Jyothi Ashok Kumar, Thotakura Balaji, C. Swathi Priyadarshini, Manickam Subramanian and Indumathi Sundaramurthi
Department of Anatomy, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chennai, Tamilnadu, India.
Corresponding Author E-mail: balajitk@yahoo.com,
DOI : https://dx.doi.org/10.13005/bpj/1781
Abstract
Every human being is exposed to the stress in one or the other form in the day to day life. Most of the existing studies on the impact of stress on the male reproduction were assessed by using single stressor, which may lead habituation to that stressor. The present study intends to estimate the consequence of stress on motor activity, sperm quality and histopathology of the testis in stress-induced male rats using multimodal stress one per day. Four weeks old Wister albino rats were randomly split into 4 groups and induced multimodal stress at different ages of life span. After induction of stress serum corticosterone levels, muscle strength and coordination, quality of sperm and histopathology of testes were estimated. Elevated serum corticosterone levels and body weight, reduced muscle strength, coordination. Sperm concentration and motility was significantly reduced and increased morphologically abnormal sperm in stress induced animals but sperm viability was not altered much. Histopathology of testes in stress received animals showed decreased tubular diameter and increased intertubular space. Multimodal stress caused elevated serum corticosterone and body weight, decreased motor activity, sperm quality and degenerative changes in the testis
Keywords
Motor Activity; Plasma Corticosterone; Sperm Quality; Stress; Wister Albino Rats
Download this article as:| Copy the following to cite this article: Kumar J. A, Balaji T, Priyadarshini C. S, Subramanian M, Sundaramurthi I. Non concurrent multimodal stress decreases sperm quality and motor activity in male Wister albino rats. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Kumar J. A, Balaji T, Priyadarshini C. S, Subramanian M, Sundaramurthi I. Non concurrent multimodal stress decreases sperm quality and motor activity in male Wister albino rats. Biomed Pharmacol J 2019;12(3). Available from: http://biomedpharmajournal.org/?p=28558 |
Introduction
Stress is an unavoidable state in day to day life of every individual. Fine balancing mechanism acts against forces of stress which is called as equilibrium. Maintaining of equilibrium is called homeostasis. Selye proposed the concepts of “eustress” and “distress”. Eustress is positive stress recognized as a pleasant form of stress caused by desirable stimuli. Distress is identified as a threat to the quality of life and homeostasis. An organism is intended to respond and adjust to surroundings in an attempt to endure. Once homeostasis has been endangered, a complex series of behavioral and physiological processes respond to restore equilibrium, known as an “adaptive stress response” or simply “stress response” [1]. Stress initiates the hypothalamus to release corticotrophic releasing hormone from paraventricular nuclei, which activates the hypophyseal gland to secrete ACTH which activates the cortex of adrenal gland to secrete of glucocorticoids into the circulation. This affects the metabolism of carbohydrates, fats and proteins which reduce blood testosterone levels and spermatogenesis. Stress causes reduced activity, interact with the environment and diminished searching behavior [2].
According to World Health Organization (WHO), Infertility is disease of reproductive system defined by failure to achieve clinical pregnancy after 12 or more months of regular unprotected sexual intercourse [3, 4, 5]. Infertility affects between 9% and 25% of couples globally [6, 7, 8]. Male infertility is the inefficiency to cause conception in a fertile female even after 12 months of regular and unprotected coitus [4]. Male infertility accounts to half of the reported infertility cases [3, 9,10]. Decrease in the quality of human sperm is increasing substantially worldwide over the past few decades [11 – 14]. The male factor alone contributes to 20–25% in infertile couples and both factors contribute to 30–40% [15]. The infertile couples in child bearing age looking for medical assistance are around 56%. [7].
The male infertility is increasing alarmingly Indian subcontinent, according to WHO estimates, the overall primary infertility rate ranges between 3.9% and 16.8% [16]. It varies extensively amongst Indian states from 3.7% in Uttar Pradesh, Himachal Pradesh, and Maharashtra [17] to 5% in Andhra Pradesh [18], and 15% in Kashmir [19]. It also varies across the tribes and castes within the same area in India [17, 20]. Reports suggest that 40% of infertility is due to male factor, 40% due to female factor and remaining 20% because of both sexes [21]. In Indian couples looking for treatment for infertility, the male factor accounts up to 23% [19]. Recent findings on the status of infertility in India, suggests that approximately 50% of infertility is due to male factor [22]. Reproductive abnormalities of male partners are an important factor in one-third of the cases and it is one of the considerable contributing factors in an additional 20% of infertile couples [23]. In male reproductive health, semen quality is the important marker. Identification of neuropsychological risk factors like stress is very essential in improving semen quality for fecundity and fertility [24].
