Kamalakshi G. Bhat1, Amrita Parida2*, Ashwin Kamath2 and Rashmi R. Rao2
1Department of Paediatrics, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, Karnataka 575001. India.
2Department of Pharmacology, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, Karnataka 575001. India.
Corresponding Author E-mail: amrita_parida@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1843
Abstract
Human intravenous immunoglobulin (IVIG) is used for diverse conditions is a relatively safe drug with fewer adverse effects. Here, we report a rare case of IVIG-induced erythema multiforme in a four-year-old female child. The child tolerated the initial doses of IVIG well; however, following the fifth dose, she developed multiple erythematous rash over the trunk, upper and lower limbs associated with itching. She was diagnosed to have IVIG-induced erythema multiforme and treated symptomatically and recovered completely within a week. There is limited literature available related to IVIG-induced erythema multiforme; hence we decided to report this case.
Keywords
Adverse Drug Reaction; Erythema Multiforme; Human IVIG
Download this article as:| Copy the following to cite this article: Bhat K. G, Parida A, Kamath A, Rao R. R. An Unusual Case of Human Intravenous Immunoglobulin Induced Erythema Multiforme in a Child. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Bhat K. G, Parida A, Kamath A, Rao R. R. An Unusual Case of Human Intravenous Immunoglobulin Induced Erythema Multiforme in a Child. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2sgNG6S |
Introduction
IVIG is currently used for varied number of immunological and inflammatory conditions such as idiopathic thrombocytopenic purpura and primary immunodeficiency. It has few adverse effects which could be mild, like headache, nausea and back pain, or serious, like aseptic meningitis, renal failure, anaphylaxis and thrombotic events.[1] We report the case of a child who developed erythema multiforme following multiple doses of IVIG.
Case History
A four-year-old girl came to the paediatric emergency department with complaint of fever of one week duration. Fever was of moderate grade and intermittent in character with occasional rigors. Since the past two days, she also had altered sensorium and generalized weakness. There was no history of seizures or vomiting, and no history of similar complaints in the past. On neurological examination, hypotonia was present in all four limbs, and the best observed power was 3/5. Deep tendon reflexes were found to be exaggerated in all four limbs. Plantar reflex was found to be extensor in both lower limbs. Based on the physical examination and laboratory investigations, a diagnosis of acute disseminated encephalomyelitis (ADEM) with a respiratory infection was made. She was started on injection ceftriaxone, IVIG and injection methylprednisolone (30 mg/kg/day) along with paracetamol and ranitidine as per the advice of the neurologist. The child had an episode of seizure in the hospital for which she was given injection sodium valproate and levetiracetam. IVIG was administered initially at the rate of 0.01 mL/kg/min for half an hour and continued at the same rate for 3 hours, after which the rate was increased to 50 mL/hr. The child tolerated the initial doses of IVIG well; however, following the fifth dose, she developed multiple erythematous rashes over the trunk, upper and lower limbs associated with itching (Figure 1). The lesions were 1–2 cm in diameter and darker in the periphery and paler in the centre. There was no mucosal or systemic involvement. A diagnosis of IVIG-induced erythema multiforme was made, and symptomatic treatment was given. The other drugs were continued.
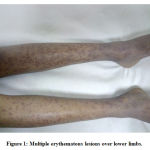 |
Figure 1: Multiple erythematous lesions over lower limbs. |
Outcome and follow-up
The skin lesions were treated with topical steroids, calamine lotion and antihistaminics. Since the child was started on methylprednisolone for the primary illness, she was continued on oral prednisolone.
After stopping IVIG, there were no additional lesions. With the above treatment, the condition of the child improved. After a week, the lesions were found to be healing, and she was discharged with the following drugs – oral sodium valproate, levetiracetam, along with oral corticosteroids (on tapering dose). One week later, the child was followed up and found to have completely recovered.
