Manuscript accepted on :02-12-2019
Published online on: 24-12-2019
Plagiarism Check: Yes
Reviewed by: Dorina Lauritano
Second Review by: Cherry Bansal
Final Approval by: Prof. em. Hans-Joachim Freisleben
Bat-Erdene Jargalsaikhan1*, Narangerel Ganbaatar1, Myadagbadam Urtnasan2, Nyamdolgor Uranbileg3 and Dagvatseren Begzsuren1
1Department of Pharmacology, Institute of Traditional Medicine and Technology, Ulaanbaatar, 17032, Mongolia
2Department of Pharmacy, Institute of Traditional Medicine and Technology, Ulaanbaatar, 17032, Mongolia
3Department of Pathology, Institute of Veterinary Medicine, Ulaanbaatar, 17024, Mongolia
Corresponding Author E-mail: Baterdene1010@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1811
Abstract
Polyherbal formulation (PHF) is composed of Artemisia santolinifolia Turcz, Saussurea salicifolia L. and Hippophae rhamnoides L., which mainly used for inflammatory disorders in traditional Mongolian medicine. The aim of the study was to evaluate the anti-inflammatory effect of PHF in carrageenan and lipopolysaccharide (LPS) induced models of inflammation. The total active constituents of 20% ethanol extract of PHF was determined, using Folin-Ciocalteu reagent and aluminum chloride reagent, respectively. Inflammation models were induced by 1% carrageenan and LPS 7.5 mg/kg in the experimental groups. The levels of serum tumor necrosis factor- α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and high mobility group box 1 protein (HMGB-1) were measured in PHF pretreatment groups by enzyme-linked immunosorbent assay (ELISA). The lungs were harvested and evaluated for histopathological assessment on 12 hours after LPS administration. The content of total phenolic was 28.5±0.12 mg/g and flavonoids 12.4±0.42 mg/g. After 60, 120, 180, 240 and 300 min, the data indicate that PHF 75, 150 and 300 mg/kg was significantly effective reducing paw edema volumes induced by carrageenan compared to control (p<0.01). PHF pretreatment significantly reduced levels of serum TNF-α, IL-1β and IL-6 at 300 minutes after carrageenan injection. Moreover, pretreated with PHF 150 mg/kg groups serum levels of TNF-α, IL-1β and HMGB-1 were significantly (p<0.01) reduced compared with the control group after LPS injection. It showed less inflammation and change of pulmonary structure compared with the LPS group at 12 hours after LPS injection. From the results of the study, it was demonstrated that PHF had sufficient potential to treat inflammatory disorders by reducing pro-inflammatory cytokines.
Keywords
Acute lung inflammation; interleukin; Mongolian herbal medicine; paw edema; pro-inflammatory cytokine
Download this article as:| Copy the following to cite this article: Jargalsaikhan B, Ganbaatar N, Urtnasan M, Uranbileg N, Begzsuren D. Anti-Inflammatory Effect of Polyherbal Formulation (PHF) on Carrageenan and Lipopolysaccharide-Induced Acute Inflammation in Rats. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Jargalsaikhan B, Ganbaatar N, Urtnasan M, Uranbileg N, Begzsuren D. Anti-Inflammatory Effect of Polyherbal Formulation (PHF) on Carrageenan and Lipopolysaccharide-Induced Acute Inflammation in Rats. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2PQMFeG |
Introduction
Inflammation is a defense mechanism to eliminate or limit the spread of injurious factor in the body, as well as local response of living mammal tissue to injury. Swelling, redness, heat, pain, and immobilities are various symptoms of inflammation occurred throughout the part of the body, especially as a reaction to injury or infection. The immune system recognizes damaged or infected cells, pathogens, and begins the healing process it self1-4. But, if the process is not regulated, or the offending agent persists, the intended protective process tends to be destructive5.
Steroidal and nonsteroidal anti-inflammatory drugs had been developed, for the management of inflammatory conditions. Usage of the drug is limited with high costs and adverse effects6, 7. World Health Organization (WHO) reported that about 70–80% of the world’s population rely on nonconventional medicine commonly from herbal sources in their primary health care and its demand is increasing day by day in developing countries8-10. Accordingly, there is a renewed interest in medicinal plant research to identify alternate agents which may be cheaper and have less or no adverse effects11.
