Snigdha Rani Panigrahy, Supriya Pradhan* and Chandra Sekhar Maharana
and Chandra Sekhar Maharana
Department of Pharmacology, MKCG Medical College, Berhampur, Odisha
Corresponding Author E-mail: drsupriyapradhan.sp3@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1830
Abstract
Oxidative stress and neuroinflammatory process are implicated in pathophysiology of epilepsy as well as epileptogenesis. The α and γ isoform of peroxisome proliferator-activated receptors (PPAR) agonist has been reported to have antioxidant and anti-inflammatory functions. We hypothesized that saroglitazar, a dual PPAR-α and PPAR-γ agonist may ameliorate oxidative stress and neuroinflammatory process in MES induced epileptic rats. A total of 36 rats were randomized to different groups (n=6). Group I served as normal control, while in the remaining groups (group IV, V and VI) animals were pre-treated with saroglitazar for 15 days prior to inducing MES. Group I animals were pre-treated with vehicle and group-III with diazepam (2mg/kg). Epilepsy was induced in rats and time taken for onset of tonic hind limb extension (THLE), duration of THLE, duration of clonic phase and recovery time in seconds were noted. Brain SOD and MDA levels were assessed and immunohistochemical analysis was done to evaluate the expression of inflammatory marker COX-2. Pre-treatment with saroglitazar was effective against tonic clonic seizure in MES treated rats. SOD levels significantly increased and a significant reduction in MDA levels with a remarkable decrease in the uptake of COX-2 antibody were reported. Saroglitazar attenuated MES induced epilepsy and the probable underlying mechanisms are due to the inhibition of oxidative stress and neuroinflammation.
Keywords
Diazepam; Neuroinflammation; Oxidative stress; PPAR α/γ; Saroglitazar
Download this article as:| Copy the following to cite this article: Panigrahy S. R, Pradhan S, Maharana C. S. Amelioration of Oxidative Stress and Neuroinflammation by Saroglitazar, a Dual PPARα/γ Agonist in MES Induced Epileptic Rats. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Panigrahy S. R, Pradhan S, Maharana C. S. Amelioration of Oxidative Stress and Neuroinflammation by Saroglitazar, a Dual PPARα/γ Agonist in MES Induced Epileptic Rats. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2DQN5eh |
Introduction
Epilepsy is a debilitating neurodegenerative condition characterized by abnormal electrical discharges from the brain leading to recurrent unprovoked seizures responsible for seizure-related disability, comorbidities, social stigma poor quality of life and economic burden of therapy.1 The mortality risk in epileptics increases by 1.2-9.3% when compared to general population.2 Nearly 65 million people of all ages, races, and social groups worldwide are affected by epilepsy.3 Current antiepileptic drugs (AEDs) are only effective in controlling seizures in about 70% cases. Moreover, the use of AEDs is often limited by the side effects and drug interactions, as a result of which quality of lives of most of the patients with epilepsy are affected adversely.4 Thus; there is still a need for developing anticonvulsant drugs having multiple mechanisms of action with minimal adverse effect.
In the past decades, significant improvements have been made in the understanding of the pathophysiological mechanisms underlying epilepsy. Several clinical and experimental studies implicate oxidative stress and neuroinflammatory process in the pathophysiology of epilepsy and subsequent epileptogenesis, a process that transform a normal brain into an epileptic one. Reactive oxygen species (ROS) can activate diverse downstream signalling pathways, and regulate expression of genes encoding a variety of proinflammatory proteins. Overexpression of cyclooxygenase-2 (COX-2) and trans-repression of transcription factors (NFk-β) have been emerged as important determinants, which contributes to the inflammatory process in epilepsy and seizures.5
The peroxisome proliferator-activated receptors (PPAR’s) are member of the nuclear receptor superfamily and belong to ligand-dependent transcription factors. Three isoforms of PPAR’s (α, β/δ, and γ) have been identified to regulate many physiological functions including glucose absorption, lipid balance, and cell growth and differentiation. PPARs form a heterodimer with retinoid X receptor (RXR) a nuclear receptor, subsequently binds to peroxisome proliferator response elements (PPREs) located within the specific target genes, causing target gene transcription (transactivation).6 PPARs also negatively regulate gene expression of pro-inflammatory genes (trans-repression) by inhibiting the activities of other transcription factors, like Activated Protein-1 (AP-1), Nuclear Factor-κB (NF-κB) and Nuclear Factor of Activated T cells (NFAT) thereby exerting their anti-inflammatory and anti-oxidative effects.7 A number of studies documented that, fenofibrate, a PPARα agonist has neuroprotective effect against epilepsy, spinal cord trauma, focal cerebral ischemia or ischemic stroke. PPAR-γ agonists (thiazolidinediones) are approved in T2DM due to their effect on decreasing insulin resistance by decreasing inflammatory cytokines and chemokines and reported to have neuroprotective properties as well.8,9 PPAR-β/δ, is expressed ubiquitously in almost all tissues, and recently implicated in the regulation of insulin sensitivity, lipid metabolism and the inflammation response, however, they are not in clinical use yet.
