Sheikh Abid Ali1*, Refaz Ahmad1, Nazir Ahmad2, Mudasir Makhdoomi3 and Qazi Parvaiz1
1Division of Biotechnology, CSIR- Indian Institute of Integrative Medicine, Srinagar-190005, (J and K) India
2Department of Microbiology and Biotechnology, Bangalore University, Bangalore, Karnataka- 560056. India
3Department of Endocrinology, SKIMS- Srinagar, 190011, Kashmir (J and K) India
Corresponding Author E-mail: abidalis2007@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1841
Abstract
A substantial and growing body of scientific research has linked Sour cherries to various biotherapeutic properties and suggested as a candidate for immunomodulation. The effects of graded doses of a chemically standardized methanolic fruit extract (PcMFE) of Prunus cerasus on the immune system and anti-oxidative status of SRBC immunized BALB/c mice were investigated. Oral administration of PcMFE (100-250 mg/kg) enhanced the expression pattern of IgM and IgG titres, stimulated cell mediated immunity reaching peak value with 200 mg/kg b. wt. Flowcytometric analysis of surface markers of T cells (CD4+ and CD8+) and B cells (CD 19+) indicated prominent enhancement in proliferation and differentiation of these lymphocytes. The extract enhanced expression of T helper cells Th1 cytokines interferon (IFN)-γ and interleukin-12 (IL-12) in the sera of treated mice compared with the control group. In vivo studies showed PcMFE increased spleen and thymus indices, activated macrophage functions ex-vivo as indicated by nitroblue tetrazolium reduction potential, inducible nitric oxide synthase activity and bactericidal property significantly. Furthermore, the oxidative stress marker studies revealed that the administration of PcMFE significantly decreased levels of LPO, increased the activities of SOD, CAT and GSH-Px as compared to the control group. These findings indicate PcMFE has immunomodulatory activity in vivo and might play an important role in prevention of oxidative damage in immunological system.
Keywords
CAT; Cytokines; Delayed Type Hypersensitivity; Haemagglutination Antibody Titre; HPLC; iNOS; Levamisole, MDA; NBT; Phagocytosis; SOD
Download this article as:| Copy the following to cite this article: Ali S. A, Ahmad R, Ahmad N, Makhdoomi M, Parvaiz Q. Augmentation of Immunocytes Functions by Prunus Cerasus Fruit and its Biotherapeutic Potential in Mice Model. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Ali S. A, Ahmad R, Ahmad N, Makhdoomi M, Parvaiz Q. Augmentation of Immunocytes Functions by Prunus Cerasus Fruit and its Biotherapeutic Potential in Mice Model. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2saYhQK |
Introduction
Clinical immunology has recently unraveled the involvement of immune system in the pathogenesis of various disease conditions due to the altered functioning of the immune system1. The immune system highly depends on accurate cell-cell communication for normal function and any damage in the signaling systems will disturb the whole immune response2. The antioxidants are central to the redox balance in the human body for maintaining the immune cells in a reducing environment and preserving their functionality3. The immunoagents which are capable of modulating (suppressing and stimulating) components of adaptive or innate immunity are considered as one of the most tools in the management of health and disease by modern medicine. Due to the adverse effects of synthetic drugs, natural immunomodulators are the potential agents to replace them in therapeutic treatment4. Presently, innovative technologies and extensive research focuses on natural products from plants, with immunomodulatory potential, to develop as novel immunomodulatory agents to supplement the present chemotherapies5.
Epidemiological studies have shown that a group fruits used in traditional medical system of remedies possess significant immunomodulation properties6. Among small soft-fleshed colourful berries, cherries configure a larger proportion that is being consumed not only in fresh and frozen forms but also as processed and derived products viz. jellies, jams and canned fruits7. Prunus cerasus L. belongs to genus Prunus and comes under Rosacea family, native to southwest of Asia and most parts of the Europe. It has a more acidic fruit than it’s closely linked wild cherry or P. avium. The fruit of sour cherries is known to possess several therapeutic uses including anti-inflammatory8 as well as hypoglycemic effects and prevention of oxidative stress related conditions9. The phytochemical studies have confirmed that sour cherries fruit contains a wide variety of unique phytonutrients and thus could be a rich source of biofunctional metabolites in our diet10.
