M.Karpakavalli1*, A.Y.Sangilimuthu2, A.Usha Raja Nanthini3, G.Nagaraja Perumal4, S. Mohan5 and T. Sivakumar6
1Dept. of Pharmaceutical Chemistry, Karpagam College of Pharmacy, Coimbatore-641032. Tamil Nadu, India.
2Dept. of Biotechnology, Karpagam University, Coimbatore-641021. Tamil Nadu, India.
3Department of Biotechnology, Mother Teresa Women’s University, Kodaikanal, India -624 101
4Dept. of Pharmacology, Karpagam College of Pharmacy, Coimbatore-641032, Tamil Nadu, India.
5Dept. of Pharmaceutical Biotechnology, Karpagam College of Pharmacy, Coimbatore-641032, Tamil Nadu, India.
6Dept. of Pharmaceutical Chemistry, Nanda College of Pharmacy, Perundurai-638052, Tamil Nadu, India.
Corresponding Author E-mail: sreemenakag@gmail.com;
DOI : https://dx.doi.org/10.13005/bpj/1809
Abstract
In the modern medicines the novel and active molecules are essential to act against various diseases and increase the needs day by day due to population increase. In view of that, we attempt to make a variety of synthetic molecules against inflammation by a new and popular greener microwave assisted and faster method such as Microwave Enhanced Chemistry assisted Vilsmeier Haack Synthesis (MEC-VHS). In this paper, we report the synthesis of nitro- dinitro- and acetyl- derivatives of 3- formyl, 7-flavonols using MEC-VHS techniques against inflammation as anti-inflammatory agent. These derivatives were synthesized via pinkish formylation complex of dimethyl formamide and phosphorous oxychloride by microwave irradiation resulted as suspension by base. The re-crystallized products were characterized through Co-TLC, λmax, IR, HPTLC, 1HNMR, CHN analysis and mass spectral studies. The HPTLC finger print profiles obtained were of with a prominent single peak and with a matching Rf values compared to that obtained by an ordinary Co-TLC technique. All the synthesized compounds were screened for their anti-inflammatory activity by in vitro protein denaturation method and in vivo carrageenan induced paw oedema method and it was found that all the compounds excepting the un-substituted 3-formyl, 7-flavonols gave an equi- or more potent activity as compared to that of the standard.
Keywords
HPTLC Profiles; in Vitro and in Vivo Anti-Inflammatory Activities; Microwave Enhanced Chemistry; Vilsmeier-Haack Synthesis; 3-Formyl, 7-Flavonols
Download this article as:| Copy the following to cite this article: Karpakavalli M, Sangilimuthu A. Y, Nanthini A. U. R, Perumal G. N, Mohan S, Sivakumar T. Anti-Inflammatory Effects of 3-Formyl, 7-Flavonols Derivatives by Microwave Enhanced Chemistry Assisted - Vilsmeier Haack Synthesis. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Karpakavalli M, Sangilimuthu A. Y, Nanthini A. U. R, Perumal G. N, Mohan S, Sivakumar T. Anti-Inflammatory Effects of 3-Formyl, 7-Flavonols Derivatives by Microwave Enhanced Chemistry Assisted - Vilsmeier Haack Synthesis. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/33GgoLE |
Introduction
Flavonoids are very good antioxidant and posses numerous health effects also it act as anticancer, anti-inflammatory, anti-analgesic and anti-microbial agents still there is a need to find good and cost effective flavonoid content to the society to overcome the scarcity1. A number of medicinal conditions were prevented with the use of flavonoids2. Hydroxy, methoxy, halogen, alkyl derivatives are common type of flavone derivatives, among which hydroxy, methoxy derivatives are more frequently synthesized derivatives3. Flavonol, present in a wide variety of fruits and vegetables, is reported as one of the non-competitive inhibitors of cytochrome P450.
In recent years, the flavonoids have grasped the full attention of a few groups of synthetic chemists owing to the challenge they face in synthesizing, purifying and characterizing them in laboratories3. Flavonoids were present in present in most of the natural sources as essential components and also the isolation of such molecule from natural source are very difficult and cost effective manner. Thus, no possibility for easy dependence of theses flavan’s family of compounds as starting material or as intermediates in the organic syntheses4.
Aromatic formylation via electrophilic aromatic substitution reaction includes Dimethylformamide and phosphorus oxychloride in the Vilsmeier-Haack reaction; Hexamine5 in the Duff reaction and the Sommelet reaction; Carbon monoxide and hydrochloric acid in the Gattermann-Koch reaction6; Anionic cyanides in the Gattermann aldehyde synthesis; Chloroform in the Reimer-Tiemann reaction; Dichloromethyl methyl ether in Rieche formylation7. Hence of all the above said formylation procedures, Vilsmeier reaction9 using dimethyl formamide and phosphorous oxychloride was used for the formylation of π-excessive heteroaromatic systems like thiophene, furan and chromene of our interest.
Therefore, we plan to address the key problems that are encountered by the medicinal chemists in academician as well as in industries to synthesis 3-formyl, 7-flavonols by aromatic formulation through microwave assisted organic synthesis (MAOS). MAOS are well advanced method with numerous advantages such as faster processing time, improved yield and quality, direct extraction capacity, lower energy consumption, reduced solvent levels, reaction rate acceleration used even in milder reaction conditions, lower energy usage and different reaction selectivity10.
Since the synthetic chemistry community has been under increased pressure to produce in an environmentally benign fashion11, outdating the too slow magnitude traditional methods called as “Bunsen Burner of 21st century” of aromatic formylation, we thus plan to avail the wonderful Microwave technology for the synthesis of 3-formyl, 7-flavonols12. In combination with digital scanning profiling, HPTLC provides accurate and precise Rf values of the fractions with peak values and chromatogram13. Since, the HPTLC fingerprint profiles for 3-formyl, 7-flavonols have not been reported in literature and thus the same has been planned to be developed in the present work for the confirmation of Rf data with that obtained by TLC method, in order to standardize them as official medicinal compounds14.