It is well known, the herbal formulations had been used preventive and therapeutic medicine for a long time. Healing properties of medicinal plants were noted for ancient medical books. PHF is containing Artemisia santolinifolia Turcz, Saussurea salicifolia L. and Hippophae rhamnoides L. which mainly used as an analgesic, antibacterial and anti-inflammatory agent in traditional Mongolian medicine12. Extract of Hippophae rhamnoides L. has potent anti-inflammatory activity that inhibited against LPS stimulation by suppressing pro-inflammatory expression13. Saussurea salicifolia L. contains mainly flavonoid glycosides like quercetin-3-O-galactoside, apigenin-7-O-rhamnoside having an anti-inflammatory effect14. Although nonsteroidal medications can be effective, herbal medications may offer a safer, and often an effective, alternative treatment for inflammatory disorders. Therefore, this study seeks to find out the possibility of anti-inflammatory activity of PHF using carrageenan-induced paw edema and LPS induced acute inflammation models.
Materials and Methods
Polyherbal formulation
PHF was prepared in the Research Center of Institute of Traditional Medicine and Technology (ITMT) of Mongolia. Briefly, Herba Artemisiae santolinifoliae and Fructus Hippophae rhamnoides were collected from the medical herb cultivation center located Dashinchilen sum, Bulgan province, Mongolia in September 2017. Herba Saussureae salicifoliae was collected in August 2017, from near Erdene sum, Tuv province, Mongolia. Those herbs were identified by Prof. Ganbold E. (Ulaanbaatar University, Ulaanbaatar, Mongolia). The herbs were dried, pulverized and stored in the dark at the room temperature. Dried herb was cold macerated with 20% ethanol, and herbal mixture ratio was 2:1:1 (Herba Saussureae salicifoliae, Herba Artemisiae santolinifoliae and Fructus Hippophae rhamnoides), respectively. The filtrate was then removed and concentrated in a vacuum evaporator. When required the paste was reconstituted in saline (0.9% w/v NaCl) and hereby referred in the pharmacological investigations.
Reagent
Carrageenan and lipopolysaccharide (Escherichia coli 055: B5 endotoxin) were purchased from Sigma Aldrich Co., (USA). Enzyme-linked immune sorbent assay (ELISA) kits were purchased from MLBio Co., (China). Folin-Ciocalteu reagent and aluminum chloride (AlCl3) of Sangon Biotech Co., (China), were used in the study.
Chemical analysis
Powdered poly herbal formulation was precisely weighted (1.0 g), extracted with 50 mL of 20% ethanol in reflux for 30 min, and then filtrated. The supernatant was used as the test solution.
Estimation of total flavonoid contents: The solution was treated with 1 mL of 5% NaNO2, 1 mL of 10% Al(NO3)3 and 10 mL of 4% NaOH solutions. The content of flavonoids in extract was reported as rutin equivalent (mg/g), using a spectrophotometer15 (UNICO UV-2102 C, China).
Estimation of total phenolic compounds: The Folin-Ciocalteu reagent (diluted 1:10 in water) and aqueous Na2CO3 (10.75%) were successively added to the extract. In 30 min, the total polyphenolic content of PHF was determined with the spectrophotometer and reported as gallic acid equivalent (mg/g)16.
Animals
Male adult Wistar rats (healthy, 12-14 weeks, 180-220 g) were randomly selected from our animal house. They were raised in the Animal house of the Research center, ITMT, Ulaanbaatar. Rats were kept in the laboratory under constant condition of light/dark (12:12) and temperature (20±2) with animal cage, free access to a standard animal diet and tap water for 7 days before and during experiment. The experimental protocols were approved by the Ethical Committee of the Ministry of Health to minimize animals suffering (№37/2018). These guidelines were in according to international principles for the care and use of laboratory animals.
Carrageenan-induced acute inflammation
The rats were randomly divided into 5 groups, each containing five rats. Control group, which received an oral administration of 0.9% saline 10 ml/kg. Ibuprofen (100 mg/kg) and PHF (75, 150 and 300 mg/kg) were dissolved in a 0.9% saline and were administrated orally as pretreatment. The paw edema was induced with 1% carrageenan to all the groups after one hour of the oral treatments17, 18. Paw edema volume was measured before and after carrageenan injection at the 30, 60, 120, 180, 240 and 300 minutes, using a plethysmometer (Ugo Basile Co., Italy). After the last paw measurement, rats were anesthetized with an injection of ketamine hydrochloride 80-90 mg/kg, and then blood were collected by cardiocentesis for serum pro-inflammatory cytokine interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor- α (TNF-α) levels by ELISA.
