intervention programme for obese children and adolescents, the mean BMI, BMI z-score, waist: hip ratio, adiposity indicators as body fat percentage (%), fat mass (kg), and body adiposity index (%) were highly significantly decreased comparative to the baseline, and control group. Moreover, the serum DPP-IV enzyme activity and serum cholesterol, triglycerides, LDL cholesterol, glucose, insulin, and HOMA-IR values were highly significantly depressed while serum level of HDL-cholesterol was significantly elevated relative to the baseline, and the control group. Our results fit with those of Malin et al.[45] who observed lower DPP-IV enzyme activity, HOMA-IR, and lipid profile following exercise training and weight loss. These authors explained this finding by the increased insulin sensitivity in adults with metabolic syndrome. Also, Reinehr et al. [46] recorded lower DPP-IV enzyme activity in obese children with weight loss. These investigators reported that exercise inducing weight loss is the cornerstone therapy for reducing metabolic syndrome and diabetes risk. Exercise training significantly reduced plasma DPP-IV enzyme activity in obese adults with metabolic syndrome, and significantly improved insulin sensitivity as well as elevated fat oxidation. These results lead us to suggest DPP-IV enzyme activity may play a fundamental role in the controlling of glucose and lipid metabolism. In contrast, Neidert et al. [42] observed high DPP-IV enzyme activity in obese adults with weight loss.
Fasting HOMA-IR is the primarily reflective indicator of liver glucose metabolism [47]. Herein, the noticeable drop in HOMA-IR after the life intervention program and the association of improved HOMA-IR value with the suppression DPP-IV enzyme activity raises the possibility that the reduction in DPP-IV enzyme activity may participate in the changes in hepatic insulin sensitivity after exercise.
As regard to lipid panel and DPP-IV enzyme activity, it has been reported that elevated DPP-IV is linked to cardiovascular disease because it is associated with dyslipidemia [48]. Therefore, the use of DPP-IV inhibitors has been proposed to produce cardio-protective effects through not only improving insulin sensitivity but also by suppressing inflammatory status [49-50].
In the current research, according to the receiver-operating characteristics (ROC) curve, high serum DPP-IV enzyme activity is considered a good predictor for obesity metabolic complications. The analysis demonstrated that serum DPP-IV could detect obesity metabolic complications at cut-off values of 835 IU/L.
Conclusively, this study offers clinical evidence for the significance of the decreased DPP-IV enzyme activity with weight loss and its association with the improvement of HOMA-IR in obese cases. Also, our data disclose that BMI z-score, waist: hip ratio, body adiposity index, and serum triglycerides are key players for inducing serum DPP-IV enzyme activity in obese cases. Therefore, we suggest that DPP-IV is accounted as a target for the management of metabolic syndrome in children and adolescents with obesity.
Competing interests
The authors have declared that no existence of competing interests.
Funding source
There is no funding source.
References
- Durinx C., Neels H., Vander Auwera J.C., Naelaerts K., Scharpé S, De Meester I: Reference values for plasma dipeptidylpeptidase IV activity and their association with other laboratory parameters, Clin. Chem. Lab. Med. 2001; 39 (155–1590.
- Raschke S, Eckardt K, Bjorklund-Holven K, Jensen J, Eckel J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS ONE. 2013; 8:e62008. [PubMed: 23637948]
- Lambeir AM, Durinx C, Scharpé S, DeMeester I: Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspect soft the enzyme DPPIV,Crit. Rev.Clin.Lab.Sci.40 (2003) 209–294.
- De Silva A., Bloom SR: Gut hormones and appetite control: A focus on PYY and GLP-1 as therapeutic targets in obesity. 2012, 6, (1) 10-20. http://refhub.elsevier.com/S2405-8440(16)30034-2/sbref0020
- Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes.2011; 60:1917–1925. [PubMed: 21593202]
- Sauve M, Ban K, Momen MA, Zhou Y, Henkelman RM, Husain M, et al. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes.2010; 59:1063–1073. [PubMed: 20097729]
- Lugari R., A.DeiCas, D.Ugolotti, A.Barilli, C.Camellini, G.Ganzerla, A. Luciani,B.Salerni,F.Mittenperger,S.Nodari: Glucagon-likepeptide1 (GLP-1) secretion and plasma dipeptidylpeptidaseIV(DPP-IV)activity in morbidly obese patients undergoing bilio pancreatic diversion,Horm. Metab.Res.36(2004)111–115.