Inflammations are a tissue reaction by infection, irritation and through foreign substances. Aging is also considered to be an inflammatory response15. Even though human endogenous anti-oxidants antagonize the oxidants and free radicals, sometimes, they are not sufficient to neutralize these reactive species. Some of the literatures16 strongly quote the increased type nitric acid production plays an important role in inflammatory diseases like arthritis, bronchial asthma and ulcerative colitis. This inspiring point led us to emphasize the importance of the mono- di- and tri- nitro derivatives of synthesized 3-formyl, 7-flavonols for testing their anti-inflammatory capabilities17. Among the many methods used for screening, one of the most commonly employed techniques is based upon the ability of such agent to inhibit the oedema produced in the hind paw of rat after injection phlogiston agent. Many phlogiston agents are used such as dextrin, carrageenan, egg albumin, formaldehyde etc.
From the detailed literature review, since no work has been carried on the formylation of 7-flavonol, and thence their anti-inflammatory activities, we therefore plan to focus our interests in working on this area and thus plan to synthesize a small collection of 3-formyl, 7-flavonol derivatives using a microwave assisted synthesis to identify their presence using HPTLC method and to explore their anti-inflammatory activity by i) protein denaturation method and ii) albumin induced rat paw oedema method so as to add value to the formyl flavonols’ traditional claim.
Materials and Methods
Chemicals and Instruments
All chemicals and solvents used were of analytical grade and obtained from SD fine chemicals, Chennai, India. A refitted microwave oven (Model No. MS-2342AE) was used for all the experiments. Melting points were determined in open capillary tubes on an Electro thermal apparatus and were uncorrected. The purity of the compounds was checked by TLC-using Silica gel-G (E-Merck) visualized by UV light. Maximum absorption data were done by using Schimadzu UV spectrophotometer (Model No. UV- 2400 PC). I.R. spectra were recorded on KBr pellets in the wave range of 4000-400 cm-1 on FTIR Schimadzu spectrophotometer (Model No.Affinity A-1). 1HNMR spectra were recorded on APTECH make (Model. No. API/W220) spectrometer in D2O as solvent. Chemical shifts were reported in parts per million down fields from Tetramethylsilane. Mass spectra were recorded on a LC Micromax mass spectrometer (Model.No.API 4000, 1034067R). The elemental analysis (C, H and N) were performed on a Leco CHNS-932 elemental analyser.
General procedure for the preparation of 3-formyl, 7- flavonol derivatives (Ia-i) by microwave technique18
In a 500 ml beaker, the freshly distilled dimethylformamide (0.16 mol) was taken and pre cooled in an ice-salt bath for about 15 minutes. To that, added the freshly distilled phosphorus oxychloride (0.14 mol) for another 15 minutes. A solution of 7-flavonols (0.12 mol) in dichloromethane (3 ml) was added to the pinkish formylation complex. The resulted yellow solution was stirred at a temperature not exceeding 10° for next 30 minutes.
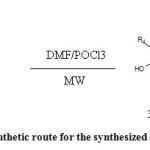 |
Scheme 1: Synthetic route for the synthesized compounds |
A refitted microwave oven was used in our laboratory with the addition of a magnetic stirrer, thermometer and water condenser for automatic chemosynthetic procedures. Another beaker containing 200 ml of water was placed next to the reaction vessel to serve as a ‘heating sink’. The viscous solution was then irradiated for one minute in a bench mate microwave synthesizer to bring up the temperature to 35 °C. To maintain the same temperature for another half an hour, the syrup was discontinuously irradiated at half a minute interval so as to obtain an opaque, paste form from a clear solution. Crushed ice was added to that to get a cherry-red aqueous solution. One third of the solution of an aqueous base, sodium hydroxide (12.4 g) in distilled water was added to above solution which was kept in crushed ice, in drop wise first and the remaining quantities were added more rapidly with vigorous stirring. The resulting suspension was irradiated rapidly to the boiling point, cooled immediately and was placed in a refrigerator overnight. Most of the inorganic material dissolved on re suspension of the crude residue in distilled water. The pure product was obtained by re-crystallization from methanol. The products (Ia–i) were confirmed by Co-TLC technique. UV spectra of compounds (20 μg / ml) were recorded using spectral grade distilled water. The infrared spectral study was done by KBr disc method (0.5-1.0 mg). All the compounds were synthesized in high yields and sufficient purity.
Table 1: Structures of 7-flavonols (Ia-i) and 3-formyl, 7-flavonols (IIa-i)
| Sl.
No. |
Name of 7-flavonol analogs (Ia-i) | Name of the 3-formyl, flavonols (IIa-i) | R1 | R2 | R3 |
| 1. |
H |
H |
H |
||
| 2. |
-NO2 |
H |
H |
||
| 3. |
– COCH3 |
H |
H |
||
| 4. |
H |
H |
– NO2 |
||
| 5. |
-NO2 |
H |
– NO2 |
||
| 6. |
– COCH3 |
H |
– NO2 |
||
| 7. |
H |
-NO2 |
– NO2 |
||
| 8. |
-NO2 |
-NO2 |
– NO2 |
||
| 9. |
– COCH3 |
-NO2 |
– NO2 |
HPTLC Finger Print profiles19-20
Development technique
Made/ Make of Instrument : Camag (Switzerland),
Sample Applicator : Linomat IV
Scanner : Camag TLC Scanner II
Development Chamber : 10X10, Camag Twin-trough chamber
Source of radiations for detection : Deuterium and tungsten lamp
Detection nm : 254 and 366 nm
Documentation System : Digi store-2 documentation system with win CATS and
Video Scan Software 3.15 version.
Photorecordings : Camag Reprostar 3
Syringe : Hamilton (USA)
Stationary phase : Pre coated silica gel 60 F254
Aluminum plates : (Merck, KgaA, Germany)
Plate thickness : 0.2 mm
Plate size : 100 x 100 mm
Syringe size : 100 μl syringe
Application rate : 10 s μl–1
Table speed : 10 mm s-1
Distance from starting : 15 mm
Distance from bottom : 10 mm
Volume applied : 2 μl
Band length : 10 mm
Distance between tracks : 10 mm
Mode of development : Ascending
Development distance : 80 mm
Reagent preparation : As per WHO & API guidelines
Mobile phase preparation : Benzene: Ethyl acetate: Formic acid 40:10:5 (v/v/v), solvent
mixture was mixed and centrifuged. The centrifugate was
used as mobile phase.