LPS induced acute inflammation
In this experiment, PHF dose of 150 mg/kg was selected from three doses of PHF by the results of carrageenan-induced inflammation. The animals were randomly divided into three groups, each containing 25 rats except normal group. Control and PHF groups were pretreated by oral administration of 0.9% saline 10 ml/kg and PHF 150 mg/kg, respectively. One hour later, acute inflammation was induced by an LPS 7.5 mg/kg (dissolved in sterile saline) intravenous injection via rats tail vein19. Rats were anesthetized with an injection of ketamine hydrochloride 80-90 mg/kg, and blood were collected by cardiocentesis for serum pro-inflammatory cytokine IL-1β, TNF-α and High mobility group box-1 protein (HMGB-1) levels by ELISA at 1, 3, 6, 9, and 12 hours after the injection. The lungs were harvested and evaluated for histopathological assessment on 12 hours after LPS administration.
Enzyme-linked immune sorbent assay (ELISA)
The blood was kept in the room temperature for 15 minutes after collection, and the samples were centrifuged at 3000 rpm for 10 min to separate the serum. The pro-inflammatory cytokines were measured in the collected serum using rat ELISA kits according to the manufacturer’s instruction by a microplate reader (ChroMate-4300, Awareness technology Co., USA).
Histopathology assessment
The lungs were fixed in 10% formaldehyde and embedded in paraffin. Then 4-6 micrometer sections were taken from the paraffin embedded blocks by microtome. Lung sections were deparaffinized and stained with hematoxylin and eosin (HE). After being stained by HE, the lung morphology was observed and photographed using an Olympus imaging system. Pulmonary histopathological score (PHS) was evaluated by the degree of intra-alveolar edema, intra-alveolar hemorrhage, and neutrophil infiltration, using grade 1 to 4 with a maximum score of 12, as previously used20.
Statistical analysis
The data were shown as the mean ± SD. An analysis of variance (One-way ANOVA and Two-way RM ANOVA followed by Tukey’s post hoc test) was performed to determine significance using software Graph Pad Prism 7.0 and a value of *p<0.05 and **p<0.01 was considered as significant.
Results
Total phenolic and flavonoids contents
The flavonoid contents of the extract in term of rutin equivalent (Standard curve equation: y = 11.815x–0.0092, r2= 1.000), and the flavonoid content in the extract of PHF was 12.4 ± 0.42 mg/g. The total content of phenolic compounds showed as Gallic acid equivalent (Standard curve equation: y =110.77x–0.0736, r2= 0.983), and the total phenolic compound was 28.5 ± 0.12 mg/g in PHF (Table. 1).
Table 1: Total phenolic and flavonoids in 20% ethanol extract of the PHF
| Bioactive substance of PHF | Standard reagent | Standard curve equation | mg/g dry mass
(n=4) |
| Total phenolic | Gallic acid | y =110.77x–0.0736, r2= 0.983 | 28.5±0.12 mg/g |
| Flavonoids | Rutin | y = 11.815x–0.0092, r2= 1.000 | 12.4±0.42 mg/g |
Effect of PHF on hind paw edema
In subplantar injection of carrageenan-induced a time-dependent increase in paw edema consisting of a relatively rapid early phase (up to 180 min), and followed by late phase (180-300 min). In the control group, the volume of hind paw edema was significantly increased by carrageenan injection in both phases and it reached peak levels at the late phase. Pretreatment of rats with PHF (75, 150 and 300 mg/kg) and Ibuprofen (100 mg/kg) administered by oral resulted in inhibition (**p<0.01) of carrageenan-induced hind paw edema in both phases (Table 2).
