Department of Pharmacology, SRM College of Pharmacy, SRMIST, Kattankulathur-603203
Corresponding Author’s E mail: k.manasa1@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1770
Abstract
Recent studies have suggested that environmental factors have a crucial role in triggering and/ or propagating the pathological changes in Parkinson’s disease (PD). Although many studies have been and being performed by utilizing MPTP like chemicals to study the effectiveness of new extracts and compounds in PD, a little focus was made on the role of pesticides. Since agricultural fields account for 37.7% of land area worldwide and the use of pesticides is an important risk factor in neurodegeneration, there is a crucial need to focus on the association between pesticides and PD. Benomyl, a benzimidazole fungicide is being widely used in India in cultivation of tropical crops. Studies prove the chronic exposure of benomyl leads to aldehyde dehydrogenase inhibition caused DOPAL toxicity, subsequently leading to dopamine degradation and Parkinson’s disease. Till date, there is no remedy for pesticide induced Parkinson’s disease. This review provides an insight of the pathophysiological aspects of pesticide induced Parkinson’s disease and also enlightens the importance of aldehyde dehydrogenase enzyme in neuroprotection.
Keywords
Alda 1; Aldehyde Dehydrogenase; Benomyl; DOPAL Toxicity; Parkinson’s Disease; Pesticide Induced PD
Download this article as:| Copy the following to cite this article: Manasa K. Chitra V. Phytoconstituents in the Management of Pesticide Induced Parkinson’s Disease - A Review. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Manasa K. Chitra V. Phytoconstituents in the Management of Pesticide Induced Parkinson’s Disease - A Review. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/2kL4BL3 |
Introduction
Parkinson’s disease (PD) is a chronic progressive, neurological disease linked to decreased dopamine production in the substantia nigra. As dopamine holds an important play in muscle tone maintenance, here in PD its depletion is well depicted with marked resting muscle tremor, rigidity, slowness of movement, impaired balance, and a shuffling gait.1 The direct and indirect pathways of dopamine experience a critical breakdown in PD. In familial PD, pathogenic mutations in Parkin, PINK1 and ATP13A2 genes are directly linked to mitochondrial dysfunction.2 In consideration with the environmental factors like chemicals, pesticides, toxic inhalants, etc. leading to the dopamine degradation, several epidemiological studies shows pesticides had a good association with PD.3
The axiom “if little is good, a lot more will be better” would be implicated in the dual role of pesticides to humans and other living systems. The use and abuse of pesticides has come into light as pesticides are not only lethal to pests, but also to non-target species like man.4 The production workers, formulators, sprayers and agricultural farm workers fall under the high risk group exposed to pesticides. Acute and chronic neurological toxicities such as headache, vomiting, dysfunctional lipid, protein, and carbohydrate metabolism are fabricated upon pesticide exposure.5 Barbeau et al. in 1987 illuminated the association between pesticide usage leads to degeneration of dopamine neurons by mitochondrial dysfunction, oxidative stress, protein aggregation, impairment of ubiquitin-proteasome process and autophagy in PD.6 The main pathological finding in pesticide associated PD is depletion of aldehyde dehydrogenase enzyme (ALDH).7,8
Certain pesticides like maneb, ziram, triflumizole, captan, etc. inhibit the aldehyde dehydrogenase-2 enzyme (ALDH2), which plays a critical role in 3,4-dihydroxy phenylacetaldehyde (DOPAL) intoxication.9 Initially, Wey and his colleagues in 2012 deigned a hypothesis to explain the decreased ALDHs (ALDH1 an ALDH2) either due to reduced aldehyde expression and or due to environmental toxins play a very prominent role in PD pathogenesis.10 According to the study performed by Fitzmaurice GA et al. in 2014, exposure to an ALDH-inhibiting pesticide (ex.benomyl) at both workplace and residential addresses was associated with a 65% increase in PD risk.11 ALDH2 is widely expressed in the frontal and temporal cortex, hippocampus, mid-brain, basal ganglia and cerebellum, glial cells and in neurons with a securing role in the brain and the spinal cord.12 Among the 19 ALDH enzymes, ALDH2 in the mitochondrial matrix holds an important role in the metabolism of ethanol,acetaldehyde, propionaldehyde, 4-HNE (4-hydroxynonenal), DOPAL and DOPEGEL13 (Figure 1). Both clinical and pre-clinical studies have shown the ALDH inhibition as one of the main pathological role in PD. Benomyl administration in mouse striatum and in vitro to PC12 cells and human cultured fibroblasts lead to DOPAL accumulation followed by ALDH inhibition, similar to rotenone.14 Six weeks treatment in rodents with Paraquat (herbicide) and maneb (fungicide) leads to a rise in 4-NHE protein that plays a major role in ALDH inhibition. MPTP and daizin administration in PC12 cells causes aldehyde load i.e., raise in 4-HNE and MDA and DOPAL concentration and causes protein modification via the inhibition of ALDH.15 Florang et al. employed the recombinant ALDH2 and ALDH2 extracts from rat brain mitochondrial fraction with 4-NHE even at low doses to explore the inhibition of DOPAL oxidation to DOPAC (3,4dihydroxyphenyl acetic acid).16