Our previous in-vitro immunoregulatory screening conducted on various extracts prepared from different parts of P. cerasus11, reported that the methanol soluble fruit extract of sour cherries showed best ability in the stimulation of T and B lymphocytes and activation of phagocytes. Moreover, the studies demand that, in order to offer immune-therapeutic potential owing to its versatile applications, here detailed investigations have been made on a standardized P. cerasus methanolic fruit extract (PcMFE) on the humoral and cell mediated immune functions in SRBC immunized BALB/c mice together with measurement of T lymphocytes subsets by flow-cytometry, as well as cytokines profile with related antibodies by enzyme linked immunosorbent assay (ELISA). Further, The NBT reduction, iNOS and bactericidal tests were demonstrated for functional ability of activated macrophages. We used a mixture of pure 4 isolated signatures as chemical markers for chemoprofiling of the fruit extract. Pertaining to antioxidant activity, CAT, SOD, GSH-Px, & MDA assays were performed.
Materials
RPMI 1640 medium, NBT, Dioxan (Himedia Bombay, India), fetal calf serum (FCS), E. coli, dimethylsulphoxide (DMSO), E. coli growth medium from Sigma, 96 V well plates and micro-tissue culture plates (96 U well) from Tarson. Assay kits for cytokines were purchased from (BD bioscience, USA). The rest of the chemicals used were of analytical grade available locally. The malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were obtained from local firms (SD Fine Chem. Ltd, Mumbai, India). The solvents used were of HPLC grade purchased from Thermofisher.
Methods
Sour cherries and preparation of methanolic fruit extract PcMFE
The Prunus cerasus L. Fruit (4 kg) was collected from Harwan village of the Kashmir valley, India and got identified by Dr. Akhtar H Malik of Center for Biodiversity and Taxonomy, University of Kashmir (India) with voucher specimen no. 2456-(KASH). Pitted fruit was lyophilized to get a gummy residue of (390 g). Lyophilized fruit (350g) was ground and percolated four times with methanol (800 ml) at room temperature. The combined extracts were filtered, centrifuged and concentrated to 1/6th of the original volume under reduced pressure in a thin film evaporator at 40±45 °C. Finally, the extract was completely dried under vacuum in the desiccator to get a red methanolic fruit extract of (145g).
Isolation of pure compounds for chemoprofiling of cherry fruit extract
Bioassay guided fractionation of P. cerasus methanolic fruit extract (PcMFE) by column chromatography afforded four major chemical signatures: Quercetin (QCTN), Daidzin (DAZ), Rutin (RUT) and Chlorogenic Acid (CHL-A), studies published in our previous research publication12. The isolated chemical signatures were used as markers for chemoprofiling of the bioactive fruit extract.
Standardization of the PcMFE
The HPLC standardization of PcMFE on the basis of isolated four compounds was performed at a flow rate of 0.8 ml/min using mobile phase consisting of 0.05% TFA in ACN: 0.05% TFA in water (gradient). The accurately weighed quantity of the dried PcMFE (110.0 mg) was taken and dissolved in 2 ml methanol HPLC grade. Pure compounds chlorogenic acid (1.2 mg/5mL), daidzin (5.0 mg/5 mL), rutin (1.8 mg/ 5mL) and quercetin (2.5 mg/ 5mL) were dissolved in methanol. The samples were centrifuged and filtered through 0.45μm Millipore filter for analysis. For markers, working solutions of 240-1000 µg/ml concentration range were prepared by diluting with methanol. These working solutions of all the marker compounds were mixed together in the ratio (CHL-A: DAZ: RUT: QCTN: 1:3:1:2) and were injected in different concentrations (10 µL, 20 µL, 30 µL, 40 µL, 50 µL). The calibration curve of each marker compound in the mixture was plotted using five levels of concentrations and linearity of each marker compound was observed in the concentration range 1.42 µg-7.10 µg for chlorogenic acid, 4.28 µg-21.40 µg for daidzin, 1.42 µg-7.10 µg for rutin and 2.85 µg-14.25 µg for quercetin. The marker compounds in the methanol fruit extract were quantified using these calibration curves.
Experimental animals and ethical clearance
Female BALB/c mice (18–23g), were procured from institute’s animal house and prior approval for their use obtained from the Institutional Animals Ethics committee. The animals were housed under standard laboratory conditions of temperature (25±20C) with 20-25 complete air changes with 100% fresh air at 50-60% relative humidity and a photoperiod of 12 h. Animals were provided pelleted feed (M/s Ashirwad Industries, Chandigarh, India) and autoclaved water ad libitum.
