Hani M Al-Shagahin*1 , Ibrahim F Kharboush2, Enas Al-Zayadneh3, Abdelrahman Alharazneh1, Eman Albataineh4 and Ala Alqatamin5
, Ibrahim F Kharboush2, Enas Al-Zayadneh3, Abdelrahman Alharazneh1, Eman Albataineh4 and Ala Alqatamin5
1Department of Special Surgery, Division of Otorhinolaryngology, Faculty of Medicine, Mutah University, Al-Karak, Jordan.
2Department of Public Health and Biostatistics, Faculty of Medicine, Mutah University, Al-Karak, Jordan.
3Department of Pediatrics, Faculty of Medicine, Jordan University, Amman, Jordan.
4Department of Microbiology and Immunology, Faculty of Medicine, Mutah University, Al-Karak Jordan.
5Department of Medicine, Al-Karak Teaching Hospital, Al-Karak, Jordan.
Corresponding Author E-Mail: hani_shagahin2@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1734
Abstract
The distribution and pattern of aeroallergens are significantly different between different countries and even in the different parts of the same country. The present study aims to evaluate the most common aeroallergens among allergic rhinitis patients in the city of Al-Karak, Jordan. A cross-sectional study was conducted at Department of Otorhinolaryngology, Mutah University, from March 2016-April 2018. Patients with a clinical diagnosis of allergic rhinitis were enrolled and Skin Prick Test (SPT) was performed using 11 common aeroallergens including, grass, weed, tree, mite, and mould in 140 patients. The results showed that the overall rate of sensitization to any allergen was 85.7%. It was shown that 69.3% of patients were poly-sensitized; while, 16.4% were sensitized to only one allergen. The majority of the common allergens were Olive tree pollen (51.4%), Dermatophagoides pteronyssinus (37.9%), respectively. Mould (Alternaria) was the least prevalent allergen (17.1%). The present study has shown the importance of Olive tree pollen which, is widely cultivated in Al-Karak, Jordan. The diagnosis of pollen allergen can be simplified by using a combination of a few common allergens.
Keywords
Aeroallergens, Allergic rhinitis, Jordan, Sensitization, Skin Prick Test
Download this article as:| Copy the following to cite this article: Al-Shagahin H. M, Kharboush I. F, Al-Zayadneh E, Alharazneh A, Albataineh E, Alqatamin A. Skin Prick Test Reactivity to Common Aeroallergens among Allergic Rhinitis Patients in Jordan. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Al-Shagahin H. M, Kharboush I. F, Al-Zayadneh E, Alharazneh A, Albataineh E, Alqatamin A. Skin Prick Test Reactivity to Common Aeroallergens among Allergic Rhinitis Patients in Jordan. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/2Pl0dkv |
Introduction
Allergic rhinitis is one of the most prevalent chronic diseases that are common in 10-30% of adults and up to 40% in children (1). Aeroallergens including pollens (plant pollens), fungi, animal dander, domestic mites, domestic animals, and insects are the most common to initiate allergic diseases (2). One of the most obvious features of pollen allergy is their seasonal nature. Each spring, summer and autumn pollens are released from trees, grasses, and weeds. As a result, individuals have started experiencing allergic symptoms only when the pollen particles are distributed in the air and reach the nose and bronchial airway (3).
Avoiding allergen exposure is the first step in the management of allergic rhinitis, even when it is not completely effective. Identifying the common allergen plays an important role in the diagnosis and treatment of allergic rhinitis. Choosing the most reliable and the most cost effective panel of allergen extracts for skin prick test (SPT) has a very important role in the diagnosis and treatment of allergic rhinitis. Moreover, the best formulation of inhalant allergen immunotherapy can be found by defining the most common allergens in each area (4). The direct and indirect effects of allergic rhinitis include; the cost of treatment, impaired quality of life, and presence of co-morbidities that cause significant impact on the public health system (5, 6). Common pollens causing allergies are different in different parts of the world and meteorological parameters (wind, temperature, solar radiation, humidity, and rainfall) have a strong impact on pollen concentration (7).
The majority of the studies have helped in identifying the most common allergens; however, studies on different parts of the body have not shown a unique pattern of sensitization (4). The majority of the detailed epidemiological studies and clinical trials have been performed in Western Europe and North America (8), unlike the Middle East region (9); although, AR and allergic asthma are global and increasing serious health problems (10). The distribution and pattern of allergens are significantly different between different countries and even in the different parts of the same country (11). A similar study was performed in Amman, the capital of Jordan to investigate the pattern of skin prick test reactivity to various aeroallergens among allergic rhinitis patients in 2011 (12). The present study aims to evaluate the prevalence of positive skin test to various common aeroallergens among allergic rhinitis patients in the City of Al-Karak, at the southern part of Jordan, to develop better methods for prevention and effective treatment of allergic rhinitis patients.