Michel TM et al., in 2014 have conducted a study in the clinical neurochemistry laboratory, University hospital Wurzburg to explain the role of ALDH2 in sporadic PD.17 They have collected 9 brain specimens of PD patient and 12 control specimens from the patients with no psychiatric illness. All the control and PD patients are matched for post-mortem time, age, gender and non-alcoholics. The frozen left hemisphere of all the brain issues were isolated and homogenized at 1000 rpm and the sediment was examined for protein quantification ad mitochondrial ALDH2 activities. An elevation in the ALDH2 activity was observed in PD patient brain tissues.
Role of Benomyl in Parkinson’s disease
Benomyl, a fungicide used for food crops and ornamental plants was shown to be a potent and rapid ALDH inhibitor, thereby associated with PD incidence in epidemiological studies. Benomyl (methyl 1-(butylamino) carbonyl-1H-benzimidazol-2-ylcarbamate) gets metabolized to carbendazim (methyl-2-benzimidazole carbamate), both acts by inhibiting microtubule assembly in fungi and has minimal effect on plant and mammalian microtubules.18 A report “Benomyl RED facts” published by U.S. Environmental Protection Agency in 2001 says benomyl as a human carcinogen and opted for a voluntary cancellation of this pesticide.19 Chronic benomyl exposure holds serious adverse effects on the male reproductive systems, causing decreased sperm counts and fertility in the rats; whereas, in female rats it was shown to induce aromatase activity in a human ovarian granulose-like tumor cell line. When studied in vitro in rat embryo cultures, it was also responsible to developmental toxicities like development of malformations in xenopus embryo’s neural tissue and decreases in yolk sac diameter. In a report published by California Environmental Protection Agency in 1999, benomyl was shown to cause hepatocellular adenomas and carcinomas in several strains of mice; hepatotoxicity and nephrotoxicity as well.20
When the living systems are exposed to benomyl, it gets dissociated to carbendazim and butyl isocyanate (BIC). BIC gets converted to S-methyl N-butylthiocarbamate (MBT) and further to MBT sulfoxide (MBT-SO) by cytochrome P450 enzymes. BIC, MBT and MBT-SO inhibit ALDH, among which MBT-SO serves as a potent ALDH inhibitor21 (Figure 2). This was confirmed by exposing the of ex vivo suspensions of nigral neurons to benomyl for 30 min exhibits a ALDH inhibition which was further reversed/minimized by pargyline – a MAO inhibitor; while, there was no significant change in α-Synuclein levels in TH+ neurons exposed to benomyl.22 Benomyl exposed zebra fish shows a deficit swimming behavior in 2 weeks. Transgenic zebrafish (Danio rerio) cell lines were employed to study the neurotoxicity of benomyl in vertebrate system by exposing to 1 μM benomyl 5 h -120 h post fertilization and found a reduction in the VMAT2+ (vesicular monoamine transporter) neuronal counts.23
Aldehyde dehydrogenase Promoters in Parkinson’s disease
Alda-1 was identified as a pharmacological chaperone to activate the human ALDH2*1 and ALDH2*2 by binding above the inhibitory site (the exact site for diazin, a potent ALDH2 inhibitor) and thus increase the efficiency of acyl-enzyme hydrolysis for smaller aliphatic substrates in ALDH2*1 and provides structural stability to the ALDH2*2 enzyme.24 This ALDH promoting role of alda 1 was studied in a pre-clinical model of PD by injecting DOPAL in the substantia nigra with the ALDH activator alda-1 to one group of rats and disulfiram (ALDH inhibitor) to 2nd group and vehicle to the control group rats.25 All the group animals were subjected to behavioral studies followed by isolating the brain samples to evaluate the levels of 4HNE ALDH and alpha-synuclein aggregates. The alda 1 treated group shows a beneficial rise in ALDH levels with a decline in the 4NHE and synuclein aggregation. This study even proves that MPTP does not have any influence on ALDH levels, although it declines dopamine levels and increases the DOPAL, 4NHE concentrations.