Antigenic challenge and experimental design
Sheep Red Blood Cells (SRBC) were used as a source of T dependent antigen. For this purpose, blood was withdrawn from a healthy sheep in Alsever’s solution in ratio of 1:2 and was centrifuged at 400×g for 10 min at 4 0C. The erythrocyte pellet obtained was washed three times with sterile saline and suspended in 0.1 M PBS (pH 7.2) for further use13. Mice were divided into six groups, with six animals in each group. PcMFE at 100 mg, 150 mg, 200 mg and 250 mg/kg (in 200μl of normal saline) was administered orally by gavage daily for 15 days. Untreated control group received normal saline whereas Levamisole was given orally to positive control group at a dose of 2.5 mg/kg body weight14. All mice were antigenically challenged with intraperitoneal administration of single dose 0.1 ml of SRBC (1×107) on 0th day of extract/drug treatment. Additional immunized groups, challenged on day 7 with SRBC were used for DTH and other immunological assays.
Humoral immune response
To assess the humoral immune response, blood samples were withdrawn from retro-orbital plexus of all SRBC challenged animals on day 7 (primary antibody titre) and day 14 (secondary antibody titre). The serum was separated and antibody titres were determined following the haemagglutination technique described by15. The reciprocal of the highest dilution of the test serum giving agglutination was taken as the antibody titre.
Cell mediated immune response
Delayed Type Hypersensitivity reaction was checked by foot pad swelling method16. PcMFE was administered 2 h after SRBC injection and once daily on consecutive days. Six days later, the thickness of the left hind food was measured with a spheromicrometer (pitch, 0.01 mm) and was considered as a control. The mice were then challenged by injecting 20 μl of 1% SRBCs intradermally into the left hind footpad and after 24 and 48 h of this challenge, the foot thickness was measured again. The pre- (control) and post-challenge difference in the thickness of footpad was expressed in mm and taken as a measure of DTH.
Total lymphocytes isolation from the spleen
Twenty-four hours after the administration of last PcMFE dose, all animals were weighed and sacrificed by cervical dislocation. The spleens were excised aseptically and lymphocytes isolated by teasing the tissue to obtain a homogeneous cell suspension. Erythrocytes present were lysed with ACK lyses solution (0.5 M NH4Cl, 10mM KHCO3 and 0.1mM disodium EDTA, pH 7.2) for 5 min. The Lymphocytes obtained were then washed thrice with PBS, counted and adjusted to desired concentration in RPMI medium,
Lymphocyte phenotyping analysis
Immunophenotyping focuses on FACS analysis of lymphocyte sub-populations: CD4, CD8 and CD19 molecules on isolated splenocytes. Briefly, splenocytes (1×106 cells/mL) were incubated with 10 μl of FITC-conjugated anti-mouse CD4 and PE-conjugated anti-mouse CD8 antibodies. For B cell analysis PE-conjugated anti-mouse CD19 antibody was used. After incubation for 30–60 min at 4 0C, the cells were washed twice with PBS and then fixed in 1% paraformaldehyde (PFA). Acquisition and analysis were performed by flow cytometry using Cell Quest Pro software (BD Bioscience).
Assay of cytokines in splenocytes cultures by ELISA
Splenocytes were isolated from different groups of PcMFE treated and untreated mice as described above. For cytokine estimation, 2×106 cells/ml were cultured with Con A (0.5 μg/ml) in 24-well tissue culture plates in RPMI–FBS (10%) medium. The plates were incubated at 37 °C in a humidified atmosphere of 5% CO2 for 48 h and the supernatants collected for cytokines assays as per the instructions of manufacturer (BD OptEIA set). The cytokines were quantified using recommended cytokines standards (BD Opt EIA set).
Nitric oxide synthase activity
Inducible nitric oxide synthase activity (iNOS) in lymphocyte suspension was evaluated by employing previously described procedure17, using arginine. Briefly the splenocytes were incubated with arginine at 37 °C for 24 hours in CO2 chamber. The colour developed indicating citruline formation from arginine, was measured spectrophotometrically at 540 nm against RPMI and Griess reagent as blank. The results were expressed as mean ± S.E.M. of percentage enzyme produced.