Spraying reagent : Sodium borohydride in alcohol (1 %) followed with ethanolic
aluminium chloride
Preparation of Solution : Sample in 10 ml of methanol (1 μgμL-1).
Anti-inflammatory activity
Animal Selection and Acclimatization
Wistar Albino rats (150-200 gm) of either sex were procured from Amrita Institute, Kerala. The experimental protocol was initially approved from the Institutional animal ethical committee under the reference No. KU/IAEC/Ph.D/065. The animals were maintained in department of animal house of our college for 7 days. They were housed in polypropylene cages and were fed with standard rodent pellet diet (Baramati Agro Ltd.,) and water ad libitum. The conditions of room temperature 21-25 ºC and relative humidity 50-60 % were maintained. They were exposed to alternate cycle of 12 hrs of darkness and 12 hrs light. The animals were fasted over night for at least 12 hrs. The standard oral feeding tubes and syringes were used for drug administration.
Acute toxicity studies- OECD 423 guidelines
Acute toxicity studies were carried out as per OECD guideline 423. The drugs in CMC suspension were administered orally. The animals were fasted overnight prior to the experimental procedure. The animals were observed individually after dosing at least once during the first 30 mins, periodically during the first 24 hrs, with special attention given during the first 4 hrs, and daily thereafter, for 14 days.
In vitro anti-inflammatory activity by Protein Denaturation Method
The egg white was separated from the whole egg. The solvent control (distilled water), standard (Diclofenac sodium, 500 µg/ml) and test samples (500 µg/ml) each 1 ml were added to egg albumin (1 ml, 1 mM) in separate sample test tubes. Denaturation of albumin was induced by keeping the reaction mixture at 70 ºC in water bath for 10 minutes. After cooling the supernatant, the turbidity was measured at 660 nm in spectrophotometer21. The percentage inhibition of protein denaturation was calculated using the formula
% Inhibition = [ A(Control) – A(Sample) / A(Control) ] X 100, where A-Absorbance
In vivo anti-inflammatory by Carrageenan induced Paw Oedema in Rats
Dose
Carrageenan: 1% w/v solution and injecting 0.1 ml underneath the plantar region. Indomethacin (20 mg/kg b. w): Prepared a stock solution of 10 ml containing 4 mg/ml concentration administrated 0.5 ml/100 g of body weight of the animal by oral route. Test samples (20 mg/kg b.w (p.o)): Prepared a stock solution of 10 ml containing 4 mg/ml concentration and administrated 0.5 ml/100 g of body weight of the animal by oral route. Solvent control: 0.3 % w/v Carboxy methyl cellulose.
Experimental Procedure
Wistar rats (both sexes) were weighed and numbered. A mark was made on both the hind paws, just beyond tibio-tarsal junction. The initial paw volume of right & left hind paw of each rat was noted by mercury displacement method. The experimental animals were divided into 5 groups; each group consisted of 6 animals. Group I received the vehicle of 1 ml, Group II received the standard of Indomethacin of 1 ml and Group III-VIII received the test samples by oral route22. After 30 minutes of the oral administration of the standard and test samples, injected subcutaneously 0.1 ml of carrageenan in the planter region of the left paw of all 7 groups by keeping the right paw as reference. The paw volume of both hind legs of all the groups were measured plethysmometrically at1, 2, 3, 4, 6, 12 and 24 hrs after the challenge of carrageenan. The results were tabulated in Table-9 and Figure -6.
Statistical analysis
Statistical analysis was carried out employing Analysis of Variance (ANOVA) and Dunnett’s multiple comparison tests.
Results and Discussion
The synthesized compounds are thoroughly characterized by physical, chemical and spectroscopic data. The compounds of different 3-formyl, 7-flavonol derivatives (IIa-i) were synthesized from different 7-flavonols (Ia-i), dimethyl formamide and phosphorous oxychloride by using a valid technique Microwave Enhanced Chemistry (MEC).
Various 3-formyl, 7-flavonols were synthesized and yielded from 60% to 92 % for 7-flavonols. The MEC-VHS formylation reacted well for both nitro- and acetyl- substituent on both 6th position of the benzene ring (B) of coumarin of 7- flavonols and also on 3’ or 4’ position of the phenyl ring was attached at 2nd position of benzene ring (A) of coumarin. The purity of the compounds were characterized and elucidated the structure as shown in Tables.3-6. Further, all the derivatives from selected mother compounds were characterized with following spectral values such as UV-Vis, FT-IR, NMR and MS.
Characterization of compound Ia: 3-formyl, 7- hydroxy flavonol
Duration of microwave irradiation 2’30 s; MF: C16H10O4; MW: 266; % yield: 68; Mp 96 ºC; Rf data 0.67; λmax 216nm; IR (Nujol) 3550-3200 cm-1 intermolecular OH stretching; 2720, 2820 cm-1 C-H streching, aldehydes; 1639 cm-1, C=C-C=O stretching, ketones; 1600 cm-1 C-C stretching, benzene nucleus; 1320-1210 cm-1, C-O stretching, Aryl; 1010 cm-1, C-O stretching, primary alcohol; 946 cm-1, Trans RCH=CHR, C-H out of plane pending; 662 cm-1, =C-H bend, medium; 268.98; 1HNMR (D2O, δ ppm): 8.7 (s, 1H, -CHO), 8.0 (d, 1H, ArH), 7.3 (d, 1H, ArH), 7.4 (d, 1H, ArH), 7.6-8.0 (m, 5H, ArH); MS (m/z): 328.28 M+; CHN analysis: Calcd.(Found)- C-74.48 (75.36); H-12.29 (11.98); O-13.23 (15.64).