Table 2: Volumes of the hind paw with carrageenan-induced edema (ml)
| Phase | Minute | Control | PHF
75 mg/kg |
PHF
150 mg/kg |
PHF
300 mg/kg |
Ibuprofen 100 mg/kg |
| Hind paw injection 0.1 ml with 1% solution of carrageenan | ||||||
| Early phase | 0 min | 2.65±0.14 | 2.81±0.17 | 2.84±0.15 | 2.61±0.31 | 2.57±0.11 |
| 30 min | 3.41±0.15 | 3.39±0.43 | 3.13±0.14 | 2.73±0.20** | 2.67±0.06** | |
| 60 min | 4.65±0.37 | 3.19±0.44** | 3.54±0.20** | 2.81±0.32** | 2.73±0.18** | |
| 120 min | 5.02±0.27 | 3.32±0.36** | 3.30±0.41** | 3.47±0.18** | 3.37±0.17** | |
| Late phase | 180 min | 5.20±0.29 | 3.59±0.20** | 3.42±0.15** | 3.85±0.27** | 3.66±0.09** |
| 240 min | 5.02±0.30 | 3.17±0.34** | 3.18±0.13** | 3.78±0.26** | 3.40±0.21** | |
| 300 min | 5.14±0.34 | 3.06±0.32** | 3.05±0.42** | 3.72±0.38** | 3.33±0.11** | |
Data were expressed as mean ± SD of 5 rats in the groups. **p<0.01 vs Control group by Two-way RM ANOVA followed by Tukey’s post hoc test.
Effect of PHF on TNF-α, IL-6, and IL-1β in Carrageenan-induced model
The levels of serum pro-inflammatory cytokine TNF-α, IL-6, and IL-1β in the control group were significantly increased by carrageenan injection after 300 minutes, compared with the normal group. The rats pretreated with PHF 75, 150 and 300 mg/kg had significantly lower levels of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β to compare control group (Table 3).
Table 3: Levels of serum pro-inflammatory cytokine with carrageenan-induced paw inflammation
| Cytokine | Normal group | Control | PHF
75 mg/kg |
PHF
150 mg/kg |
PHF
300 mg/kg |
| Hind paw injection 0.1 ml with 1% solution of carrageenan | |||||
| TNF-α pg/ml | 24.75±3.77 | 48.86±2.58# | 28.02±0.71** | 29.08±4.04** | 34.50±3.53* |
| IL-6 pg/ml | 15.51±4.43 | 59.98± 6.40# | 19.38±7.56** | 31.02±1.92** | 42.70±3.02* |
| IL-1β pg/ml | 8.50±3.11 | 14.46±1.02# | 5.440±1.53** | 7.360±1.39* | 11.46±1.10* |
Data were expressed as mean ± SD of 5 rats in the groups. #p<0.05 vs Normal group, **p<0.01; *p<0.05 vs Control group by One-way ANOVA followed by Tukey’s post hoc test. Abbreviations: IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α
Effect of PHF on IL-1β, TNF-α, and HMGB-1 in LPS-induced model
Results showed that levels of pro-inflammatory cytokine IL-1β, TNF-α and HMGB-1 were significantly (#p<0.05) increased in the control compared with normal group (Table 4). Therefore, the oral administration of PHF 150 mg/kg to pretreated group resulted in significant (**p<0.01) suppression of TNF-α compared with the control group during 1 to 9 hours. The level of IL-1β was significantly decreased to compare between PHF and control group after LPS administration from 1 to 3 hours. The level of HMGB-1 had significantly lower expression in pretreated with PHF compared to control (Table 4).
Table 4: Levels of serum pro-inflammatory cytokine with LPS-induced inflammation
| Cytokine | Group | After LPS 7.5 mg/kg administration via tail vein (n=5) | ||||
| 1 h | 3 h | 6 h | 9 h | 12 h | ||
| TNF-α (pg/ml) | Normal | 24.75± 3.77 | 24.75± 3.77 | 24.75± 3.77 | 24.75± 3.77 | 24.75± 3.77 |
| Control | 56.46±14.33# | 63.50±7.93# | 47.25±10.6# | 48.00±11.97# | 31.50±4.20 | |
| PHF | 33.80±5.37** | 41.10±7.23** | 36.75±8.91* | 26.77±1.67** | 26.81±1.17 | |
| IL-1β (pg/ml) | Normal | 8.5±3.11 | 8.5±3.11 | 8.5±3.11 | 8.5±3.11 | 8.5±3.11 |
| Control | 50.4±11.6# | 47.5±6.2# | 30.7±6.8# | 29.2±4.3# | 26.75±5.37# | |
| PHF | 23.9± 3.5** | 28.5±8.3** | 37.8±2.1 | 29.3±1.2 | 27.5±7.8 | |
| HMGB-1 (µg/ml) | Normal | 1.18±0.09 | 1.18±0.09 | 1.18±0.09 | 1.18±0.09 | 1.18±0.09 |
| Control | 3.49±0.79# | 3.68±0.54# | 4.91±0.65# | 6.78±1.21# | 4.47±0.88# | |
| PHF | 3.092±0.19 | 3.00±0.22 | 3.51±0.39* | 2.54±0.99** | 2.11±0.35** | |
Data were expressed as mean ± SD of 25 rats in the experimental groups and 5 rats in the normal group. #p<0.05 vs Normal group, *p<0.05; **p<0.01 vs Control group by Two-way RM ANOVA followed by Tukey’s post hoc test. Abbreviations: TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; HMGB-1, High mobility group box-1.