Alda 1 treatment in the male C57BL/6 mice has shown a positive improvement in the intestinal injuries in intestinal ischemia.26 As Alda-1 treatment suppressed the serum levels of TNF-α, IL-1β and IL-6, a remarkable decrease in the morphological damage in the lung and intestine was seen. In a rat model of myocardial infarction, alda 1 treatment shows a decline in the number of TNF-α-positive cells and NO.27 These studies have shown the promising positive role of alda 1 in cellular damage, but there is no documental evidence on the trials which enlightens its application in Parkinson’s disease.
Phytoconstituents with aldehyde dehydrogenase promoting activity
Herbal medicine or phytomedicine is the use of plants for medicinal and therapeutic purpose for curing of diseases and improve human health.28 Although Parkinson’s disease is being treated with a number of synthetic compounds effectively, till date there is no synthetic drug except alda 1 to treat ALDH deficient PD. Alda 1 has been utilized in the pre-clinical studies of DOPAL induced PD and delineated a positive role in promoting ALDH levels, but still was not been employed in human trails. So far, Pesticide induction in PD was treated with MAO inhibitors like seligiline, pargyline i.e., drugs acting by inhibiting the DOPAL synthesis and concentrations.29 Some studies shows the protective role of Coenzyme Q10 in rotenone and paraquat-induced Parkinsonian rat models,30-32 Ginkgo biloba extract paraquat-induced apoptosis in vitro33 and acetyl–L-carnitine, sodium butyrate, vildagliptin, Hipercium perforatum, etc. had neuroprotective effects in rotenone-induced Parkinsonism in animals.34-40 All these studies are yet to be confirmed for their efficiency in humans and even none of the above agents were proved to be treated the ALDH PD model. Hence, we can inquest the alternatives which play the similar role of alda 1 in promoting ALDH. Phytochemicals are active ingredients which possess therapeutic properties that are considered as a medicine or drug and can be employed successfully as an alternative to the synthetic marketed drugs.
Certain plants have aldehyde dehydrogenase genes and are termed as “ALDH gene superfamily” and are mentioned in the table 1.41-45 Twenty four gene families among different plant taxa have been identified to encode ALDH. Aldehyde dehydrogenase enzyme in plants are meant to carry the homeostasis of aldehydes by eliminating the toxic aldehydes (DOPAL, 4NHE), hence termed as “aldehyde scavengers”.46 Among such plants, Arabidopsis thaliana (Brassicaceae) has ALDH genes of 9 families.47,48 In my project, the active constituents of this plant were identified and are to be utilized in studying for the presence of aldehyde dehydrogenase promoting character.
Insilico docking studies of eight compounds in Arabidopsis thaliana shows Rapalexin, Camalexin and Arvelexin has good interaction with the ALDH receptors, almost similar values to that of alda 1 (Table 2).