Nitroblue tetrazolium reduction assay
NBT reduction test, (a measure of respiratory burst in leucocytes) was carried out by slight modification of previous procedure18. The lymphocyte suspension was incubated with NBT and formazon thus formed was extracted in Dioxan. The reduction in NBT was measured spectrophotometrically at 520 nm (Shimadzu, UV-1650 PC) against Dioxan as blank. The results were expressed as mean ± S.E.M. of percentage dye reduced to Formazon.
Bactericidal activity
The macrophage function was determined by phagocytosis of microorganism (Bactericidal activity) using the method given by19. Briefly, the lymphocyte suspension was incubated with bacterial suspension (Escherichia coli) at 37 °C for 60 min. The lymphocytes were lysed with sterile distilled water, spread on agar plate and incubated at 37 °C for 24 h. Bacterial cell suspension was spread in the control plate. Number of colony forming units (CFU) developed in control and test plates were counted and results were expressed as mean ± S.E.M. of percentage bactericidal activity.
Biochemical assay
The liver, spleen, and thymus were excised and spleen and thymus indices were calculated according to the formula: thymus or spleen index (mg/g) = (weight of thymus or spleen/body weight). A small portion of liver was removed and kept on ice and homogenized with 0.1 g/mL wet weight of isotonic physiological saline. The sample was centrifuged at 3000 rpm/min at 4 0C for 15 min, and the supernatants were used to measure SOD, CAT, MDA, and GSH-Px. The assay for SOD was based on its ability to inhibit the oxidation of oxymine by the xanthine–xanthine oxidase system. The CAT activity was tested using spectrophotometric determination of hydrogen peroxide (H2O2) which form stable complex with ammonium molybdate. Further, thiobarbituric acid reaction (TBAR) was used to determine the MDA content, whereas, GSH-Px activity was checked by a modified glutathione exhaustion assay with modifications.
Statistical analysis
All the results were expressed as m ean ± S.E.M. with six animals per group. The results were statistically analysed using one-way analysis of variance ANOVA Bonferroni test. A value of p < 0.05 was regarded as statistical significance.
Results
Quantification of chemical signatures in PcMFE
The HPLC chromatogram of P. cerasus fruit PcMFE with reference to percentage of isolated phytochemicals is shown in Figure 1B. Protocol used resolved the mixture of four chemical signatures: CHL-A, RUT, DAZ and QCTN and quantities of marker compounds in PcMFE determined by using standard curve. PcMFE explored to be rich in daidzin (0.110%) followed by quercetin (0.084%), chlorogenic acid (0.037%) and rutin (0.018%) Figure 1A.
 |
Figure 1
|
Effects of PcMFE on lymphoid organs weight
The weight and cellularity of lymphoid organs being good indicators of toxicity and indicate immune functional shown in table 1, at increasing doses of 100 to 200 mg/kg, there was moderate increase in the body, spleen and thymus weights. The effect of PcMFE (200 mg/kg) was comparable to the standard drug levamisole (2.5 mg/kg). Interestingly, PcMFE did not provoke any gross pharmacological changes or manifestations of toxic symptoms in the animals and nothing abnormal was detected in all the animals.
Table 1: Effect of PcMFE on the body and lymphoid organs weight.
| Treatment | Dose mg/kg | Body weight | Relative organ wt. in grams
Spleen Thymus |
Mortality | |
| Control (N.S.) | 200 μl | 22.35 ± 0.63 | 0.61 ± 0.02 | 0.23 ± 0.01 | 0/6 |
| Levamisole | (2.5) | 22.47 ± 0. 71 | 0.79 ± 0.04 | 0.41 ± 0.03 | 0/6 |
| PcMFE | 100 | 22.31 ± 0. 68 | 0.65 ± 0.03 | 0.28 ± 0.03 | 0/6 |
| PcMFE | 150 | 22.28 ± 0.39 | 0.71 ± 0.02 | 0.34 ± 0.03 | 0/6 |
| PcMFE | 200 | 22.17 ± 0.12 | 0.77 ± 0.04 | 0.39 ± 0.02 | 0/6 |
| PcMFE | 250 | 21.86 ± 0.41 | 0.67 ± 0.02 | 0.31 ± 0.02 | 0/6
|
Data are Mean ± S.E. (N=6). * p < 0.05, ** p < 0.01, compared with normal control.Effect of PcMFE on anti-SRBC antibody titer
Increasing doses of PcMFE (100, 150 and 200mg/kg) significantly augmented the antibody titre after seven days when compared with normal control. A similar pattern was seen after 14 days with IgG predominating over IgM (Table 2). The maximum effect both in primary and secondary antibody titres was observed at 200 mg/kg extract, even higher than standard drug levamisole. Further increase in dose (250mg/Kg) showed a decreased response.