Materials and Methods
Study Population and Design
A cross-sectional study was conducted at Al-Karak Teaching Hospital in the southern part of Jordan. It is the largest public and teaching hospital in the south affiliated with the Faculty of Medicine at The University of Mutah, Jordan. A total of 140 patients, including children and adults clinically diagnosed to have allergic rhinitis were recruited. These patients attended the Ear, Nose, and Throat Clinics at Al-Karak Teaching Hospital during March 2016 – April 2018.
Inclusion and Exclusion Criteria
The inclusion criteria were the diagnosis of current allergic rhinitis as defined by having sneezing or runny nose or nasal obstruction in the absence of a cold or flu in the last 12 months. The diagnosis of the patients was performed in the outpatient clinic of ENT by a specialist, patients included in the study if they agreed to participate and signed informed consent. The exclusion criteria included underlying disease such as cold, acute and chronic sinusitis, and patients taking antihistamine and corticosteroids drugs over the last two weeks or patients with a history of anaphylaxis and severe persistent asthma.
Ethical Consideration
This study was approved by the ethics (Ref. number 201829) and scientific committees of the faculty of medicine at Mutah University. The patients were provided with a consent form, research scientific details, and informative sheet before performing the test. The consent form was filled and signed by the patients.
Data Collection
The demographic details of the patients, smoking history, and family history of allergic disease was obtained by questionnaire.
Skin Prick Test
Skin prick testing with 11 standardized allergen extract from a commercial test kit (Stallergenes) was performed on all patients in accordance with published guidelines (13). Skin test findings have a sensitivity of 80-97% and a specificity of 70-95%. The eleven tested inhaled allergens were; Cat pelt, Sasola-Kali, two strains of House dust mites (Dermatophagoides petronyssinus and Dermatophagoides farina), Cereal- mix (oat, wheat, barley, maize), Olive pollen, Grass mix, Mould (Alternaria), Dog fur, Compositae, and wall-pellitary. Allergens used in this study were chosen according to the regional plant species (14) and regional studies tested the common aeroallergens (12). The tests were performed by a single trained and experienced physician to insure uniformity. Histamine hydrochloride (10mg/ml) and glycerol saline were used as positive and negative controls, respectively.
The skin prick test was performed on healthy skin on the volar surface of the forearm. The test sites were placed 20-30mm apart approximately 5 cm below the elbow and 5 cm above the wrist. A drop from each extract was applied to the skin (6 on the right and 5 on the left arm) and then the skin was pricked through each drop using a sterile lancet (Stallerpoint, Stallergenes). The mean wheal size was recorded after 15 minutes and SPT was regarded as positive with a wheal size of minimum 3-mm larger than the negative control.
Data Analysis
Microsoft excel was used to process and analyze data. The chi-square test was used to compare frequencies and independent sample t-test was used to compare means. A p-value of less than 0.05 was considered significant. The odds ratio and its 95% confidence intervals for potential risk factors for sensitization were assessed by univariate analysis and variables significantly associated with sensitization outcome in univariate analysis (P< 0.05) were included in the multivariate logistic regression model. The exposure variables included age group, gender, education level, smoking, and family history of allergy.
Results
One hundred and forty patients were evaluated for skin prick test against 11 allergens. There were 73 females and 67 males evaluated for skin prick test response against common aero-allergens. The mean age of women and men were 51.86 and 45.61, respectively. Demographic and clinical characterization of the patients is presented in table 1. The median duration of allergic rhinitis was 3.5 years. Concomitant asthma was found in 23%, allergic conjunctivitis in 38%, eczema and atopic dermatitis in 15%, and food allergy in 4% of those patients with allergic rhinitis. A positive family history of allergic disease was found in 55% of the patients; while, 22.9% of the patients were active smoker and 39.3% of the patients were passive smokers.
Table 1: Demographic Profile of the Respondents.
| Demographic and Clinical characteristics of patients of Allergic Rhinitis |
||
| Measure | Items | N (%) |
| Mean age ± SD | Male | 45.6 % (± 23.6) |
| Female | 51.8 % (± 21.8) | |
| Gender | Male | 67 |
| Female | 73 | |
| Education level | Primary school | 24 (17.1 %) |
| High school | 40 (28.6 %) | |
| College | 22 (18.7 %) | |
| University degree | 54 (38.6 %) | |
| Clinical history | Previous family history of allergic rhinitis | 77 (55 %) |
| Concomitant asthma | 38 (27 %) | |
| Allergic conjunctivitis | 32 % | |
| Concomitant eczema | 15 % | |
| Food allergy | 4 % | |
| Seasonal | 102 (73 %) | |
| Perennial | 38 (27 %) | |
| Smoking status | Current | 32 (22.9 %) |
| Passive | 55 (39.3 %) | |
| Never | 52 (37.1 %) | |
| X-smoker | 1 (0.7 %) | |
| Severity of disease | Mild | 25 % |
| Moderate | 30 % | |
| Severe | 45 % | |
Among patients with positive skin prick test, seasonal pattern was observed in 73% of the patients and perennial pattern was observed in 27% of the patients. Overall rating of sensitization to any allergen was observed in 85.7% of the patients, with 14.3% of the patients showing no reaction to any of the tested allergens. Out of all the patients, 69.3% were poly-sensitized; while, 16.4% were sensitized to only one allergen (Figure 1). The mean and median numbers of positive reactions were 3.5 ±1.8 and 3, respectively, among patients with positive skin prick tests. Moreover, Olive tree, Dermatophagoides pteronyssinus, and weed pollens (Compositae) were the most prevalent allergens (51.4%, 37.9%, 32.9%), respectively among the patients with allergic rhinitis (Table 2).