Table 1: Plants with Aldehyde dehydrogenase (ALDL) genes
| Plant Species | Number of ALDL genes |
| Arabidopsis thaliana | 16 |
| Oryza sativa | 20 |
| Vitis vinifera | 25 |
| Vitis carteri | 7 |
| Zea mays | 22 |
| Clamydomonas reinhardtii | 9 |
| Physcomitrella patens | 21 |
| Physcomitrella trichocarpa | 26 |
| Selaginella moellendorffii | 24 |
| Setaria italica | 22 |
| Homo sapiens | 19 |
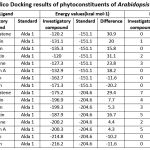 |
Table 2: Insilico Docking results of phytoconstituents of Arabidopsis thaliana |
 |
Figure 1: Aldehyde dehydrogenase enzyme in Parkinson’s disease |
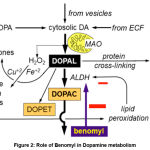 |
Figure 2: Role of Benomyl in Dopamine metabolism |
Conclusion
We need to comprehend the importance of the medicinal plants which are commonly found all over the country as the synthetic drugs and agents may contain harmful chemicals leading to various side effects and drug reactions. Due to minimal side effects and cost effectiveness, traditional medicinal plants containing natural sources of anti-oxidants and neuroprotective effects may be helpful in the long run. It also provides a novel insight on a disease pathology which provides different treatment strategies using natural sources decreasing the risk of drug interactions or adverse effects and increasing the quality of life and safety of patients.
Acknowledgement
I am grateful to my Guide, Dr. V. Chitra, SRM College of Pharmacy and Dean Dr. K. S. Lakshmi, SRM College of Pharmacy, SRMIST for their immense support in my research.
Conflict of Interest
The authors declare no conflict of interest
Funding Source
This is a review of the self-funded project (PhD).
Summary
Parkinson’s disease is due to several environmental and genetic factors leading to neurodegeneration of substania nigra dopaminergic neurons. Aldehyde dehydrogenase (ALDH) is one of the important liver enzyme which also synthesized in mitochondrial matrix of SN neurons and has a pronounced effect in protecting dopamine and this ALDH will be depleted by exposure to pesticides The pathophysiology of aldehyde dehydrogenase related Parkinson’s disease has gained much insight in agricultural countries like India, but still there is a need to identify the pharmaceutical compound/ phytoconstituent that act by acting through promotion of this ALDH enzyme.
References
- Sulzer D, Surmeier JD. Neuronal vulnerability, pathogenesis and Parkinson’s disease. Mov Disord., 2013; 28(1): 41–50.
- Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr. Opin .Cell. Biol., 2004; 16(6): 653-62.
- Francesca M, Alessandro P, Pierluigi T. Pathophysiology of motor dysfunction in Parkinson’s disease as the rationale for drug treatment and rehabilitation. Parkinson. Dis., 2016; 1: 1-18.
- Kamel F, Hoppin JA. Association of Pesticide Exposure with Neurologic Dysfunction and Disease. Environ. Health Perspect,. 2004; 112(9): 950-8.
- Gomes J, Lloyd O, Revitt DM. The influence of personal protection, environmental hygiene and exposure to pesticides on the health of immigrant farm workers in a desert country. Scand. J. Work. Environ. Health., 1999; 24: 213-9.
- Barbeau A, Roy M, Bernier G, Campanella G, Paris S.Ecogenetics of Parkinson’s disease: prevalence and environmental aspects in rural areas. Can J Neurol Sci., 1987; 14(1):36-41.
- Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson’s disease pathogenesis. Brain. res., 2003; 989(2):205-13.
- Marchitti SA, Deitrich RA, Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxy phenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol. rev., 2007; 59 (2): 125-150.
- Burke WJ, Li SW, Chung HD, et al. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicol., 2004; 25(1-2):101-15.
- Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, et al. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem., 2013; 126:591-603.
- Fitzmaurice AG, Rhodes SL, Cockburn M, Ritz B, Bronstein JM. Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease. Neurol., 2014; 82(5):419-26.