Saroglitazar, a novel dual PPAR α/γ agonist was developed in India and approved by Drug Controller General (DCGI) of India for the treatment of diabetic dyslipidemia and hypertriglyceridemia with T2DM not controlled by statin therapy.10 Keeping this fact that both PPARα and PPARγ agonists having neuroprotective effects basically in epilepsy, we hypothesized that saroglitazar may also have a positive effect in epilepsy by acting on both PPARα and γ receptors. Therefore, the present study was designed to explore the antiepileptic effect of saroglitazar alone and in combination with diazepam, a standard drug for epilepsy, and also, to investigate if those effects are mediated through anti-oxidative and anti-inflammatory mechanisms in maximal electroshock (MES) induced epileptic rats.
Materials and Methods
The study was carried out as per the CPCSEA guidelines after obtaining approval from the Institutional Animal Ethics Committee dated 29.04.2016.
Animals
Thirty-six adult wistar albino rats, six weeks old of either sex weighing between 150-200 grams were procured from a registered breeder and housed at department animal house (Regd. No. 472/CPCSEA) in 12:12 hour light: dark cycle under standard laboratory conditions and at a temperature of 24-28°C with a relative humidity of 50-60%. The animals were provided with standard laboratory pellet diet and water ad libitum and kept for 1 week to acclimatize with the laboratory condition before starting the experiment.
Drugs and Chemicals
All the drugs and chemicals were of analytical grade and procured from Himedia Lab Ltd. COX-2 antibody for IHC (Product code: HPA001335) was obtained from Sigma Aldrich. The test drug Saroglitazar was obtained from Zydus Pharmaceuticals Ltd.
Acute Toxicity Studies
Acute toxicity studies were performed on rats as per OECD guidelines 423. In acute dose studies, the maximum tolerated dose (MTD) in wistar albino rat was 1200 mg/kg. There was no behavioural changes or mortality observed during the study period.11
Grouping
The rats were randomly distributed into six groups (n=6)
Group I- Normal rats (Treated with Tween 80)
Group II- MES induced rats (Treated with Tween 80)
Group III- MES induced rats treated with diazepam(2mg/kg)
Group IV- MES induced rats with saroglitazar (1mg/kg)
Group V- MES induced rats with saroglitazar (3mg/kg)
Group VI-MES induced rats treated with diazepam(0.5mg/kg) and saroglitazar (1mg/kg)
MES model was used to evaluate the anticonvulsant activity of saroglitazar. Animals were pre-treated with the respective vehicle, standard or test drugs. The dose of the standard and test drug was selected from standard published literature.12, 13 Saroglitazar was given in a dose of 1mg/kg and 3mg/kg per orally once daily for a period of 15 days whereas diazepam (single dose) was injected intraperitoneally 30 minutes before the induction of epilepsy. Epilepsy was induced in rats of group 2 to 6 by delivering electroshock of 50 mA, trans-auricularly for 0.2 seconds using an electro-convulsiometer. Time taken for onset of tonic hind limb extension (THLE), duration of THLE, duration of clonic phase and recovery time in seconds were noted for 30 minutes. The decrease in duration of hind limb extension was considered as a protective action of the drug.
Biochemical assessments
The animals were sacrificed and the whole brain was removed and was held in 0.9% cold saline. 100mg of brain tissue was taken along with 2 ml of phosphate buffer saline and tissue homogenate was prepared for estimation of various biochemical parameters.
Antioxidant Parameters
Estimation of Superoxide dismutase activity (SOD)
Estimation of Superoxide dismutase (SOD) activity was assayed according to the method of Kakkar et al.14
Estimation of Malondialdehyde (MDA)
The MDA levels in brain tissues were analyzed by a method based on the reaction with thiobarbituric acid at 90-100ºC.15 In the thiobarbituric acid test reaction, MDA or MDA-like substances and thiobarbituric acid react together to produce a pink pigment with an absorption maximum of 532nm.