Histological changes in lung structure
The figure shows the structure of the lungs with healthy rats (Figure 1A and 1B). The LPS control group showed that increased alveolar wall thickness, edema, and overwhelming diffuse alveolar hemorrhage, severe infiltration of inflammatory cells near bronchial at 12 hours after LPS administration (Figure 1C and 1D). Pretreated with PHF 150 mg/kg groups showed less inflammation and change of pulmonary structure compared with the LPS control group (Figure 1E and 1F). The score of pulmonary histopathological changes in the groups indicated that pretreated with PHF 150 mg/kg was significantly (*p<0.05) lower than the LPS control group (Table 5).
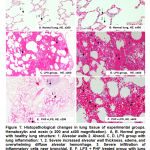 |
Figure 1: Histopathological changes in lung tissue of experimental groups. Hematoxylin and eosin (x 200 and x400 magnification). |
Table 5: Pulmonary histopathological score (PHS) of LPS induced acute lung inflammation
| Group | Histopathological changes | Pulmonary histopathological score |
| Normal | – Healthy lung structure | 0.4±0.54 |
| Control | – Severe increased alveolar wall thickness and edema
– Overwhelming diffuse alveolar hemorrhage – Severe infiltration of inflammatory cells near bronchial |
8.8±1.09# |
| PHF | – Mild increased alveolar wall thickness and edema
– Moderate alveolar hemorrhage – Moderate infiltration of inflammatory cells near bronchial. |
4.6±1.14* |
Data were expressed as mean ± SD of 5 rats in the groups. #p<0.05 vs Normal, *p<0.05 vs Control by One-way ANOVA followed by Tukey’s post hoc test.
Discussions
This investigation was the first experimental study designed to evaluate polyherbal formulation (combination of Saussurea salicifolia L., Artemisia santolinifolia Turcz., and Hippophae rhamnoides L.) would have anti-inflammatory effect on carrageenan and LPS-induced acute inflammation models in rats. From the results of the study, the PHF had sufficient potential to treat inflammatory disorders by reducing pro-inflammatory cytokines.
The present study showed that the carrageenan-induced inflammation an elevation in the levels of serum IL-1β, TNF-α, and IL-6 with hind paw edema. These observed results are the major symptoms of inflammatory conditions21-25. In contrast, current investigations indicated that LPS-induced inflammation which confirmed by the increases of serum TNF-α, IL-1β and HMGB-1 levels with lung histopathological changes. Also, TNF-α and IL-1β can stimulate a release of HMGB-1 by monocytes, and there was a reciprocal functional relationship between the activities of the early phase related to TNF-α and IL-1 β and late phase related to HMGB-1 cytokines26, 27. Moreover, many studies demonstrated that these parameters were significantly increased with lung histopathological changes in experimental animals treated with LPS26- 29.
In the current study, it was observed that both phases of the carrageenan-induced hind paw edema were significantly reduced by pretreatment of rats with PHF, due to decrease in serum levels of pro-inflammatory cytokine IL-1β, TNF-α, and IL-6. Also, the serum levels of IL-1β and TNF-α in LPS-induced inflammation rats were suppressed by pretreatment of rats with PHF in the early phase, and the serum level of HMGB-1 was suppressed by pretreatment with PHF in the late phase. It had preventive effect with less inflammation and change of pulmonary structure compared with the LPS group at 12 hours after LPS injection. The content of total phenolic was 28.5±0.12 mg/g and flavonoids 12.4±0.42 mg/g founded in 20% ethanol extract of PHF.