Effect of PcMFE on delayed type hypersensitivity DTH
Effect of PcMFE on cell mediated DTH response to SRBC antigen challenge was measured in terms of footpad thickness of mice following oral administration of PcMFE at indicated doses Table 2. The PcMFE treatment (100, 150 & 200 mg/kg) elicited a significant DTH response compared to control group, with maximum effect being observed in 200 mgkg extract treated group by 48 hr. This dose elicited a marginally better response when compared to the standard drug levamisole.
Table 2: Effect of PcMFE on Humoral and cell mediated immune response in mice
| Samples | Dose (mg/kg) | Antibody Titre Mean ± S.E. | Foot Pad Thickness | ||
| Primary (IgM) (After 7 days) | Secondary IgG) (after 14 days) | Mean± S.E. (24 h) | Mean± S.E. (48 h) | ||
| Control N.S. | 200 μl | 7.0 ± 0.424 | 6.33 ± 0.447 | 1.44 ± 0.041 | 1.35 ± 0.038 |
| Levamisole | 2.5 | 10.83 ± 0.00*a | 8.2 ± 0.22* | 1.70 ± 0.056* | 1.55 ± 0.040* |
| PcMFE | 100 | 9.2 ± 0.20*a | 8.5 ± 0.20*a | 1.78 ± 0.0*a | 1.60 ± 0.063*a |
| 150 | 10 ± 0.00*a | 9.8 ± 0.20*a | 1.86 ± 0.02*a | 1.68 ± 0.02*a | |
| 200 | 11.55 ± 0.82*a | 10.1 ± 0.32*a | 1.97 ± 0.02*a | 1.74 ± 0.02*a | |
| 250 | 8.7 ± 0.24*a | 7.6 ± 0.31*a | 1.67 ± 0.03*a | 1.53 ± 0.01*a | |
Antibody titres (IgM and IgG) in mice sera measured on 7th and 14th day after immunization. DTH response was determined in SRBC immunized & PcMFE treated mice at 24 & 48 h after antigen challenge. *p<0.01 compared with control group; ap<0.05 compared with levamisole group.
Effects of PcMFE on lymphocyte phenotyping in mice
We also determined T and B cells sub-populations by flowcytometric study of cell surface markers: CD4, CD8 & CD19, which revealed that PcMFE treatment exhibited impact in increasing the population of CD4+ and /CD8+ T cells (P<0.01) compared to control group. The increase was not only in T-cell subpopulations but B cells (CD19+) also showed same pattern (P<0.01). The dose dependent increase was registered through 200 mg/kg, Table 3.
Table 3: Effect of PcMFE on T and B lymphocyte subsets from spleen
| Group | Dose (mg/kg BW) | CD4+ (%) | CD8+ (%) | CD19+ (%) |
| Normal control | 200 μl | 50.10±3.53 | 38.45±4.45 | 45.17±3.22 |
| Levamisole | 2.5 | 63.9±23 | 49.8±82.5 | 55.3±62 |
|
PcMFE |
100 | 42.09±16.5 | 34.30±17.16 | 38.51±11.83 |
| 150 | 54.25±15.8 | 39.57±21.4 | 52.12±30.5 | |
| 200 | 67.58±15 | 51.14±21.07 | 60.49±14.3 | |
| 250 | 55.07±6.5 | 43.28±23.25 | 51.11±17.5 |
Flow-cytometric analysis of T and B cell surface markers expression in splenocytes of PcMFE treated mice. ** p < 0.01, compared with normal control. # p < 0.05, compared with levamisole group. model values are means S. E. N = 6.
PcMFE influences the polarizations of lymphocytes to Th1 immunity
Oral administration of graded doses of PcMFE influenced positively on production of IL-12 and IFN-γ as compared to untreated control. the modulatory effect of PcMFE observed at 200mg/kg was higher than positive control levamisole treated group (Figure 2). Further increase in extract dose 250mg/kg demonstrated decrease in the level of selected cytokines.
 |
Figure 2
|
Effect of PcMFE on nitric oxide synthase (iNOS) activity
It may be seen that 100-200mg/kg\ PcMFE treatment supported high expression of iNOS with maximum effect observed at 200mg/kg, better in comparison to levamisole (P < 0.05) treated group as shown in Figure 3. The influence on magnitude of iNOS declined with higher dose (250 mg/kg) of the PcMFE extract.