- Kieburtz K, Wunderle KB. Parkinson’s disease: evidence for environmental risk factors. Mov. Disord., 2013; 28:8-13.
- Harada S, Agarwal DP, Goedde HW. Electrophoretic and biochemical studies of human aldehyde dehydrogenase isozymes in various tissues. Life. Sci., 1980; 26: 1773-80.
- Casida JE, Ford B, Jinsmaa Y, Sullivan P, Cooney A, Goldstein DS. Benomyl, Aldehyde Dehydrogenase, DOPAL, and the Catecholaldehyde Hypothesis for the Pathogenesis of Parkinson’s disease. Chem. Res. Toxicol., 2014; 27:1359-61.
- Lamensdorf I, Eisenhofer G, White JH, Hayakawa Y, Kirk K Kopin IJ. Metabolic stress in PC12 cells induces the formation of the endogenous dopaminergic neurotoxin, 3,4- dihydroxyphenylacetaldehyde. J. Neurosci. Res., 2000; 60: 552-8.
- Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S, Doorn JA. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem. Res. Toxicol,. 2009; 22: 835-841.
- Michel TM, Kasbauer L, Gsell W, Jecel J, Sheldrick AJ, Cortese M, et al. Aldehyde dehydrogenase 2 in sporadic Parkinson’s disease. Parkinsonism. Relat. Disord., 2014; 20(1): 68-72.
- Marchitti SA,Deitrich RA, Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4- dihydroxyphenylacetaldehyde and 3,4-dihydroxy phenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol. Rev., 2007; 59: 125-150.
- https://archive.epa.gov/pesticides/reregistration/web/html/benomyl_fs.html.
- Rull RP, Ritz B. Historical pesticide exposure in California using pesticide use reports and land-use surveys: An assessment of misclassification error and bias. Environ. Health. Perspect,. 2003; 111(13):1582-1589.
- Tanner CM, et al. Rotenone, paraquat, and Parkinson’s disease. Environ. Health. Perspect., 2011; 119 (6):866-872.
- Chou AP, Li S, Fitzmaurice AG, Bronstein JM. Mechanisms of rotenone-induced proteasome inhibition. Neurotoxicol., 2010; 31(4):367-372.
- Burgess HA, Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. J. experiment. biol., 2007; 210: 2526-2539.
- Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting Aldehyde Dehydrogenase 2: New Therapeutic Opportunities. Physiol. Rev., 2014 Jan; 94(1): 1-34.
- Ohta S, Ohsawa I. Dysfunction of mitochondria and oxidative stress in the pathogenesis of Alzheimer’s disease: on defects in the cytochrome c oxidase complex and aldehyde detoxification. J. Alzheimers. Dis., 2006; 9(2):155-166.
- Perez-Miller SM, Younus H, Vanam R, Chen CH, Rosen DM, Hurley TD. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat. Struct. Mol. Biol., 2010; 17(2): 159-164.
- Zhu Q , HeG , Wang J , Wang Y,Wei Chen W. Pretreatment with the ALDH2 agonist Alda-1 reduces intestinal injury induced by ischaemia and reperfusion in mice. Clin. Sci., 2017; 131 1123-1136.
- Andrade C. The Herbal Treatment of Parkinson’s disease: A Possible Role for BR-16A (Mentat), Ind. J. Psychol. Med., 1996; 19(2): 82.
- Schapira AHV. Present and future drug treatment for Parkinson’s disease. J. Neurol. Neurosurg. Psychiat., 2005; 76:1472-1478.
- Somayajulu-Nitu M, Sandhu JK, Cohen J, et al. Paraquat induces oxidative stress, neuronal loss in substantia nigra region and parkinsonism in adult rats: neuroprotection andamelioration of symptoms by water-soluble formulation of coenzyme Q10. BMC neuroscience., 2009; 10: 88.
- Muthukumaran K, Leahy S, Harrison K, et al. Orally delivered water soluble Coenzyme Q10 (Ubisol-Q10) blocks on-going neurodegeneration in rats exposed to paraquat: potential for therapeutic application in Parkinson’s disease. BMC neuroscience., 2014; 15: 21.