- Kirino Y, Sei M, Kawazoe K, Minakuchi K, SatoY :Plasmadipeptidyl peptidase 4 activity correlates with body mass index and the plasma adiponectin concentration in healthy young people,Endocr.J.59(2012) 949–953.
- Bateman LA, Slentz CA, Willis LH, Shields AT, Piner LW, Bales CW: Comparison of Aerobic Versus Resistance Exercise Training Effects on Metabolic Syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise – STRRIDE-AT/RT). Am J Cardiol.2011; 108:838–844. [PubMed: 21741606]
- Joseph LJ, Prigeon RL, Blumenthal JB, Ryan AS, Goldberg AP: Weight loss and low-intensity exercise for the treatment of metabolic syndrome in obese postmenopausal women. J Gerontol A BiolSci Med Sci. 2011; 66:1022–1029.
- Villareal DT, Miller BV, Banks M, Fontana L, Sinacore DR, Klein S: Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J ClinNutr.2006; 84:1317–1323. [PubMed: 17158411]
- Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults a randomized clinical trial.J Gerontol A Biol Med Sci. 2009; 64:90–95.
- You T, Arsenis NC, Disanzo BL, LaMonte MJ: Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med. 2013; 43:243– 246. [PubMed: 23494259]
- Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH: Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994.Arch Pediatr Adolesc Med 2003, 157:821-827.
- Duncan GE, Li SM, Zhou XH: Prevalence and trends of a metabolic syndrome phenotype among U.S.: Adolescents, 1999-2000. Diabetes Care 2004; 27: 2438– 2443.
- WHO, Anthro Plus for personal computers. Manual Software for assessing growth of the world’s children, and adolescents.Geneva, 2009. (http://www.who.int/growthref/tools/en/).
- Tanner JM, Hiernaux J, Jarman S, Weiner JS, Lourie JA: Growth, and physique studies. Human biology: A guidance to fields methods. Eds.Weiner JS, Lourie SA, IBP. London, Blackwell Scientific Publications.Oxford Blackwell Scientific Publications, 1969: 273-275.
- Lohman, TG, Roche AF, Martorell R: Anthropometric standardization reference manual. Champaign, IL: Human kinetics Publishers, 1988.
- Centers for Disease Control and Prevention. Weight, height, and BMI growth charts. Available from: http://www.cdc.gov/growthcharts/ (cited 18 January 2009)
- Bergman RN, Stefanovski D, Buchanan TA, Sumner AE, Reynolds JC, et al. (2011) A better index of body adiposity. Obesity (Silver Spring) 19: 1083–1089.
- Livingstone MB, Robson PJ: Measurement of dietary intake in children. Proc Nutr Soc 2000, 59: 279–293.
- Burrows TL, Martin RJ, Collins CE: A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc. 2010, 110:1501–1510.
- Matthews, D., Hosker, J., Rudenski, A. et al: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28, 412–419.
- Keskin M, Kurtoglu S, Kendirci M, Atabek M E, and Yazici C : Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents, Pediatrics, 2005; 115 (4), 500–503,.
- Eckel RH, Alberti KG, Grundy SM, Zimmet PZ (2010) The metabolic syndrome. Lancet 375: 181–183.
- Magge SN, Goodman E, Armstrong SC: Committee on nutrition, section on endocrinology, section on obesity. The Metabolic Syndrome in Children and Adolescents: Shifting the Focus to Cardiometabolic Risk Factor Clustering. Pediatrics. 2017; 140(2):e20171603
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW et al: Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362-74.
- Ferreira AP, Oliveira CE, França NM. Metabolic syndrome and risk factors for cardiovascular disease in obese children: the relationship with insulin resistance (HOMA-IR). J Pediatr (Rio J) 2007;83:21-6.
- da Silva RC, Miranda WL, Chacra AR, Dib SA. Metabolic syndrome and insulin resistance in normal glucose tolerant brazilian adolescents with family history of type 2 diabetes. Diabetes Care 2005;28:716-8.
- Janssen I, Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS: Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005; 115: 1623–1630.
- Després JP, Lemieux I: Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887.
- Katzmarzyk PT: Waist circumference percentiles for Canadian youth 11–18y of age. Eur J ClinNutr 2004; 58: 1011–1105.