Many studies have stated that there is a correlation between psychosomatic stress and sperm quality such as concentration, motility and morphometry [25, 26, 27]. In human beings, emotionally stressed individuals had reduced sperm count and motility [28]. Stress during academic examinations altered luteinizing hormone (LH) and testosterone levels [29]. Various studies have suggested decreased testicular weight, viability and motility in epididymal sperm and increased adrenal gland weight because of stress in rats [30, 31]. Earlier studies carried out to estimate the effect of corticosterone was by either administration of exogenous corticosterone [32] or by inducing stress like noise stress [33], forced swimming stress[34], immobilization stress [35] etc. One major limitation of this method is the animal might get acclimatized or adapted to that stress. Moreover during application of single stressor for prolonged time might harm the animal. In order to avoid these five variable stressors as multimodal stress were used in the present study in random manner. The novelty of this study lies in the fact that application of variable stressor and its results can be extrapolated to stressful condition in humans rather than using single stressor which is purely applicable only in experimental animals. Thus present study variable stressors have been applied to assess the consequences of stress on motor activity, sperm quality and morphology of testes. Assessment of semen analysis in experimental animals after inducing stress using variable stressors yet to be reported and hence this study.
Materials and methods
Animals
24 male Wister albino rats 4 weeks old were acquired from Biogen, Laboratory Animal Facility, Bangalore – 562107. Karnataka, India. Registration number: 971/bc/06/CPCSEA, for the present study. They were housed in clean transparent polypropylene cages, in a 12 hr light/12 hr dark cycle with ambient temperature around 23°C and relative humidity of 55 – 60%, free access to food and water with provided ad libitum.
Statement of Ethics
Animal experiments were conducted according to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The experimental protocol was approved by the Institutional Animal Ethics Committee of Chettinad Hospital and Research Institute (Approval number: IAEC 1/Desp.No.7/Dt.02.03.17)
Study design
Animals were randomly split into 4 groups of six males in each. Group 1 was control (n=6), Group 2 animals received 42 days of multimodal stress from post – natal day 28 to 70 (n=6), Group 3 animals received 42 days of multimodal stress from post – natal day 70 to 112 ( n=6), Group 4 animals received 84 days of multimodal stress from post – natal day 28 to 112 (n=6).
Stressing Paradigm
Stressors were carefully selected so as to they don’t get habituating, do not cause pain and do not influence food or water intake. The following stressors namely 36 hours constant light, the new object in the cage during the nighttime, restraint in a conical tube for 15 minutes, multiple cage changes and Saturated bedding overnight were employed for this study at the rate of one stressor for each day [36].
The body weight, serum corticosterone levels and motor activity was measured prior to induction of stress. All the above mentioned parameters were again repeated after induction of stress, subsequently all animals were sacrificed and analyzed semen parameters and histopathology of testes.
Serum corticosterone assay
Serum corticosterone levels were assessed by using ELISA kit (Cayman Chemical, Catalogue No: 501320). Determination of serum corticosterone was according to the instructions given along with the kit.
Motor activity
Actophotometer
Spontaneous motor activity was done by using Actophotometer (Inco Instruments & Medical Devices). Apparatus contains an activity chamber of 30 x 30 x 30 cm in dimension with a grid floor. Twelve light transmitters and receivers were arranged in the walls of the activity chamber at a suitable height from the floor of the chamber. When the animal interrupts any light beams, it was recorded as a locomotor activity. Animals were introduced in the chamber and recorded number of counts recorded per minute [37].
Rotarod
This instrument (Inco Instruments & Medical Devices) contains a metal rod of diameter 4 cm with a roughened surface for the animals to grip. It rotates on a horizontal axis by a motor and pulleys which can be tuned to rotate at various speeds (5 – 25 rpm/ min). Rats were made to cling to the rod, which rotates forwards at 15 rpm until they fall off from the rod. The rationale of this test was to assess the latency of an animal maintains itself on a moving rod, animals having defective motor coordination, dropped off early from the rod. Three trials were given per animal and the duration was recorded in seconds [38, 39].
Inverted screen test
The inverted screen (Inco Instruments & Medical Devices) is a wire mesh containing 12 mm squares of 1 mm diameter wire. The animal was placed on the screen and turned upside down. The time of an animal maintains itself holding and gripping is recorded. Latency to fall from the screen was recorded in seconds [40].