Immunohistochemical analysis (IHC)16
IHC analysis was performed to evaluate the expression of inflammatory marker COX-2. Brain tissue was fixed and embedded after which cutting and mounting of the sections were done. Then the sections were deparaffinized and rehydrated. Subsequently, antigen retrieval was done followed by immunohistochemical staining with COX-2 antibody (Sigma Aldrich). Counterstaining was done with haematoxylin and the slides were viewed under the microscope.
Statistical analysis
All values obtained were expressed as Mean +/- Standard deviation. Statistical analysis of all the data obtained was done by one-way analysis of variance followed by Tukey’s post-hoc test using SPSS 20.0 statistical software. Values with p<0.05 were considered as statistically significant.
Results
Effect on THLE (Onset & duration)
Administration of saroglitazar at doses 1mg/kg and 3mg/kg resulted in dose dependent effect on THLE (Table-I). There was a significant increase in the latency and decrease in the duration of THLE (p<0.001) in saroglitazar (3mg/kg) as well as combination of saroglitazar 1mg/kg with diazepam 0.5mg/kg as compared to MES induced control rats.
Effect on Duration of Clonus and Recovery Time
A significant decrease in the duration of clonus, as well as the recovery time, was found in saroglitazar 1mg/kg and 3mg/kg, diazepam and saroglitazar (1mg/kg + diazepam 0.5mg/kg) treated groups as compared to MES control rats(p<0.001) (Table-I).
Table I: Effect of Saroglitazar on the duration of onset of THLE, total duration of THLE, duration of clonus and recovery time in MES induced epileptic rats(n=6)
|
Drug |
Onset of THLE(sec) |
Duration of THLE(sec) |
Duration of clonus(sec) |
Recovery time(sec)
|
|
MES control (Tween 80) |
3.5±0.54 |
18.33±1.20 |
18.67±2.26 |
122.67±15.47 |
|
Diazepam(2mg/kg) |
203±28.90** |
4.83±0.75** |
1.33±0.51** |
6.17±0.4** |
|
Saroglitazar(1mg/kg) |
51±5.29* |
11.33±1.21** |
10.5±1.04**
|
60.67±8.09**
|
|
Saroglitazar(3mg/kg) |
176.17±16.72** |
6.83±0.75** |
4.67±0.81**
|
36±3.89**
|
| Saroglitazar(1mg/kg)
+ Diazepam(0.5mg/kg) |
266.83±22.99** |
4.17±0.75** |
2.5±0.54**
|
13.67±2.16**
|
(n=6). Data expressed as Mean ± SD. One way ANOVA with Tukey’s posthoc test –
*: p<0.01 drug treatment vs MES control, **: p<0.001 drug treatment vs MES control
Effect on SOD and MDA levels of brain
There was a significant decrease in SOD and a significant increase in MDA levels ofthe brain in MES control rats when compared to normal vehicle treated rats (p<0.001). The mean SOD levels of saroglitazar 1mg/kg, 3mg/kg and saroglitazar 1mg/kg along with diazepam was found was significantly increased when compared to MES control. The mean brain MDA levels of MES induced epileptic rats was found to be significantly increased (p<0.001) when compared with normal control group. The results also revealed (Table-II) a significant reduction in MDA levels (p<0.05 in saroglitazar 1mg/kg and p<0.001 in all other drug treated groups) as compared to MES control
Table II: Effect of drugs on SOD levels and MDA levels of brain in MES model
|
Drug |
SOD(Brain)
(Units / mg tissue) |
MDA(Brain)
(nmol MDA/mg tissue) |
|
Normal control |
15.69±1.88
|
2.9±0.55 |
|
MES control |
8.86±0.95** |
8.65±0.9** |
|
Diazepam(2mg/kg) |
12.54±0.82***
|
4.79±0.69*** |
|
Saroglitazar (1mg/kg) |
9.96±0.80
|
5.57±0.8* |
|
Saroglitazar(3 mg/kg) |
12.47±0.83***
|
3.34±0.23b*** |
| Saroglitazar(1mg/kg)
+ Diazepam (0.5mg/kg) |
14.88±1.31*** |
3.01±0.22*** |
(n=6); Data expressed as Mean ± SD. One way ANOVA with Tukey’s posthoc test –
*: p<0.05 drug treatment vs MES control,**: p<0.001 MES control vs normal control, ***: p<0.001 drug treatment vs MES control
Immunohistochemical analysis of inflammatory maker (COX-2 antibody)
The immuno-histochemistry findings suggested that the uptake of COX-2 antibody was minimum in normal control group (original bluish colour retained) as depicted in figure no.1(a) and maximum in MES induced vehicle control groups which were depicted as brown colour in Fig no.1(b). No remarkable decrease in the expression of COX-2 antibody was found in saroglitazar 1mg/kg treated groups (Fig no.1d). However, in saroglitazar 3mg/kg treated group there is decreased intensity of brown colouration with some bluish stained neuronal cells (Fig no.1e). There was a remarkable decrease in the intensity of brown colour with a characteristic bluish appearance of the well-defined neuronal cells in diazepam (fig no.1c) and combination of saroglitazar 1mg/kg and diazepam 0.5mg/kg treated rats (fig no.1f) as compared to MES induced vehicle treated epileptic rats. Thus, saroglitazar attenuates the inflammatory changes which are depicted in the form of decreased expression of inflammatory markers like COX-2.