- McCarthy HD, Jarrett KV, Crawley HF: The development of waist circumference percentiles in British children aged 5.0–16.9 y Eur J ClinNutr 2001; 55: 902–907.
- Schisterman EF, Faraggi D, Reiser B: Adjusting the generalized ROC curve for covariates. Statist Med 2004; 23: 3319–3331.
- Maffeis C, Banzato C, Talamini G; Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology: Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J Pediatr 2008; 152: 207–213.
- Freedman DS, Kahn HS, Mei Z, Grummer-Strawn LM, Dietz WH, Srinivasan SR, Berenson GS: Relation of body mass index and waist-to-height ratio to cardiovascular disease risk factors in children and adolescents: the Bogalusa Heart Study. Am J ClinNutr 2007; 86: 33–40.
- McCarthy HD, Ashwell M: A study of central fatness using waist-to-height ratios in UK children and adolescents over two decades supports the simple message – ‘keep your waist circumference to less than half your height’. Int J Obes 2006; 30: 988–992.
- Santoro N, Amato A, Grandone A, BrienzaC ,Savarese P, Tartaglione N, Marzuillo P, Perrone L, Miraglia E, del Giudice :Predicting Metabolic Syndrome in Obese Children and Adolescents: Look, Measure and Ask. Obes Facts 2013;6:48–56. DOI: 10.1159/000348625
- Inas R. El-Alameey, Nevein N. Fadl, Enas R. Abdel Hameed, Lobna S. Sherif, Hanaa H. Ahmed: Clinical Relevance of Transforming Growth Factor-β1, Interleukin-6 and Haptoglobin for Prediction of Obesity Complications in Prepubertal Egyptian Children. Macedonian Journal of Medical Sciences. 2015 Mar. 15; 3 (1): 105- 110.
- Hsieh SD, Yoshinaga H, Muto T : Waist-to-height ratio, a simple and practical index for assessing central fat distribution and metabolic risk in Japanese men and women. Int J ObesRelatMetabDisord. 2003; 27: 610–616.
- Sangun O, BuminDundar, MuhammetKoflker, OzgurPirgon, NihalDundar: Prevalence of Metabolic Syndrome in Obese Children and Adolescents using Three Different Criteria and Evaluation of Risk Factors. J Clin Res Ped Endo 2011; 3(2):70-76. DOI: 10.4274/jcrpe.v3i2.15
- Neidert Leslie E. Neidert, Katherine S.Wainright, ChenZheng, Jeganathan Ramesh Babu, HeidiA.Kluess : Plasma dipeptidyl peptidase IV activity and measures of body composition in apparently healthy people. Heliyon 2 (2016). http://dx.doi.org/10.1016/j.heliyon.2016.e00097
- Lugari R, Dei Cas A, Ugolotti D, Barilli AL, Camellini C, Ganzerla GC, Luciani A, Salerni B, Mittenperger F, Nodari S, Gnudi A, Zandomeneghi R. Glucagon-like peptide 1 secretion and plasma dipeptidyl peptidase IV (DPP-IV) activity in morbidly obese patients undergoing biliopancreatic diversion. HormMetab Res 2004; 36: 111-115.
- Meneilly GS, Demuth HU, Mclntosh CH, Pederson RA. Effect of ageing and diabetes on glucose dependent insulinotropic polypeptide and dipeptidyl peptidase IV responses to oral glucose.Diabet Med 2000; 17: 346-350.
- Malin SK, Huang H, Mulya A, Sangeeta R. Kashya P, Kirwan JP: Lower Dipeptidyl peptidase-4 following exercise training plus weight loss is related to increased insulin sensitivity in adults with metabolic syndrome. Peptides. 2013 September ; 47: . doi:10.1016/j.peptides.2013.07.008.
- Reinehr T, Roth CL, Pablo J, Masur K: Changes of dipeptidyl peptidase IV (DPP-IV) in obese children with weight Loss: Relationships to Peptide YY, Pancreatic Peptide, and Insulin Sensitivity. Journal of Pediatric Endocrinology & Metabolism, 23, 101-108 (2010)
- Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA: Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care.2007; 30:89–94. [PubMed: 17192339]
- Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, OuwensDM :Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes.2011; 60:1917–1925.
- Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, Wagner JA: Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J ClinPharmacol.2008; 48:592–598.
- Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP: DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging.2010; 3:195–201. [PubMed: 20075143]