Semen Analysis
The animals were sacrificed by the overdose of halothane. Removal of the testis (Orchidectomy) and collection of spermatozoa was performed [41, 42]. The incision was made ventral to the scrotal sac and the testes were taken out of the incision site. The testes were exposed by cutting the tunica vaginalis. The spermatic cord was identified, ligated and incised [43]. The sperm were acquired from caudal epididymis by incising longitudinally and squeezed thoroughly then minced by scissors in 5 ml of phosphate buffer saline (PBS), placed in the Petri dish, incubated at room temperature for 2 minutes and analyzed immediately for sperm concentration, motility, morphology and viability of spermatozoon. The investigator was blinded while performing all the investigations of the study to avoid bias. The result was again confirmed by another assessor.
Sperm concentration
1 ml supernatant was mixed with 100 ml solution consisting of 5 g sodium bicarbonate, 1 ml of 35% formalin and 25 mg of eosin per 100 ml H2O (1: 100 ratio). 10 µl of this preparation was pipetted and charged into the counting chamber after placing the cover slip on it and left for 5 minutes to allow even distribution. Sperms were counted under a light microscope at 40× magnification. Total count represented as N x 106.
Motility
10µl sperm supernatant was placed on a sterile slide and covers with 22 x 22mm cover slip and incubated for 1 – 2 minutes. Slides were observed under a light microscope (Carl zeiss Axio lab.A1 microscope) at 40x, 200 spermatozoa were counted in total from several fields. Sperms were classified as PR = Progressive motile (forward movement, large circles), NP = Non progressive motile (on the spot movement, twitching), IM = Immotile (no movement).
Sperm Morphology
Sperm analysis for head and tail deformities was done as per WHO Laboratory Manual [44] for semen analysis. Around 20 µl of sperm suspension was placed on the slides and smeared. Slides were dried and fixed with fixative then stained with hematoxyline and eosin. 200 spermatozoa (intact sperm) per slide were analyzed under the light microscope at 40× magnification.
Vitality
Around 20 µl of sperm suspension was placed on the slide and mixed with same volume of 0.4% trypan blue and incubated for 2 minutes at room temperature then smeared with another slide. Slides were viewed by bright-field microscope under 40 × magnifications. Dead sperm loses their membrane permeability, allowing the dye to enter into the cytoplasm making it appear thick blue in color. Whereas the cytoplasm of live sperm is not stained with trypan blue and appear lighter. 200 sperms were counted for each slide and viable sperm percentage was calculated.
Histology of testes
Tissues were fixed with 10% buffered formalin, dehydrated with alcohol, cleared with xylene, impregnated with paraffin, embedded in paraffin wax, sectioned with microtome to obtain 4-5 µm thick paraffin sections, Dewaxed sections were stained with hematoxylin and eosin and microphotographs were quantified with image J software.
Statistical analysis
Data obtained were expressed in Mean ± SD. Statistical analysis was done by one-way ANOVA followed by multiple comparisons by two-tailed t-test by SPSS 21.0 software. The values for P < 0.05 were considered to be statistically significant.
Results
Body weight
All stress induced groups gained more body weight than control. Among the four groups, group 4 gained more body weight when compared with control and other stress-induced groups (group 2 and 3) [Fig-1].
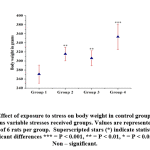 |
Figure 1: Effect of exposure to stress on body weight in control group compared with various variable stresses received groups. |
Corticosterone levels
Serum corticosterone levels were much elevated in group 4, followed by group 3 and group 2 stress induced rats [Fig- 2].
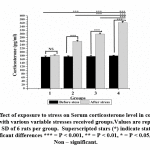 |
Figure 2: Effect of exposure to stress on Serum corticosterone level in control group compared with various variable stresses received groups. |
Motor activity and coordination
Rotarod, Actophotometer and inverted screen test result showed reduced motor activity in group 3 and 4 but not in group 2 as compared to that of control [Fig- 3, 4, 5]. All stress received groups showed a significant reduction of motor coordination. Moreover, the result indicates that adult stress causes a considerable reduction in motor coordination than pubertal or prepubertal stress.