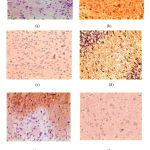 |
Figure 1: Immunohistochemistry of rat brain depicting COX-2 uptake (x400). (a)- Normal vehicle control, (b)- MES vehicle control, (c)- Diazepam (2mg/kg), (d) –Saroglitazar (1mg/kg), (e)- Saroglitazar(3mg/kg), (f)- Saroglitazar (1mg/kg) + Diazepam(0.5mg/kg)
|
Discussion
In this study, the anticonvulsant effect of a dual PPARα/γ activator saroglitazar, was evaluated on MES induced epilepsy model by measuring the onset of THLE, duration of THLE, duration of clonus and recovery time. Oxidative stress, as well as neuroinflammation, is major contributing factors in epilepsy, both as an epileptogenic, as well as a post-injury response that drives the progression of the disease.17 We have selected MES-induced convulsions method to induce epilepsy in experimental animals since this model represents grand mal epilepsy in human beings. MES stimulation generates high-frequency repetitive potentials, thus opening the Na+ channels and increasing the intracellular Ca++ levels which leads to depolarization of the cell and seizure.18 Continuous seizures induce increased production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which cause oxidative damage of biomolecules. The brain is highly susceptible to damage by ROS/RNS due to its high oxygen requirement, and high metabolic rate.19 Increased amount of ROS also results in elevated intracellular Ca2+concentration, and the vicious cycle of seizure continues inducing neuronal death either through necrosis or apoptosis. Furthermore, ROS can initiate diverse downstream signalling pathways, such as mitogen-activated protein kinases (MAPKs) or the transcription factor nuclear factor- κB (NF-κB). Subsequently, producing neuroinflammation by expressing proinflammatory cytokines (IL-1β, TNF-α and IL-6) and increasing the expression of cyclooxygenase-2 (COX-2) and of inducible nitric oxide synthase (iNOS) in microglia, astrocytes and neurons.20 In agreement with these mechanisms, our study observed a decrease in SOD and an increase in MDA levels in the brain tissues of MES induced rat along with increased uptake of COX-2 antibody in brain neurones suggesting of oxidative damage and neuroinflammation in rat brains after MES induced seizure.