Characterization of compound Ib: 3-formyl, 6-nitro, 7-hydroxy flavonol
Duration of microwave irradiation 2’30 s; MF: C16H9 NO6; MW: 311; % yield: 67; Mp 99 ºC; Rf data 0.75; λmax 228nm; IR (Nujol) 3600-2600 cm-1 OH stretching; 2900-2695 cm-1 C-H stretching, aldehydes; 1639 cm-1, C=C-C=O stretching, ketones; 1600 cm-1 C-C stretching, benzene nucleus; 1550-1510 cm-1 -NO2 asymmetric stretching & aromatic nitro group; 1435-1405 cm-1,
CH2-C=O, acyclic; 1320-1210 cm-1, C-O stretching, Aryl; 1010 cm-1, C-O stretching, primary alcohol; 946 cm-1, C-H out of plane pending; 662 cm-1, =C-H bend, medium; 1HNMR (D2O, δ ppm): 8.7 (s, 1H, -CHO), 6.70 (s, 2H, ArH), 7.45 (d, 1H), 6.48 (m, 1H), 7.40-7.50 (m, 5H, ArH); MS (m/z): 313.78 M+; CHN analysis: Calcd.(Found)- C-68.14 (70.23); H-11.06 (13.06); N-2.65 (3.12); O-18.15 (19.78).
Characterization of compound Ic: 6- acetyl, 3-formyl, 7-hydroxy flavonol
Duration of microwave irradiation 2’30 s; MF: C18H12O5; MW: 308; % yield: 91; Mp 119 ºC; Rf data 0.70; λmax 219 nm; IR (Nujol) 3600-2600 cm-1 -OH stretching; 2900-2695 cm-1 C-H stretching, aldehydes; 1639 cm-1, C=C-C=O stretching, ketones1600 cm-1 C-C stretching, benzene nucleus; 1320-1210 cm-1, C-O stretching, Aryl; 1280 cm-1 C=O stretching dimer; 1010 cm-1 C-O stretching, primary alcohol; 946 cm-1 C-H out of plane pending; 662 cm-1, =C-H bend, medium; 1HNMR (D2O, δ ppm): 8.68 (s, 1H, -CHO), 7.95 (s, 1H, ArH), 2.59 (d, 1H, -COCH3), 7.70 (dd, 2H, ArH), 7.62 (dd, 2H, ArH), 7.35 (s, 1H, ArH); MS (m/z): 308.94 M+; CHN analysis: Calcd.(Found) – C-73.09 (74.68); H-11.69 (12.63); O-15.21 (17.02).
Characterization of compound Id: 3-formyl, 4’- nitro, 7-hydroxy flavonol
Duration of microwave irradiation 2’00 s; MF: C16H9 NO6; MW: 311; % yield: 79; Mp 89 ºC; Rf data 0.69; λmax 213 nm; IR (Nujol) 3600-2600 cm-1 OH stretching; 2900-2695 cm-1 C-H stretching, aldehydes; 1639 cm-1, C=C-C=O stretching, ketones 1600 cm-1 C-C stretching, benzene nucleus; 1550-1510 cm-1 -NO2 asymmetric stretching & aromatic nitro group; 1320-1210 cm-1, C-O stretching, Aryl; 1280 cm-1 C=O stretching dimer; 1010 cm-1 C-O stretching, primary alcohol; 946 cm-1, C-H out of plane pending; 662 cm-1,=C-H bend, medium; 1HNMR (D2O, δ ppm): 8.72 (s, 1H, -CHO), 7.44 (d, 1H, ArH), 7.43 (d, 1H, ArH), 7.68 (d, 2H, ArH), 8.30 (dd, 2H, ArH); MS (m/z): 311.27 M+; CHN analysis: Calcd.(Found) – C-68.14 (70.23); H-11.06 (13.06); N-2.65 (3.12); O-18.15 (19.78).
Characterization of compound Ie: 3-formyl, 4’,6- dintro, 7-hydroxy flavonol
Duration of microwave irradiation 2’00 s; MF: C16H8 N2O8; MW: 356; % yield: 66; Mp 104 ºC; Rf data 0.69; λmax 210 nm; IR (Nujol) 3600-2600 cm-1 -OH stretching; 3250-2500 cm-1 -OH stretching (Intra molecular with C=O, NO2 etc.,) ; 2900-2695 cm-1 C-H stretching, aldehydes; 1639 cm-1, C=C-C=O stretching, ketones 1600 cm-1 C-C stretching, benzene nucleus; 1550-1510 cm-1 -NO2 asymmetric stretching & aromatic nitro group 1320-1210 cm-1, C-O stretching, Aryl; 1280 cm-1 C=O stretching dimer; 1010 cm-1, C-O stretching, primary alcohol; 946 cm-1, C-H out of plane pending; 662 cm-1,=C-H bend, medium; 1HNMR (D2O, δ ppm): 8.92 (s, 1H, -CHO), 8.41 (s, 1H, ArH), 6.20 (s, 1H, ArH), 7.60-7.75 (m, 2H, ArH), 8.34-8.40 (m, 2H, ArH); MS (m/z): 357.02 M+; CHN analysis: Calcd.(Found) – C-62.80 (64.31); H-10.01 (11.03); N-4.88 (5.23); O-22.31 (24.58).
Characterization of compound If: 6- acetyl, 3-formyl, 4’-nitro, 7-hydroxy flavonol
Duration of microwave irradiation 2’00 s; MF: C18H11 NO7; MW: 322; % yield: 75; Mp 115 ºC; Rf data 0.66; λmax 218 nm; IR(Nujol) 3600-2600 cm-1, OH stretching; 3250-2500 cm-1 OH stretching (Intra molecular with C=O, NO2 etc.,) ; 2900-2695 cm-1 C-H stretching, aldehydes; 1639 cm-1, C=C-C=O stretching, ketones1600 cm-1 C-C stretching, benzene nucleus; 1550-1510 cm-1 -NO2 asymmetric stretching & aromatic nitro group; 1435-1405 cm-1, CH2-C=O, acyclic; 1320-1210 cm-1, C-O stretching, Aryl; 1280 cm-1 C=O stretching dimer; 1010 cm-1, C-O stretching, primary alcohol; 946 cm-1, C-H out of plane pending; 662 cm-1,=C-H bend, medium; 1HNMR (D2O, δ ppm): 8.70 (s, 1H, -CHO), 7.94 (s, 1H, ArH), 2.62 (s, 3H, -COCH3), 7.60 (m, 2H, ArH), 8.25 (m, 2H, ArH); MS (m/z): 321.55 M+; CHN analysis: Calcd.(Found); C-67.33 (69.87); H-10.59 (12.63); N-2.45 (4.01); O-19.62 (21.09).