Discussion
Human IVIG was initially approved by the US Food and Drug Administration for six clinical conditions: primary immunodeficiency, idiopathic thrombocytopenic purpura, prevention and treatment of infections in patients with hypogammaglobulinemia, B-cell chronic lymphocytic leukemia, bone marrow transplantation, paediatric HIV infection and prevention of coronary artery aneurysms associated with Kawasaki disease. However, IVIG currently has more than 50 off label uses.[2] With such a wide range of clinical uses, monitoring the efficacy and safety of IVIG becomes imperative. The adverse effects of IVIG are usually divided into immediate (headache, nausea), delayed (skin eruptions, renal dysfunction) or late (viral infections), depending upon the time gap between the administration of the drug and onset of the adverse effect.[1] These effects can be further classified into mild and severe depending on the severity of the adverse reaction. Mild reactions include headache, low back pain, nausea, injection site reactions and skin eruptions. The serious reactions include aseptic meningitis, tubular necrosis, thrombotic events and anaphylaxis.[3] The drug characteristics that are likely to be involved in the side effects include volume, pH, osmolality, rate of infusion, solvents and the purification processes used in the products.[4] The most important patient characteristic that affects the occurrence of adverse effects is the underlying disease itself. It is seen that IVIG given to patients suffering from active infections are at increased risk of developing an adverse drug reaction. This could be because of the interaction between the microbial antigens and IVIG leading to activation of the complement system. Treatment of the infection with adequate antibiotics before start of IVIG infusion is also recommended to reduce adverse effects.[5]
Dermal eruption is a rare adverse effect of IVIG. Most of these reactions reported earlier in the literature are eczematous in nature.[6] In the current case, the reaction was non-eczematous and extremely pruritic and was diagnosed to be erythema multiforme. To the best of our knowledge, till date, there is only one reported case of IVIG-induced erythema multiforme in a 27-year-old female treated for idiopathic thrombocytopenic purpura.[7] The most common cause of drug-induced skin lesions is hypersensitivity.[8] As per the previous published articles, it was earlier thought that the allergic reactions to IVIG were because of stabilizers or other constituents which is present along with the active drug. However it was found that allergic reactions were seen with IVIG preparations by different manufactures that use dissimilar types of excipients and solvents. Hence, the researchers started looking for other mechanisms behind IVIG induced hypersensitivity. Currently, it is believed that IVIG itself sometimes acts a super antigen and activates certain B-cells resulting in such reactions.[6] Drugs are responsible for 50% of the cases of erythema multiforme as per literature. The other predominant cause of erythema multiforme is viral infection. In this child, based on the history, clinical presentation and investigations, it was most likely to be drug induced rather than due to viral infections. However possibility of viral cause cannot be completely ruled out. Hence, the adverse drug reaction was assessed to be “probable/likely” according to the WHO/UMC and also “probable” according to the Naranjo’s causality assessment scale .[9,10]
Conclusion
Though human IVIG is a relatively safe drug, physicians should be aware of its possible adverse effects including rare ones like erythema multiforme.
Acknowledgment
There is no Acknowledgement.
Conflict of interest
There is no Conflict of interest
Funding Source
There is no Funding source
References
- Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: A review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 2005; 117: 525-53.
- Iagaru N, Cinteza E, Cochino AV. Adverse reactions to intravenous immunoglobulin therapy. Mædica – a Journal of Clinical Medicine 2007; 2(4):294-99.
- Feldmeyer L, Benden C, Haile SR et al. Not all intravenous immunoglobulin preparations are equally well tolerated. Acta Derm Venereol 2010; 90: 494–7.
- Bonilla FA. Intravenous immunoglobulin: Adverse reactions and management. J Allergy Clin Immuno 2008; 122(6):1238-9.
- Carbone J. Adverse Reactions and Pathogen Safety of Intravenous Immunoglobulin. Current Drug Safety 2007;2:9-18.
- Vecchietti G, Kerl K, Prins C, et al. Severe Eczematous Skin Reaction After High-Dose. Intravenous Immunoglobulin Infusion. Arch Dermatol. 2006;142:213-7.
- Rodeghiero F, Castaman G, Vespignani M et al. Erythema multiforme after intravenous immunoglobulin. Blut. 1988;56:145.
- Ardern-Jones MR, Friedman PS. Skin manifestations of drug allergy. Br J Clin Pharmacol 2011; 71(5):672–83.
- The use of the WHO-UMC system for standardised case causality assessment. [Internet] [cited 2019 June 03] Available from: http://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al.. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther 1981;30 (2): 239–45.