Therefore, several publications revealed that a polyherbal formulation had beneficial effects in animal models of inflammatory disorders. The Gardi-5 has been used as an antibacterial and analgesic agent in traditional Mongolian medicine. It significantly inhibited paw edema and secretion of inflammatory mediators in the carrageenan-induced model30. Xuan-Ju has been used to rheumatoid arthritis in traditional Chinese medicine, and the results showed that an inhibitory effect on edema which induced by carrageenan31. Lider-7 tang Mongolian polyherbal medicine has exerted a preventive effect against LPS-induced acute lung inflammation mediated by inhibiting the release of pro-inflammatory cytokines32. These anti-inflammatory effects of polyherbal formulations related to inhibiting pro-inflammatory mediators and cytokines and it was similar to our result.
Previous study showed that medical herbs Saussurea salicifolia L. with detected anti-inflammatory effect, contained mainly flavonoid glycosides33. Unfortunately, there are not enough reports in literature to support Artemisia santolinifolia Turcz in inflammatory disorders, but generally, Artemisia species have anti-inflammatory effects34. Hippophae rhamnoides L. contains ursolic acid and oleanolic acid which have anti-inflammatory effects including inhibit COX-2, reduce paw edema and inhibit histamine release that demonstrated by previous studies35-38.
According to the results of current investigation, pretreatment of PHF inhibited the release of pro-inflammatory cytokines induced by carrageenan and LPS in rats. It ameliorated histopathological changes in acute lung inflammation. Hence, the anti-inflammatory properties of PHF might have to chemical compounds which inhibiting pro-inflammatory mediators and cytokines. In-vivo method was used to perform the study. Therefore, in order to overcome the limitations of the model, further investigations needed to clarify various models of inflammatory disorders and to utilize in-vitro studies as well.
Conclusion
Present study concluded that pretreatment of PHF in rats with LPS-induced acute inflammation, which reduced the levels of serum pro-inflammatory cytokines (IL-1β, TNF-α, and HMBG-1) to preserve lung structures. Moreover, it significantly reduced hind paw edema mediated by inhibiting the release of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) induced by carrageenan. This action may be useful in the treatment of some inflammatory conditions.
Acknowledgments
We thank the colleagues of the Institute of Traditional Medicine and Technology of Mongolia for their support during this study.
conflict of interest
No conflict of interest
Funding Source
This study was funded by the Mongolian Foundation for Science and Technology (Research of Science and Technology under project number: SHUTZ-2017/05)
References
- Mahat, M.A. and Patil, B.M. Evaluation of antiinflammatory activity of methanol extract of Phyllanthus amarus in experimental animal models. Indian Journal of Pharmaceutical Sciences., 2007; 69:33–36.
https://doi.org/10.4103/0250-474X.32104 - Libby, P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev., 2007; 65:140-6.
https://doi.org/10.1111/j.1753-4887.2007.tb00352.x - Sosa, S., Balick, M.J., and Arvigo, R. et al. Screening of the topical anti-inflammatory activity of some Central American plants. Journal of Ethnopharmacology., 2002; 81:2:211–215.
https://doi.org/10.1016/S0378-8741(02)00080-6 - Sherwood, ER. and Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol.,2004; 18:3:385-405.
https://doi.org/10.1016/j.bpa.2003.12.002 - Helenius, I. Lumme, A. and Haahtela, T. Asthma, airway inflammation and treatment in elite athletes. Sports Medicine., 2005; 35:7:565–574.
https://doi.org/10.2165%2F00007256-200535070-00002 - Burisch, J. Jess, T. Martinato, M. and Lakatos, P. L. The burden of inflammatory bowel disease in Europe. Journal of Crohn’s and Colitis., 2013; 7:4:322–337.
https://doi.org/10.1016/j.crohns.2013.01.010 - Tabas, I. and Glass, C. K. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science., 2013; 339:6116:166–172.
https://Doi.org/10.1126/science.1230720
https://science.sciencemag.org/content/339/6116/166 - WHO, 2002. Traditional Medicine Strategy 2002–2005. WHO, Geneva, Switzerland.
http://www.wpro.who.int/health_technology/book_who_traditional_medicine_strategy_2002_2005.pdf - Roy, P. Amdekar, S. Kumar, A. and Singh, V. Preliminary study of the antioxidant properties of flowers and roots of Pyrostegia venusta (Ker Gawl) Miers. BMC Complementary and Alternative Medicine., 2011; 11:69.
https://doi.org/10.1186/1472-6882-11-69 - Barua, C. C. Talukdar, A. and Begum S. A. Antinociceptive activity of methanolic extract of leaves of Achyranthes aspera Linn. (Amaranthaceae) in animal models of nociception. Indian Journal of Experimental Biology., 2010; 48:8:817–821.