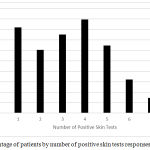 |
Figure 1: Percentage of patients by number of positive skin tests responses.
|
Table 2: Prevalence of Positive Skin Prick Test to selected Allergens.
| Aeroallergen name | All patients
(n=140) |
Sex | p-value
(Male vs. Female) |
|
| Male
(n=67) |
Female
(n=73) |
|||
| Mites Dermatophagoides petronyssinus | 37.9% *(53) | 44.8 %
*(30) |
31.5 %
*(23) |
0.13 |
| Mites Dermatophagoides farina | 30.7% *(43) | 29.9 %
*(20) |
31.5 %
*(23) |
0.21 |
| Cereal-mix | 17.9% *(25) | 20.9 %
*(14) |
15.1 %
*(11) |
0.22 |
| Olive pollen | 51.4% *(72) | 55.2 %
*(37) |
47.9 %
*(35) |
0.25 |
| Grass mix | 24.3% *(34) | 17.9 %
*(12) |
30.1 %
*(22) |
0.15 |
| Mould (Alternaria) | 17.1% *(24) | 14.9 %
*(10) |
19.2 %
*(14) |
0.16 |
| Dog fur | 23.6% *(33) | 26.9 %
*(18) |
20.5 %
*(15) |
0.22 |
| Compositae | 32.9% *(46) | 37.3 %
*(25) |
28.8 %
*(21) |
0.21 |
| Wall-pellitary | 23.6% *(33) | 31.3 %
*(21) |
16.4 %
*(12) |
0.055 |
| Sasola-kali | 32.1 % *(45) | 35.8 %
*(24) |
28.8 %
*(21) |
0.23 |
| Cat pelt | 30 %
*(42) |
31.3 %
*(21) |
28.8 %
*(21) |
0.26 |
*(- ) Number of patients.
Among the study sample of patients, the percentage of the allergen group showed that outdoor allergens (80%) were the most common allergen group followed by indoor allergens (68.6%), weed pollen (60%),tree allergens (51.4%), mite (47.9%) and animal allergens (39.3%) (Figure 2). Among indoor allergens, Dermatophagoides pteronyssinus was the most common allergen (37.9%) followed by Dermatophagoides farina (30.7%), Animal dander (Cat hair 30% and Dog epithelia 23.6%); whereas, mould made the least prevalent allergens (17.1%) (Table 3). Among outdoor allergens, olive tree pollen (51.4%) made the most prevalent allergen followed by Weed pollen (Compositae (32.9%), Salsola Kali (32%)), and Grasses mixtures (24.3%). The least prevalent allergens in this group were Cereals mixture (17.9%). Among weed pollens, Compositae (32.9%) made the most frequent allergen; while, Wall pellitory (23.6%) was the least prevalent (Table 3). There was no statically significant difference observed in the rate of sensitization to other allergens between male and female patients (Table 2).
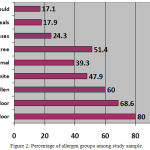 |
Figure 2: Percentage of allergen groups among study sample.
|
Table 3: Prevalence of Positive Skin Prick Test to selected Allergens among different age groups.
| Aeroallergen name | Age (year) | p-value | ||||
| 1-17 (n=11) |
18-30 (n=22) |
31-45 (n=37) |
46-65 (n=37) |
66+ (n=33) |
||
| Mites Dermatophagoides petronyssinus | 27.3% *(3) | 40.9% *(9) | 40.5% *(15) | 35.1% *(13) | 39.4%
*(13) |
0.39 |
| Mites Dermatophagoides farina | 18.2% *(2) | 31.8% *(7) | 32.4% *(12) | 21.6% *(8) | 42.4 %
*(14) |
0.24 |
| Cereal-mix | 27.3% *(3) | 18.2% *(4) | 21.6% *(8) | 10.8% *(4) | 18.2 %
*(6) |
0.41 |
| Olive pollen | 72.7% *(8) | 40.9% *(9) | 37.8% *(14) | 64.9 %
*(24) |
51.5 %
*(17) |
0.035 |
| Grass mix | 27.3% *(3) | 22.7% *(5) | 21.6% *(8) | 16.2% *(6) | 36.4 %
*(12) |
0.33 |
| Mould (Alternaria) | 18.2% *(2) | 22.7% *(5) | 16.2% *(6) | 10.8% *(4) | 21.2 %
*(7) |
0.42 |
| Dog fur | 18.2% *(2) |  Visited 924 times, 1 visit(s) today
| ||||