Characterization of compound Ig: 3-formyl, 3’4’,- dintro,7-hydroxy flavonol
Duration of microwave irradiation 2’10 s; MF: C16H8 N2O8; MW: 356; % yield: 86; Mp 121 ºC; Rf data 0.67; λmax 219 nm; IR (Nujol) 3600-2600 cm-1 -OH stretching; 3250-2500 cm-1 -OH stretching (Intra molecular with C=O, NO2 etc.,) ; 2900-2695 cm-1 C-H stretching, aldehydes; 1639 cm-1, C=C-C=O stretching, ketones 1600 cm-1 C-C stretching, benzene nucleus; 1550-1510 cm-1 -NO2 asymmetric stretching & aromatic nitro group; 1320-1210 cm-1, C-O stretching, Aryl; 1280 cm-1 C=O stretching dimer; 1010 cm-1, C-O stretching, primary alcohol; 946 cm-1, C-H out of plane pending; 662 cm-1,=C-H bend, medium; 1HNMR (D2O, δ ppm): 8.70 (s, 1H, -CHO), 8.0 (d, 1H, ArH), 7.30 (d, 1H, ArH), 7.40(m, 1H, ArH), 8.40 (m, 1H, ArH), 9.0-9.2 (m, 2H, ArH); MS (m/z): 356.99 M+; CHN analysis: Calcd.(Found); C-62.80 (64.31); H-10.01 (11.03); N-4.88 (5.25); O-22.31 (24.58).
Characterization of compound Ih: 3-formyl, 6,3’4’,- trinitro,7-hydroxy flavonol
Duration of microwave irradiation 2’10 s; MF:C16H7 N3O10;MW: 401; % yield: 60; Mp 135 ºC; Rf data 0.74; λmax 220 nm; IR(Nujol) 3600-2600 cm-1 OH stretching; 3250-2500 cm-1 OH stretching (Intra molecular with C=O, NO2 etc.,) ; 2900-2695 cm-1 C-H stretching, aldehydes; 1639 cm-1, C=C- C=O stretching, ketones 1600 cm-1 C-C stretching, benzene nucleus; 1550-1510 cm-1 -NO2asymmetric stretching & aromatic nitro group; 1320-1210 cm-1, C-O stretching, Aryl; 1280 cm-1 C=O stretching dimer; 1010 cm-1, C-O stretching, primary alcohol; 946 cm-1, C-H out of plane pending; 662 cm-1,=C-H bend, medium; 1HNMR (D2O, δ ppm): 10.25 (s, 1H, -CHO), 8.74 (s, 1H, ArH), 6.80 (d, 1H, ArH), 7.95 (m, 2H, ArH), 7.60 (m, 1H, ArH); MS (m/z): 403.44 M+; CHN analysis: Calcd.(Found); C-58.23 (61.01); H-9.12 (10.78); N-6.79 (8.54); O-25.86 (27.45).
Characterization of compound Ii: 6- acetyl, 3-formyl, 3’4’,- dinitro,7-hydroxy flavonol
Duration of microwave irradiation 2’10 s; MF: C18H10 N2O9; MW: 398; % yield: 73; Mp 128 ºC; Rf data 0.72; λmax 215 nm; IR (Nujol) 3600-2600 cm-1 OH stretching; 3250-2500 cm-1 OH stretching (Intra molecular with C=O, NO2 etc.,) ; 2900-2695 cm-1 C-H stretching, aldehydes; 1639 cm-1, C=C-C=O stretching, ketones 1600 cm-1 C-C stretching, benzene nucleus; 1550-1510 cm-1 -NO2 asymmetric stretching & aromatic nitro group; 1435-1405 cm-1, CH2-C=O, acyclic; 1320-1210 cm-1, C-O stretching, Aryl; 1280 cm-1 C=O stretching dimer; 1010 cm-1, C-O stretching, primary alcohol; 946 cm-1, C-H out of plane pending; 662 cm-1, =C-H bend, medium; 1HNMR (D2O, δ ppm): 9.51 (s, 1H, -CHO), 8.19 (s, 1H, ArH), 6.73 (m, 1H, ArH), 2.76 (s, 3H, -COCH3), 7.42 (m, 1H, ArH), 7.14 (m, 1H, ArH); MS (m/z): 399.24 M+; CHN analysis: Calcd.(Found); C-62.41 (64.12); H-9.66 (10.97); N-4.55 (5.66); O-23.38 (25.12).
HPTLC studies were carried out to confirm the presence and purity of the reported compounds. This study revealed that the solvent system Benzene: Ethyl acetate: Formic acid 40:10:5 (v/v/v) was ideal and gave a single spot with Rf 0.2-0.25. The Rf values (Table-6) obtained for all synthesized 3-formyl, 7-flavonol (IIa-i) derivatives by HPTLC technique were found to be almost similar to that observed by conventional TLC method and thus a fast and most appropriate Rf values with area and area percentage data were obtained for further confirmation and characterization of the synthetic 3-formyl, 7-flavonol derivatives.