http://nopr.niscair.res.in/handle/123456789/9987 - Rafieian-Kopaei, M. Medicinal plants and the human needs. Journal of HerbMed Pharmacology., 2002; 1:1:1-2.
http://eprints.skums.ac.ir/4790/1/2.pdf - , 2013. Medical plants in Mongolia. WHO, Geneva, Switzerland
http://www.wpro.who.int/publications/Medicinal_Plants_in_Mongolia_VF.pdf - Tanwar, Himanshi, Shweta Karthik Ananth, M Singh, Divya Narsimhan, S Singh, Shashi Ganju, Lilly. Anti-inflammatory activity of the functional groups presents in Hippophae rhamnoides (Seabuckthorn) leaf extract. Inflammopharmacology., 2017; 26:1-11.
https://doi.org/10.1007/s10787-017-0345-0 - Chunsriimyatav, G. Hoza, I. Valášek, P. Skrovanková, S. Banzragch, D. and Tsevegsuren, N. Anticancer Activity of Lignan from the Aerial Parts of Saussurea salicifolia (L.) DC. Czech J. Food Sci., 2009; 27:256-258
https://doi.org/10.17221/1097-CJFS - Quettier, DC. Gressier B, Vasseur J, Dine T, Brunet C, Luyckx MC, et al. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol., 2000: 72:35–42. doi:10.1016/S0378-8741(00)00196-3
https://doi.org/10.1016/S0378-8741(00)00196-3 - Singleton, VL. Orthofer, R. Lamuela-Raventos, RM., Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol., 1999; 299:152–178.
https://doi.org/10.1016/S0076-6879(99)99017-1 - Winter, C. A. Risley, E. A. and Nuss, G. W. Carrageenan-induced edema in the hind paw of rat as an assay for anti-inflammatory activity. Proceedings of the Society for Experimental Biology and Medicine., 1962; 111:544–547.
https://doi.org/10.3181/00379727-111-27849 - Hiroe Maruyama., Takashi Sakamoto., Yoko Araki., and Hideaki Hara. Anti-inflammatory effect of bee pollen ethanol extract from Cistus of Spanish on carrageenan-induced rat hind paw edema. BMC Complementary and alternative medicine., 2010; 10:30.
https://doi.org/10.1186/1472-6882-10-30 - Sha Li., Liu Han et al. Effects of propofol on early and late cytokines in lipopolysaccharide-induced septic shock in rats. Journal of Biomedical Research., 2010; 24(5):389-94.
https://doi.org/10.1016/S1674-8301(10)60052-8 - Chen, F. Liu, Z. Wu, W. Rozo, C. Bowdridge, and S. Millman, A. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. NatMed., 2012; 18: 260–266.
doi: 10.1038/nm.2628 - Willis, A.L., Davison, P., Ramwell, P.W., Brocklehurst, W.E., Smith, B. Release and Actions of Prostaglandins in Inflammation and Fever: Inhibition by Anti-Inflammatory and Antipyretic Drugs. In: Ramwell P.W., Pharriss B.B. (eds) Prostaglandins in Cellular Biology. Alza Conference Series, 1972; vol 195B. Springer, Boston, MA
https://doi.org/10.1007/978-1-4684-2844-5_12 - Bhukya, B., R. N. Anreddy, C. William, and K. Gottumukkala. Analgesic and Anti-Inflammatory Activities of Leaf Extract of Kydia Calycina Roxb. Bangladesh Journal of Pharmacology., 2009; 4:2:101-4. DOI:10.3329/bjp.v4i2.2112
https://www.banglajol.info/index.php/BJP/article/view/2112/25317 - Ramprakash Tandon, Vishal. and Kumar Gupta, Rajesh. Anti-inflammatory Activity and Mechanism of Action of Vitex negundo Linn. International Journal of Pharmacology., 2006; 2:303-308.