Effect of fruit extract on NBT reduction potential
Treatment of mice with different doses of PcMFE produced a significant (P < 0.05) increase in NBT reduction potential of macrophages as compared to untreated control (Figure 3). 200 mg/kg dose showed an impact comparable to standard drug levamisole. Further increase in the dose 250 mg/kg mg showed a less response.
Effect of PcMFE on phagocytic property of macrophages
Pre-treatment of animals with selected doses of PcMFE (100-250mg/kg) considerably reduced the number of bacterial colonies and thus enhanced the phagocytic property of the macrophages as compared to untreated control Figure 3. The extract showed effectively greater phagocytic index at the high dose of 200 mg/kg dose, which decreased with 250 mg/ kg dose, yet not lower than normal control.
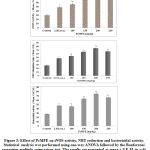 |
Figure 3
|
Effects of PcMFE on Antioxidant enzymes in mice
Maintaining an antioxidant status provided a useful approach in securing immune cell injury and dysfunction, wherein cherry fruit PcMFE showed a moderate effect on LPO, SOD, CAT, and GSH-Px at lower doses. But at 200 mg/kg, PcMFE dose, MDA level in the liver decreased significantly comparable to the level of Levamisole group. The activities of CAT and SOD in the liver of PcMFE treated animals significantly increased as compared with the normal control (Table 4). Antioxidant profile of GSH-Px in different groups of animals showed a pattern similar to the (CAT and SOD) with a significant increase in the activities of antioxidant enzymes (Table 4)..
Table 4: Modulatory influence of PcMFE on the MDA level and the activities of SOD, CAT and GSH-Px in control and experimental animals.
| Group
(mg/kg b. w.) |
CAT
(U/mg) |
SOD
(U/mg) |
GSH -Px
(mg1-1) |
MDA
(nmol (mg protein)-1) |
| Control N.S.
200 μl |
149.8±5.9
|
140.49±19.44 | 263.1±18.4 | 0.562 ± 0.08 |
| Levamisole
2.5 |
162.3±2.6** | 188.31±34.72** | 365 ± 69.5** | 0.51±0.140** |
| PcMFE
100 |
91.68±6.0 | 192.28±35.19* | 349 ± 74.1 | 0.600 ± 0.077 |
| PcMFE
150 |
181.2±5.1** | 201.23±45.42** | 410 ± 83.2** | 0.527 ± 0.074** |
| PcMFE
200 |
208.6±9.9** | 259.2±4.8** | 597 ± 97.7** | 0.468 ± 0.061** |
| PcMFE
250 |
194.0±5.4** | 217.1±9.7** | 525.1±18.6** | 0.488 ± 0.096** |
Values are means S E, N = 6. * p < 0.05, ** p < 0.01, *** p < 0.001, compared with normal control. # p < 0.05, ## p < 0.01, ### p < 0.001, compared with positive model control.
Discussion
Based on our previous study and nutraceutical values, the immunomodulatory activity of standardized methanolic fruit extract of P. cerasus (PcMFE) was explored in experimental animal model. The results of the present study indicate that PcMFE is highly efficient in augmenting the immune responses to T-dependent antigen under in vivo conditions. This is evidenced from our findings that PcMFE increased lymphocyte sub-populations, supported Th1 type immunity, and enhanced macrophage activation and immunoglobulins secretion as well as increased the DTH reaction thereby suggesting that PcMFE stimulates cell mediated and humoral immunity. Antibody molecules (IgG and IgM) are the major immunoglobulin in humoral immune responses, were determined by the haemagglutination titre. The PcMFE treatment exerted a direct effect on responsiveness of T and B cells subsets resulting in an enhanced production of IgM and IgG immunoglobulins in blood sera of SRBC immunized mice, with optimal effect at 200 mg/kg dose, reflecting an overall elevation of humoral response. Since the antibody production was closely associated with the co-operation of macrophages, T and B lymphocyte responsiveness. The T cells in turn participate in the expression of cell mediated immunity contributing to Delayed type hypersensitivity (DTH) aspect of host defense system 20. The treatment with PcMFE enhanced the DTH reaction, as compared to the control group, suggesting infiltration of macrophages to the inflammatory site and induction becomes apparent within 24-72 h. Increase in the DTH response indicates that drug has a stimulatory effect on lymphocytes. Further exploring as an immunomodulator, the effects of PcMFE on both T and B lymphocytes sub-populations in SRBC immunized BALB/c mice were analyzed by flow cytometric assay. The results of present investigation revealed that the percentage of CD4+, CD8+ lymphocytes and CD19+ cells were greatly augmented by PcMFE, indicating the immunostimulatory potential of sour cherries.