Table 2: HPTLC data of the synthesized 3-formyl, 7-flavonols (IIa-i)
| Sl. No. | Name of the compounds (Ia-i) | Rf values | Height of the peak (AU) | Area (AU) | Area percentage |
| 01. | Compound (Ia) | 0.67 | 645.5 | 9563.70 | 57.76 |
| 02. | Compound (Ib) | 0.73 | 840.0 | 11856.90 | 44.79 |
| 03. | Compound (Ic) | 0.68 | 564.7 | 3475.80 | 36.20 |
| 04. | Compound (Id) | 0.64 | 645.8 | 5437.40 | 41.20 |
| 05. | Compound (Ie) | 0.71 | 952.7 | 12853.90 | 52.50 |
| 06. | Compound (If) | 0.63 | 487.2 | 3011.10 | 33.30 |
| 07. | Compound (Ig) | 0.61 | 654.5 | 4563.90 | 51.70 |
| 08. | Compound (Ih) | 0.70 | 701.7 | 3434.70 | 41.60 |
| 09. | Compound (Ii) | 0.66 | 765.3 | 6879.60 | 53.70 |
Ia:3-formyl, 7-flavonol;Ib:6-nitro, 3-formyl, 7-flavonol;Ic:6-acetyl, 3-formyl, 7-flavonol; Id:3-formyl, 4’-nitro, 7 flavonol; Ie:4’6-dinitro, 3-formyl, 7 flavonol; If:6-acetyl, 3- formyl, 4’-nitro, 7 flavonol;Ig:3-formyl,3’4’-dinitro,7flavonol; Ih:3-formyl,3’4’6-trinitro,7F; Ii:6-acetyl, 3-formyl, 3’4’- dinitro,7F.
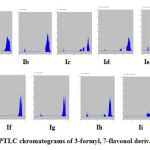 |
Figure 1: HPTLC chromatograms of 3-formyl, 7-flavonol derivatives (IIa-i) |
Based on the mortality results of sighting study, dose levels of 5, 50, 300 and 2000 mg/kg were decided as starting doses for the main study and carried out with five animals. They were found safe without exhibiting any toxic effects on animals’ up to 300 mg / kg, body weight by oral. Based on the result on 14th day of observation, the dose for in vivo study was selected as 300 mg/kg.
Table 3: Acute toxicity study data (OECD guidelines)
| Sl. No. | Dose levels
(mg/kg) |
Weight of the animal (in gms) | Signs of toxicity | Weight of the animal after the study (in g) | Onset of toxicity | Duration of study |
| 01. | 2000 | 230±10 | Nil | 232±11 | Nil | 14 days |
| 02. | 300 | 230±10 | Nil | 222±12 | Nil | 14 days |
| 03. | 50 | 210±15 | Nil | 213±10 | Nil | 14 days |
Denaturation of proteins is a well documented cause of inflammation. Most NSAIDs have shown dose dependent ability to thermally induced protein denaturation. As a part of the investigation on the mechanism of the anti-inflammatory activity of 3-formyl, 7-flavonols to inhibit protein denaturation was studied with egg white test due to sensitivity by orally active anti-inflammatory agents particularly in the acute phase of inflammation. The nitro derivatives of the test compounds (500 µg/ml) showed significant inhibition of protein denaturation of egg albumin (91 %), whereas the standard Diclofenac sodium showed 93 % inhibition compared with the control. Maximum inhibition was observed with nitro derivatives (91 %).
Table 4: In vitro anti-inflammatory activity of the synthesized 3-formyl, 7-flavonols by protein denaturation method.
| Sl. No | Samples | Dose (µg/ml) | Absorbance at 660 nm | % inhibition |
| 01. | Solvent Control | – | 2.181 | nil |
| 02. | Standard, Diclofenac sodium | 500 | 0.151 | 93 |
| 03. | 3-formyl, 7-flavonol (Ia) | 500 | 1.721 | 17 |
| 04. | 3-formyl, 6-nitro, 7-flavonol (Ib) | 500 | 1.614 | 26 |
| 05. | 6-acetyl, 3-formyl, 7-flavonol (Ic) | 500 | 1.158 | 47 |
| 06. | 3-formyl, 4’-nitro, 7-flavonol (Id) | 500 | 0.721 | 67 |
| 07. | 3-formyl, 4’,6-dinitro, 7-flavonol (Ie) | 500 | 1.263 | 42 |
| 08. | 3-formyl, 4’-nitro,6-acetyl, 7-flavonol (If) | 500 | 1.172 | 47 |
| 09. | 3-formyl, 3’4’,-dintro, 7-flavonol (Ig) | 500 | 0.431 | 81 |
| 10. | 3-formyl, 3’4’6,-trinitro, 7-flavonol (Ih) | 500 | 0.199 | 91 |
| 11. | 3-formyl, 3’4’-dinitro, 6-acetyl, 7-flavonol (Ii) | 500 | 1.022 | 54 |
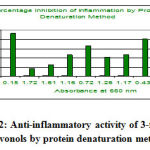 |
Figure 2: Anti-inflammatory activity of 3-formyl, 7-flavonols by protein denaturation method |
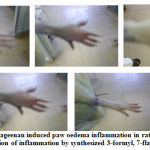 |
Figure 3: Carrageenan induced paw oedema inflammation in rats and gradual inhibition of inflammation by synthesized 3-formyl, 7-flavonols |
Stage-1 (Hind paw before induction of inflammation); Stage-1I (Inflammation induced); Stage-1II (Bulged appearance of Inflammation induced Hind paw); Stage-1V (Gradual subsiding of Inflammation) and Stage-V (Inhibitory activity shown by the synthesized 3-formyl, 7-flavonols)
The carrageenan induced paw oedema method in rats was used to comparatively evaluate the anti-inflammatory activity of 3-formyl, 7-flavonol derivatives. The results revealed that the all synthesized flavonols and its derivatives have shown anti inflammatory activity (*P<0.05, **P<0.01 and ***P<0.001) when compared to control and positive control group. The dinitro derivatives say, 3’4’-dinitro, 7-flavonol and 6, 4’-dinitro, 7-flavonol have expressed (**P<0.05) and (***P<0.001) reduction in paw volume as that of the standard Indomethacin the second and
Table 5: Percentage of inhibition of inflammation by 3-formyl, 7- flavonols in Carrageenan induced paw oedema in rats method.