DOI: 10.3923/ijp.2006.303.308 - Nantel, F. Denis, D. Gordon, R. Northey, A. Cirino, M. Metters, KM. and Chan, CC. Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Br J Pharmacol., 1999; 128:853–859.
https://doi.org/10.3329/bjp.v4i2.2112 - Mohan, M. Gulecha, VS. Aurangabadkar, VM. Balaraman. R. Austin, A. and Thirugnanasampathan, S. Analgesic and anti-inflammatory activity of a polyherbal formulation. Oriental Pharmacy and Experimental Medicine., 2009; 9:3:232-237.
https://doi.org/10.3742/OPEM.2009.9.3.232 - Wang, H. Bloom, O. Zhang, M. Vishnubhakat, JM. Ombrellino, M. and Che, J. HMG-1 as a late mediator of endotoxin lethality in mice. , 2009; 285:248-251.
https://doi.org/10.1126/science.285.5425.248 - Andersson, U. Wang, H. Palmblad, K. Aveberger, AC. Bloom, O. Erlandsson-Harris, H. High mobility group 1 protein (HMGB1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med., 2000; 192:565–70.
https://doi.org/10.1084/jem.192.4.565 - Wu G, Dai X, Li X, Jiang H. Antioxidant and anti-inflammatory effects of rhamnazin on lipopolysaccharide-induced acute lung injury and inflammation in rats. Afr J Tradit Complement Altern Med., 2017; 14(4):201–212.
doi:10.21010/ajtcam.v14i4.23
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5471467/pdf/AJTCAM-14-201.pdf - Li, G. and Zhou, CL. Galantamine protects against lipopolysac-charide-induced acute lung injury in rats. Braz J Med BiolRes., 2016; 49: e5008.
http://dx.doi.org/10.1590/1414-431×20155008 - Dejidmaa, B. Uuganbayar, B. Erdenechimeg, Ch. Chimedragchaa, Ch. and Munkhzul, G. The anti-inflammatory effect of gardi patch in carrageenan-induced paw edema of rats. Proceedings of the Mongolian Academy of Sciences., 2018;57:4:50-54.
https://doi.org/10.5564/pmas.v57i4.923 - Jia, W. Gao, W. Cui, N. and Xiao, P. Anti-inflammatory effects of an herbal medicine (Xuan-Ju agent) on carrageenan and adjuvant induced paw edema in rats. Journal of Ethno pharmacology., 2003; 89:39-141.
https://doi.org/10.1016/S0378-8741(03)00272-1 - Erdenechimeg, Ch. Guiqide, A. Buyantogtokh, Dejidmaa. Chimedragchaa, Ch. Sodnomtseren, Purevsuren. Total phenolic, flavonoid, alkaloid and iridoid content and preventive effect of Lider-7-tang on lipopolysaccharide-induced acute lung injury in rats. Brazilian Journal of Medical and Biological Research. 2017;
http://dx.doi.org/10.1590/1414-431×20175916 - Chunsriimyatav, G. Hoza, I. Valášek, P. Skrovanková, S. Banzragch, D. and Tsevegsuren, N. Determination of Phenolic Compounds in Saussurea salicifolia (L.) DC by HPLC. Czech J. Food Sci., 2009; 27:259-261
https://doi.org/10.17221/947-CJFS - Eunjeong Choi., Heesook Park., Jehyuk Lee., and Gunhee Kim. Anticancer, antiobesity, and anti-inflammatory activity of Artemisia species in vitro. J Tradit Chin Med., 2013; 15;33(1):92-97.
http://www.journaltcm.com/modules/Journal/contents/stories/131/16.pdf
https://www.ncbi.nlm.nih.gov/pubmed/23596819 - Gautam, R. and Jachak, SM. Recent development in anti-inflammatory natural products. Med Res Rev., 2009; 29:767–820
https://doi.org/10.1002/med.20156 - Yasukawa K, Kitanaka S, Kawata K and Goto K. Anti-tumor promoters phenolics and triterpenoid from Hippophae rhamnoides. Fitoterapia., 2009; 80: 164–167
https://doi.org/10.1016/j.fitote.2009.01.006 - Rédei D, Kúsz N, Jedlinszki N, Blazsó G, Zupkó I, Hohmann J. Bioactivity-Guided Investigation of the Anti-Inflammatory Activity of Hippophae rhamnoides Fruits. Planta Med., 2018; 84(1):26-33.
https://doi.org/10.1055/s-0043-114424 - Tsuruga, T. Chun, YT. Ebizuka, Y. and Sankawa, U. Biologically active constituents of Melaleuca leucadendron: Inhibitors of induced histamine release from rat mast cells. Chem Pharm Bull., 1991; 39:3276-3278 https://doi.org/10.1248/cpb.39.3276