Cytokines are major factors involved in regulation of the immune response to antigens and infectious agents, and immunologic intervention with fruit extract that helps specifically modulating Th1 and Th2 response may have important implications in eliciting desired type of immune response 21. The enhanced secretion of selective cytokines IL-12 and IFN-γ by PcMFE may be because of various chemical constituents present in the extract. In response to cytokines secreted by activated macrophages, IFN-γ elaborated by T cells further activates the macrophages to produce higher levels of nitric oxide by inducing the expression of iNOS activity 22. Our study demonstrated that production of iNOS and activation of macrophages are correlated and PcMFE treatment is responsible for high expression of iNOS. Moreover, NBT reduction test is an indirect marker of the oxygen dependent bactericidal activity of the phagocytes and granulocytes 23. Treatment of mice with different doses of PcMFE showed a significant, but dose dependent rise in NBT reduction potential of macrophages. Thereafter, PcMFE elevated phagocytic activity of macrophages that eventually help in the generation of effective immune response by secreting various chemokines.
In order to defense against infection, phagocytes produce ROS and cause injury to target cells, which is harmful to the immune system 24. Therefore, maintaining adequate antioxidant status is useful in preventing immune cell injury and dysfunction observed in some inflammatory/autoimmune disorders. The glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and catalase (CAT) are important enzymes, which detoxify lipid hydroperoxides, superoxide radicals and hydrogen peroxide respectively 25. Here, PcMFE significantly elevated the SOD, GSH-Px, and CAT levels and decreased the MDA level. When the PcMFE dose is 200 mg/kg, MDA in the liver reached the level of the Levamisole group. The results suggest that the lipid peroxidation inhibition by PcMFE might be due to its effects on the antioxidant enzyme system (Table 4), which showed that it has the ability to improve the immunomodulatory activity. These findings indicated that PcMFE can protect the immune organs, cells by increasing the activities of antioxidant enzymes.
Conclusion
Overall the present study showed that PcMFE played an important role in the modulation of the immune response as well as exhibited significant therapeutic bioactivity and thus may have applications as an effective immunotherapeutic agent.
Acknowledgment
Sheikh Abid Ali would like to thank Dr. Ram A. Director, IIIM Jammu for providing necessary facilities and support.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- M.D. Hagerstown, L. Williams, Wilkins, Essentials of pathophysiology: concepts of altered health states. Porth Carol. 270 (2007) ISBN 0–7817–7087–4.
- V.M. Victor, M. Rocha, M. Fuente, Immune cells: Free radicals and antioxidants in sepsis. IntImmunopharmacol. 4 (2004) 327–347.
- J.M. Gutteridge, J. Michell, Redox imbalance in the critically ill. Br Med Bull. 55 (1999) 49–75.
- F.L. Kong, F.E Li, Z.M. He, Y. Jiang, Y.R Hao, X. Sun, H.B. Tong, Anti-tumor and macrophage activation induced by alkali-extracted polysaccharide from pleurotusostreatus. Int. J. Biol. Macromol. 69 (2014) 561–566.
- N. Raje, T. Hideshima, K.C. Anderson, Therapeutic use of immunomodulatory drugs in the treatment of multiple myeloma.Expert Rev Anticancer Ther. 6 (9) (2006) 1239-47.
- A. Bafna, S. Mishra, Antioxidant and immunomodulatoryactivity of the alkaloidal fraction of cissampelospareiralinn. SciPharm, 78 (2010) 21–31.
- G. Ferretti, T. Bacchetti, A. Belleggia, D. Neri, Cherry antioxidants, from farm to table. Molecules, 15 (2010) 6993–7005.