The primary results of the present study show that pre-treatment of rats with a dual PPARα/γ activator saroglitazar can provide protection against the deleterious consequences of excitotoxicity induced by MES seizure. Antiepileptic parameters of saroglitazar were evaluated by measuring the onset of THLE, duration of THLE, duration of clonus and recovery time. Though both the doses of saroglitazar (1mg/kg and 3mg/kg) were effective against epilepsy, the effect of saroglitazar 3mg/kg was almost similar to the test drug diazepam (2mg/kg). Similar antiepileptic effects of PPARγ agonists, pioglitazone and rosiglitazone were reported by Lee et. al. and they have suggested that probably the anti-inflammatory and anti-oxidant properties are responsible.21 Puligheddu et al. even demonstrated that adjunctive therapy with PPARα agonist fenofibrate displayed a reduction of seizure frequency both in pre-clinical studies and patients affected by NFLE (nocturnal frontal lobe epilepsy).22
To elucidate the mechanism of the saroglitazar, we explored the antioxidant and anti-inflammatory effect of the drug. As the major biological effects of PPARs is down-regulation of oxidative stress-sensitive and inflammatory signalling pathways, so, reduces inflammation by decreasing inflammatory mediators like cytokines, adhesion molecules and nitric oxide synthase-2, COX-2 and reduces oxidative stress by increasing antioxidant enzymes superoxide dismutase (SOD) expression and catalase activity. Also, most of the research suggested that PPARs exert their therapeutic effects by anti-inflammatory and anti-oxidant properties. Brain SOD levels were increased significantly in rats treated with standard drug diazepam and saroglitazar 3mg/kg. Collino et. al. reported a similar increase in SOD levels while rats were pre-treated with of PPAR-γ agonists for neuroprotection.23 No significant increase in SOD levels was found in saroglitazar 1mg/kg treated rats, suggesting that low dose, do not produce an antioxidant effect, however, when the low dose was combined with diazepam low dose, the SOD levels was almost comparable with that of the normal rats. (Table no II)
Similarly, oxidative stress produced by electrical stimulation in rats also significantly increase brain MDA levels as compared to normal rats. Diazepam, saroglitazar 1mg/kg and 3mg/kg reversed this increase in brain MDA levels which was found to be at par with studies done by Anwer et.al. on the antioxidative properties of bezafibrate.24 Combination of diazepam and saroglitazar in MES model decreased the MDA levels to a significant extent which was almost in the range of MDA in normal rats.
From the immunohistochemical studies of the brain, we found out there is a certain role of inflammation in epilepsy. Cyclooxygenase-2 is known to play a key role in the early inflammatory response to an insult, and consequently a significant role in post-seizure inflammation and hyperexcitability of the brain. Our studies revealed the upregulated expression of COX-2 antibody in the brain tissue which is depicted as brownish colour in epileptic control rats (fig no.1b) which was due to increased uptake of the COX-2 antibody by the brain tissue which corroborates the study done by Collino et al. 23 Upregulation of COX-2 was inhibited by diazepam 2mg/kg, saroglitazar 3mg/kg pre-treated rats due to which there is decrease in the intensity of brown colour indicating expression of COX-2 antibody has been decreased (fig no. 1c and 1e respectively). Similar studies have shown the effect of pioglitazone on glial inflammation25 and rosiglitazone on global cerebral ischaemia.26 Though no remarkable effect was seen in saroglitazar 1mg/kg treated rats (fig no. 1d), a combination of saroglitazar 1mg/kg and diazepam treated rats showed a remarkably decreased expression of COX-2 as compared to epileptic controls (fig no. 1f). These findings can be correlated to the fact that epilepsy causes neuroinflammation which can be expressed by COX-2 and saroglitazar attenuates the inflammatory changes which are depicted in the form of decreased expression of inflammatory markers like COX-2.
Conclusion
In conclusion, the present study demonstrated that saroglitazar attenuated MES induced epilepsy and the probable underlying mechanisms are due to the inhibition of oxidative stress and neuroinflammation. Moreover, saroglitazar when combined with a lower dose of diazepam produced significant effect, indicating it can decrease the dose of antiepileptic drugs. However, further clinical correlation is required with proper study design to take advantage of this novel drug in clinical conditions.
References
- Fisher RS, Boas WE, Blume W, Elger C, Genton P, Lee P et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46:470-72.
- Shin HW, Jewells V, Hadar E, Fisher T, Hinn A. Review of Epilepsy – Etiology, Diagnostic Evaluation and Treatment. Int J Neurorehabilitation. 2014;1:130.
- Giussani G, Cricelli C, Mazzoleni F, Cricelli I, Pasqua A, Pechhioli S et al. Prevalence and Incidence of Epilepsy in Italy Based on a Nationwide Database. 2014;43(3-4):228-32.
- Svein J, Johannessen L. Antiepileptic drug interactions- Principles and clinical implications. Curr Neuropharmacology. 2010;8(3):254-267.
- Citaro R, Leo A, Marra R, De Saro G, Russo E. Antiepileptogenic effects of the selective COX-2 inhibitor etoricoxib, on the development of spontaneous absence seizures in WAG/Rij rats. Brain Res Bull. 2015.113:1-7.
- Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2(4):236–40.
- Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771(8):926–35.
- Saha L, Swati B, Alka B, Dibyajyoti B, Amitava C. Anti-kindling Effect of Bezafibrate, a Peroxisome Proliferator-activated Receptors Alpha Agonist, in Pentylenetetrazole Induced Kindling Seizure Model. Journal of Epilepsy Research. 2014;4:45-54.