| Treatment (mg/Kg oral route) | OEDEMA (ml) [Time after carrageenan 30 µg/paw (h)] | |||||||
| 0.5 | 1 | 2 | 3 | 4 | 6 | 12 | 24 | |
| Control | 1.40±0.07 | 1.50±0.06 | 1.80±0.08 | 1.90±0.14 | 1.90±0.04 | 1.80±0.09 | 1.70±0.11 | 1.60±0.13 |
| Standard, Indomethacin (20) | 0.70±0.02
(50.0 %) |
0.60±0.01*
(60.0 %) |
0.70±0.03**
(61.1 %) |
0.70±0.06
*** (63.2 %) |
0.60±0.01
*** (68.8 %) |
0.42±0.02
*** (78.80 %) |
0.47±0.00
*** (72.2 %) |
0.47±0.01
*** (70.60 %) |
| Sample IId (30) | 1.19±0.11
(15.0 %) |
1.17±0.07
(22.0 %) |
1.17±0.02*
(35.0 %) |
0.80±0.04
* (42.0 %) |
0.93±0.01
** (51.1 %) |
0.86±0.05
** (52.2 %) |
0.92±0.03
** (45.9 %) |
0.94±0.07
** (41.3 %) |
| Sample IIe (30) | 1.08±0.08
(22.9 %) |
1.06±0.05
(29.3 %) |
1.21±0.06*
(32.8 %) |
0.97±0.03
* (49.0 %) |
0.80±0.02
** (57.9 %) |
0.70±0.04
** (61.2 %) |
0.80±0.01
** (53.0 %) |
0.88±0.02
** (45.1 %) |
| Sample IIf (30) | 1.10±0.02
(21.4 %) |
1.06±0.01
(29.3 %) |
1.08±0.10
(40.0 %) |
0.99±0.08
* (48.0 %) |
0.85±0.01
** (55.3 %) |
0.70±0.03
** (61.1 %) |
0.82±0.06
** (51.8 %) |
0.83±0.07
** (48.1 %) |
| Sample IIg (30) | 0.98±0.04
(28.6 %) |
0.80±0.03
(46.7 %) |
0.88±0.06*
(51.1 %) |
0.83±0.02
** (56.3 %) |
0.71±0.04
*** (62.6 %) |
0.50±0.01
*** (72.2 %) |
0.57±0.00
*** (66.5 %) |
0.64±0.01
*** (60.0 %) |
| Sample IIh (30) | 0.78±0.02
(44.3 %) |
0.76±0.01
(49.3 %) |
0.83±0.03*
(53.9 %) |
0.79±0.04
** (58.4 %) |
0.65±0.00
*** (65.8 %) |
0.43±0.02
*** (76.1 %) |
0.54±0.03
*** (68.2 %) |
0.63±0.04
*** (60.6 %) |
| Sample IIi (30) | 1.02±0.07
(27.2 %) |
0.96±0.08
(36.0 %) |
1.04±0.09*
(42.2 %) |
0.82±0.06
** (56.8 %) |
0.74±0.05
*** (61.1 %) |
0.56±0.01
*** (69.0 %) |
0.63±0.02
*** (63.0 %) |
0.77±0.03
*** (52.0 %) |
Samples IId: 3-formyl, 4’-nitro,7-flavonol; IIe: 3-formyl, 4’,6-dinitro,7-flavonol; IIf: 3-formyl, 4’nitro,6-acetyl,7-flavonol; IIg: 3-formyl, 3’,4’-nitro, 7-flavonol; IIh: 3-formyl, 3’,4’,6-trinitro,7-flavonol; IIi: 3-formyl, 3’,4’-dintro,6-acetyl,7-flavonol. Values are expressed as mean+ SD; Values are from triplicate readings; and are statistically significant at p<0.05*, p<0.01**, p<0.001***, when compared to the standard, Indomethacin.
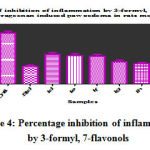 |
Figure 4: Percentage inhibition of inflammation by 3-formyl, 7-flavonols |
third hours respectively. In next level, the trinitro- and mononitro-derivatives, say, 3-formyl, 4’-nitro, 7-flavonol and 3-formyl, 6, 3’4’-trinitro, 7-flavonol started showing a good anti-inflammatory activity at third hr (***P<0.001); The 6-acetyl, 4’-nitro, 7- flavonol and 6-acetyl, 3’4’-dinitro 7-flavonol were shown moderate reduction in paw volume when compared each other groups at second hr. The anti inflammatory action when compared for next 24 hrs in the above specified same manner, it was overall found that dinitro-, then trinitro- & mononitro- derivatives of 3-formyl, 7-flavonols worked well as equi- or more potent as that of the standard Indomethacin. The anti-inflammatory activity study was satisfactory as it gave a significant in reduction of paw volume.
The comparison of the factors time and yield for the current compounds can thus be well imagined as most favorable when compared to the general formylation procedure of the other compounds, as there is no specific formylation reactions reported for any synthetic flavnoids or for flavonols too. Thus, the present method provides i) a new approach for the synthesis of 3-formyl, 7-flavonols and ii) the high yield of the same in the shorter duration. The proposed HPTLC fingerprint method combined with digital scanning profiling was used for standardization of 3-formyl, 7- flavonol derivatives.
Conclusion
Finally we concluded that, synthesized 10 series of molecules by Microwave Enhanced Chemistry assisted Vilsmeier Haack Synthesis with 3-formyl, 7-flavonols as mother molecule. Among the synthesized compounds, 3-formyl, 3’4’-dinitro, 6-acetyl, 7-flavonol (Ii); 3-formyl, 4’-nitro, 7-flavonol (Id); 3-formyl, 3’4’,-dintro, 7-flavonol (Ig) and 3-formyl, 3’4’6,-trinitro, 7-flavonol (Ih) were found to be possessing high anti-inflammatory potential. These derivatives may use as anti-inflammatory agents in future. Further pharmacological investigations needs to prove the effectiveness of the derivatives.
Acknowledgements
The authors wish to place on record their heartfelt thanks to the Principal and the management of Karpagam College of Pharmacy, Othakalmandapam, Coimbatore – 641032, India for providing the facilities to carry out the research successfully.