- A. Sarić, S. Sobocanec, T. Balog, B. Kusić, V. Sverko, V. Dragović-Uzelac, B. Levaj, Z. Cosić, Z. MacakSafranko, T. Marotti, Improved antioxidant and anti-inflammatory potential in mice consuming sour cherry juice (Prunuscerasus cv. Maraska). Plant Foods Hum Nutr. 64(4) (2009) 231-237.
- T. Traustadóttir, S.S. Davies, A.A. Stock, Y. Su, C.B. Heward, L.J. Roberts, Tart cherry juice decreases oxidative stress in healthy older men and women. J Nutr. 139 (2009) 1896-1900.
- A. Kirakosyan, E.M. Seymour, K.R. Noon, D.E. UrcuyoLlanes, P.B. Kaufman, S.L. Warber, S.F. Bolling, S Interactions of antioxidants isolated from tart cherry (Prunuscerasus) fruits. Food Chem. 122 (2010) 78–83.
- A.A. Sheikh, A. Bhatia, Q. Parvaiz, H.M. Bhat, S.F. Ahmad, N. Khera, R. Ahmad, In vitro immunomodulatory study of different parts of Prunuscerasus L. (sour cherry) Plant. Asian Journal of Plant Science & Research. 3(3) (2013) 35-43.
- A. Sheikh, K. Anamika, P. Qazi, S. Tabasum B. Aruna, S. Surjeet, A.R. Shabir, M.K. hawa, N.K Satti, P. Dutt, Immunomodulatory studies of a bioactive fraction from the fruit of Prunuscerasus in BALB/c mice. Int. Immunopharamacol. 12 (2012) 626–634.
- J.B. Alsever, R.B. Ainslie, A new method for the preparation of dilute blood plasma and the operation of a complete transfusion service. New York State. Jr of Medi. 41 (1941) 126–131.
- M. A. Tempero, Y. Haga, C. Sivinsk, D. Birt, L. Klassen, G. Thiele, S Immunologic effects of levamisole in mice and humans: evidence for augmented antibody response without modulation of cellular cytotoxicity. J Immunother. 17 (1995) 47-57.
- A. Gupta, A. Khajuria, J. Singh, K.L Bedi N. K. Satti, P. Dutt, Immunomodulatory activity of biopolymericfraction RLJ-NE-205 from Picrorhizakurroa. Int.Immunopharmacol. 6 (2006) 1543-1549.
- A.R. Bafna, S.H. Mishra, Protective effect of bioactive fraction of Sphaeranthusindicus Linn against cyclophosphamide induced suppression of humoral immunity in mice. J Ethnopharm. 104 (2006) 426-429.
- D.J. Stuehr, M.A. Marletta, Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 47 (1987) 5590–5594.
- L. Hudson, F.C. Hay, F.C. A Handbook of Practical Immunology, 3rd ed. Blackwell Scientific Publication. London Oxford. 1989.
- N. Raghuramulu, K.N. Madhavan, S. Kalyansundham, A Manual of Laboratory Techniques. NIN, ICMR, Silver Prints, Hyderabad, India. 1983.
- H.H. Mu, W.A. Sewell, Regulation of DTH and IgE responses by IL-4 and IFN-gamma in immunized mice given pertussis toxin. Immunol. 83 (4) (1994) 639–645.
- L.C. Yang, C.C. Hsieh, W.C. Lin, Characterization and immunomodulatory activity of rice hull polysaccharides. Carbohydr. Polym. 124 (2015) 150–156.
- X.B. Yang, Y. Lv, L.M. Tian, Y. Zhao, Composition and systemic immune activity of the polysaccharidesfrom an herbal tea (LysopusiucidusTrucz). J. Agric. Food Chem. 58 (2010) 6075–6080.
- E. Vivier, D.H. Raulet, A. Moretta, M.A. Caligiuri, L. Zitvogel, L.L. Lanier, W.M. Yokoyama, S Ugolini, Innate or adaptive immunity. The example of natural killer cells. Science. 331 (2011) 44–49.
- S.A. Weitzman, L.I. Gordon, Inflammation and cancer: Role of phagocyte-generated oxidants in carcinogenesis. Blood. 76 (1990) 655–663.
- J. Lovric, M. Mesic, M. Macan, M. Koprivanac, M. Kelava, V. Bradamante, Measurement of malondialdehyde (MDA) level in rat plasma after simvastatin treatment using two different analytical methods. Period. Biol. 110 (2008) 63–67.