- Abdin AA, Hamouda HE. Mechanism of the neuroprotective role of coenzyme Q10 with or without L-dopa in rotenone-induced Parkinsonism. Neuropharmacol., 2008; 55(8): 1340-1346.
- Kang X, Chen J, Xu Z, Li H, Wang B. Protective effects of Ginkgo biloba extract on paraquat-induced apoptosis of PC12 cells. Toxicol, in vitro., 2007; 21(6):1003-1009.
- Zaitone SA, Abo-Elmatty DM, Shaalan AA. Acetyl-Lcarnitine and alpha-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain, implication for Parkinson’s disease therapy. Pharmacol. Biochem. behav., 2012; 100(3):347-360.
- St Laurent R, O’Brien LM, Ahmad ST. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neurosci, 013; 246:382-390.
- Ning X, Yuan M, Guo Y, et al. Neuroprotective effects of (E)-3,4-diacetoxystyryl sulfone and sulfoxide derivatives in vitro models of Parkinson’s disease. J. Enzyme Inhib. Med. Chem., 2016; 31(3):464-469.
- Guo B, Xu D, Duan H, et al. Therapeutic effects of multifunctional tetramethyl pyrazine nitrone on models of Parkinson’s disease in vitro and in vivo. Biol. Pharm. Bul.,l 2014; 37(2):274-285.
- Gomez del Rio MA, Sanchez-Reus MI, Iglesias I, et al. Neuroprotective Properties of Standardized Extracts of Hypsericum perforatum on Rotenone Model of Parkinson’s Disease. CNS. Neurol. Disord. Drug. Targets., 2013; 12(5):665-679.
- Gokul K, Muralidhara. Oral supplements of aqueous extract of tomato seeds alleviate motor abnormality, oxidative impairments and neurotoxicity induced by rotenone in mice: relevance to Parkinson’s disease. Neurochem. Res., 2014; 39 (7): 1382-1394
- Abdelsalam RM, Safar MM. Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: role of RAGE-NFkappaB and Nrf2-antioxidant signaling pathways. J.neurochem., 2015; 133(5):700-707.
- Ma WH, Qin QLP. Chemical Constituents of Arabidopsis thaliana. Chem. Nat. Comp., 2014; 50(4):776-777.
- Tesniere C, VerrieS QC. Molecular cloning and expression of cDNAs encoding alcohol dehydrogenases from Vitis vinifera L. during berry development. Plant. Sci., 2000; 157: 77-88.
- Huang W, Ma X, Wang Q, Gao Y, Xue Y, Niu X, et al. Significant improvement of stress tolerance in tobacco plants by overexpressing a stress-responsive aldehyde dehydrogenase gene from maize (Zea mays). Plant. Mol. Biol., 2008; 68: 451-463.
- Sophos NA, Vasiliou V. Aldehyde dehydrogenase gene superfamily: the 2002 update. Chem. Biol. Interac., 2003; 143-144: 5-22.
- Chen Z, Chen M, Xu ZS, Li LC, Chen XP, Ma YZ. Characteristics and expression patterns of the aldehyde dehydrogenase (ALDH) gene superfamily of foxtail millet (Setaria italica L.).PLoS One., 2014 2;9(7): 1-13.
- Doorn JA, Florang VR, Schamp JH, Vanle BC. Aldehyde dehydrogenase inhibition generates a reactive dopamine metabolite autotoxic to dopamine neurons. Parkinsonism. Relat. Disord., 2014; 20(1): 73-75.
- Kirch HH, Schlingensiepen S, Kotchoni S, Ramanjulu S, Bartels D. Detailed expression analysis of selected genes of the aldehyde dehydrogenase (ALDH) gene superfamily in Arabidopsis thaliana. Plant. Mol. Biol., 2005; 57, 315-332.
- Fitzgerald TL, Waters DL, Henry RJ. Betaine aldehyde dehydrogenase in plants. Plant. Biol., 2009; 11:119-130.