References
- Lotito SB, Frei B. Consumption of flavanoid rich foods and increased plasma anti-oxidant capacity in humans; cause, consequence or epiphenomenon. Free Rad Boil Med., 41:1727-46 (2006).
- Jain VR, Jain B. Synthesis and evaluation of antibacterial activity of some carbazone derivatives of flavanol. J Pharm Res., 3: 2745-6 (2010).
- Lozanoab C, Torresb JL, Jimenezb A, Centellesa JJ, Cascantea M. Effect of new antioxidant cysteinyl-flavanol conjugates on skin cancer cells. FEBS let., 579: 4219-25 (2005).
- Magarino SP, San Jose MLG. Evolution of flavanols, Anthocyanins and their derivatives during the aging of red wines elaborated from grapes harvested at different stages of ripening. J Agri Food Chem., 52:1181-9 (2004).
- Quiroga J¸ Trilleras J, Insuasty B, Abonía R, Nogueras M, Cobo J. Regioselective formylation of pyrazolo[3,4-b]pyridine and pyrazolo[1,5-a]pyrimidine systems using Vilsmeier–Haack conditions. Tetrahed Lett., 49: 2689-91 (2008).
- William E, Smith. Formylation of aromatic compounds with hexamethylenetetramine and trifluoroacetic acid. J Org Chem., 37: 3972-3 (1972).
- Jalmes LS, Rochin C, Janin R, Morel M. Formylation of aromation compounds in superacidic medium. Ind Chem Lib., 8: 325-35 (1996).
- Yakubov AP, Tsyganov DV, Belenkii LI, Krayuskin MM. Synthesis of sterically hindered aromatic aldehydes. Russian Chem Bull., 40: 1427-32 (2004).
- Fieser FL, Hartwell JL, Jones JE, Wood JH, Bost RW. Formylation of anthracene. Org Syn Collin., 3: 98 (1955).
- Gmouh S, Ait M, Meziane A, Mireille BD. Practical and Efficient Synthesis of Tris(4-formylphenyl)amine, a Key Building Block in Materials Chemistry. Olivier Mongin Syn., 1771-4 (2005).
- Vivek P, Varma RS. Microwave assisted organic synthesis and transformations using Benign reaction Media. Accelrys Chem Res., 41: 629-39 (2008).
- Oliver K. Controlled Microwave Heating in modern organic synthesis, Angewandte Chemie Int edn., 2004; 43: 6250-84.
- Kappe OC. High speed combinatorial synthesis utilizing microwave irradiation,Current Opinion in Chem Biol., 6: 314-20 (2002).
- Lew A, Krutzik PO, Hart ME, Chamberlin AR. Increasing rates of reaction; Microwave assisted organic synthesis for Combinatorial Chemistry. J Combinat Chem., 4: 95-105 (2002).
- Molteni, Valentine, Ellis, David A. Recent advances in Microwave assisted synthesis of Heterocyclic compounds. Curr Org Syn., 2: 333-75 (2005).
- Gupta AP, Verma RK, Gupta MM, Kumar S. Estimation of Plumbagin using High performance Thin Layer Chromatography. J Med Anal Pharmaceu Sci., 21:661-3 (1999).
- Kulkarni VS, Pathak SP. A laboratory handbook of organic qualitative analysis and separations. Aurangabad: Chaaya Publishing house. 15-66 (2006).
- Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR. Vogel’s textbook of practical organic chemistry 5th ed. Pearson education publication. 1196-233 (2005).
- Di X, Chan KK, Leung HW, Huie CW. Fingerprint profiling of acid hydrolyzates of polysaccharides extracted from the fruiting bodies and spors of Lingzhi by high-performance thin layer chromatography. J Chromatogr A., 2003.
- Yamunadevi M, Wesely EG, Johnson M. Chromatographic finger print analysis of steroids in Aerva lanata L by HPTLC technique. Asian Pacific J Tropic Biomed., 1: 428-33 (2011).
- Laurence L. General Principles of Antimicrobial Therapy in: Hardman LE. Goodman and Gillman’s, the pharmacological basis of therapeutics. 11th ed. New York: Medical publishing division; 2006, 709.
- Wagner H, Baldt S, Zgainski EM. Plant drug anal Berlin:Springer; 1996.
- Bistrian B. Systemic response to inflammation. Nutri Rev. 2007; 65: S170–2.
- Kueh JTB, O’Connor PD, Hugel H, Brimble MA. Synthetic studies towards the anti-inflammatory agent, oleocanthal using a Johnson-Claisen (orthoester) rearrangement strategy. Archi Org Chem., 58-71 (2009).
- Lim H, Kim SB, Park H, Chang HW, Kim HP. New anti-inflammatory synthetic biflavonoid with C-C (6-6″) linkage: differential effects on cyclooxygenase-2 and inducible nitric oxide synthase. Archi Pharm Res., 32:1525-31 (2009).
- Lu M, Patsouris D, Li P. A new antidiabetic compound attenuates inflammation and Insulin resistance in Zucker diabetic fatty rats. American J Physiol., 298: E1036–48 (2010).
- Niraldo P et.al., Evaluation of anti-inflammatory effect of synthetic 1,5-bis(4-acetoxy-3-methoxyphenyl)-1,4-pentadien-3-one, HB2. Bioorg Med Chem., 17: 4290-5 (2009).
- Meth Cohn O, Stanforth SP. Vilsmeier-Haack reactions of Tertiary alcohols: Synthesis of Functionalized Pyridines and Napthyridines. Composition Org Syn. 2: 777-94 (1991).
- Sethi PD, High Performance Thin Layer Chromatography, I ed. Kongposh Publications Pvt. Ltd: 61-5 (2011).
- Seidler NW, Yeargans GS. Effects of thermal denaturation on protein glycation. Life Sci., 70:1789-99 (2002).
- Kulkarni SK, A Handbook of Experimental Pharmacology, 3rd ed. New Delhi: Vallabh Prakashan, 172-5 (1999).