- Esmaeili MA, Yadav S, Gupta RK, Garrett RW, Abigail D,Noel YC et al.. Preferential PPAR-α activation reduces neuroinflammation, and blocks neurodegeneration in vivo. Human Molecular Genetics. 2016;25(2):317–27.
- Krishna, Ayesha V, Ravinder K, Mazher A. Saroglitazar: a novel dual acting peroxisome proliferator activated receptor (PPAR) in dyslipidemia associated with T2DM . European Journal of Pharmaceutical and Medical Research.2017;4(2):680-84.
- http://lipaglyn.com/downloads/Lipaglyn_Product_Monograph.pdf. last accessed on May 12th,2019
- Muralidhar C, Sridhar I; Kavitha M. Anti Convulsant Effect Of Nifedipine, Diazepam And In Combination On MES Induced Epilepsy In Rats. Int J Clin Biomed Res. 2017; 3(4):22-6.
- Jain MR, Giri SR, Trivedi C, Bhoi B, Rath A, Vanage G et al. Saroglitazar, a novel PPARα/γ agonist with predominant PPARα activity, shows lipid-lowering and insulin-sensitizing effects in preclinical models. Pharmacol Res Perspect. 2015;3(3).
- Kakkar P, Das B & Viswanathan PN. A modified spectroscopic assay of superoxide dismutase. Indian Journal of Biochemistry and Biophysics . 1984;21:130-2
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction.Anal Biochem. 1979;95(2):351–8.
- San Y, Liu Y, Zhang Y, Shing P, Yan LZ. Peroxisome proliferator‑activated receptor‑γ agonist inhibits the mammalian target of rapamycin signaling pathway and has a protective effect in a rat model of status epilepticus. Molecular Medicine Reports. 2015;12:1877-83.
- Ho YH, Lin YT, Wu CW, Chao YM, Chang AY, Chan JY. Peripheral inflammation increases seizure susceptibility via the induction of neuroinflammation and oxidative stress in the hippocampus. J Biomed Sci. 2015;22(1):46.
- Krishna C, Shanmugam D, Elsani M. Antiepileptic activity of Alstonia scholaris Linn. on MES, PTZ, Strychnine induced convulsions in rats. International Journal of Pharmacy and Biological Sciences. 2016;6(1):207-213.
- Aguiar CC, Almeida AB, Araújo PV, Rita Neuma D, Edna MC, Otoni CV et al. Oxidative stress and epilepsy: literature review. Oxid Med Cell Longev. 2012.
- Yoshikawa K, Kita Y, Kishimoto K, Shimizu T. Profiling of eicosanoid production in the rat hippocampus during kainic acid-induced seizure: dual phase regulation and differential involvement of COX-1 and COX-2. Biol. Chem. 2006;281:14663–69.
- Lee C H, Min Hee Yi, Dong J C , Enji Z, Sang Ha Oh, Dong Woon Kim. Biomol Ther (Seoul). 2015;23(3): 261–267.
- Puligheddu, M., Melis, M., Pillolla, G., Milioli, G., Parrino, L., Terzano, G. M., et al. Rationale for an adjunctive therapy with fenofibrate in pharmacoresistant nocturnal frontal lobe epilepsy. Epilepsia. 2017;58:1762–1770.
- Collino, M., Aragno, M., Mastrocola, R., Gallicchio, M., Rosa, A.C., Dianzani, C., Danni, O., Thiemermann, C., & Fantozzi, R. Modulation of the oxidative stress and inflammatory response by PPAR-gamma agonists in the hippocampus of rats exposed to cerebral ischemia/reperfusion. European journal of pharmacology. 2006;530;1-2: 70-80
- Anwer T, Sharma M, Pillai KK, Haque SE, Alam MN, Zaman MS. Protective effect of bezafibrate on streptozotocin-induced oxidative stress and toxicity in rats. Toxicology. 2007;229:165-72.
- Heneka MT. Acute treatment with the PPARγ agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ1–42 levels in APPV717I transgenic mice. Brain. 2005;128(6):1442–1453.
- Jing J, Jennifer A, Wenzhan D. Neuroprotective effects of PPAR-γ agonist rosiglitazone in N171-82Q mouse model of Huntington’s disease. J Neurochem. 2013 ; 125(3):410–19.