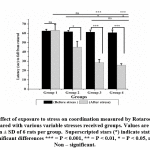 |
Figure 3: Effect of exposure to stress on coordination measured by Rotarod in control group compared with various variable stresses received groups. |
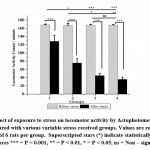 |
Figure 4: Effect of exposure to stress on locomotor activity by Actophotometer in control group compared with various variable stress received groups. |
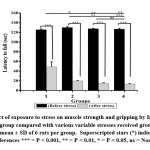 |
Figure 5: Effect of exposure to stress on muscle strength and gripping by Inverted screen test in control group compared with various variable stresses received groups. |
Sperm Concentration
Sperm count showed considerably lower concentration in all stress induced groups when compared with control. Among the stress-induced groups, group 4 animals had a severe reduction in sperm count [Table – 1].
Sperm Motility
Sperm motility was graded as progressive motile, non-progressive motile, immobile. Progressive motile sperm number was reduced in all stress induced groups compared with control. There is no considerable change in non-progressive motile sperms between control and stress-induced animals but the significantly high amount of immotile sperms in stress-induced groups [Table 1].
Sperm morphology
All stress induced groups showed significantly less number of normal sperms than control. Tail abnormalities were substantially higher in stress-induced groups than control. Teratozoospermia was markedly observed in group 4 animals with head and tail abnormality. The percentage of normal sperm was severely reduced [Table -1].
Sperm viability
In trypan blue staining, control animal had significantly more number of live spermatozoa than the stress induced groups, group 4 shown significantly less number of viable cells than group 2 and group3 [Table -1].
Table 1: Caudal epididymal sperm count, motility, morphology and viability in control and stress received groups. Values are represented as mean ± SD of 6 rats per group.
| Sperm parameters | Group 1 | Group 2 | Group 3 | Group 4 | p-value | |
| Sperm concentration ( × 106) (mean ± SD) | 66.6 ± 2.9 | 64.0± 0.91 | 47.61 ± 1.31 | 39.0 ± 0.65 | < 0.00001 | |
| Sperm Motility | Progressive motile (mean ± SD) | 61.7 ± 4.2 | 57.8 ± 4.5 | 46.8 ± 2.6 | 36.8 ± 1.2 | < 0.00001 |
| Non- progressive motile (mean ± SD) | 19.3 ± 2.1 | 19.7 ± 3.1 | 19.2 ± 3.1 | 18.2 ± 1.9 | < 0.84868 | |
| Immotile (mean ± SD) | 19.5 ± 1.8 | 22.5 ± 4.1 | 34.3 ± 3.8 | 45 ± 2.8 | < 0.00001 | |
| Sperm morphology | Normal sperm (mean ± SD) | 66.8 ± 3.2 | 64.3 ± 2.9 | 55.0 ± 1.4 | 47.0 ± 1.2 | < 0.00001 |
| Sperms with tail abnormality (mean ± SD) | 26.5 ± 2.4 | 27.5 ± 3.4 | 30.8 ± 1.1 | 39.8 ± 1.3 | < 0.00001 | |
| Sperm with head abnormality (mean ± SD) | 6.6 ± 2.1 | 8.1 ± 1.7 | 13.8 ± 1.1 | 14.8 ± 1.8 | < 0.00001 | |
| Viability | Live sperm (mean ± SD) | 96 ± 1.4 | 91.3 ± 1.2 | 91.3 ± 2.0 | 83.2 ± 2.5 | < 0.00001 |
| Dead sperm (mean ± SD) | 4 ± 1.4 | 8.7 ± 1.2 | 9 ± 1.5 | 16.8 2.5 | < 0.00001 |
Weight of the testes
Testicular weight was significantly reduced in stress received rats than the control; the weight of group 4 animal’s testes was much lower than the remaining stress induced groups [Table – 2].
Table 2: Histomorphometry of testes in control and stress received groups. Values are represented as mean ± SD of 6 rats per group.
| Testes parameters | Group 1 | Group 2 | Group 3 | Group 4 | p-value | |
| Weight of the testes | 1.7 ± 0.05 | 1.4 ± 0.04 | 1.3 ± 0.03 | 0.85 ± 0.04 | < 0.004957 | |
| Histomorphometry of testes | Diameter of seminiferous tubules | 53.8 ± 0.2 | 46.9 ± 0.4 | 42.8 ± 0.2 | 32.06 ± 0.6 | < 0.00001 |
| Intertubular space | 1.6 ± 0.2 | 11.1 ± 0.16 | 21.7 ± 0.4 | 33.9 ± 0.4 | < 0.00001 | |
| Number of spermatogonia | 353.6 ± 6.02 | 265.6 ± 6.1 | 230 ± 4.0 | 60 ± 3.0 | < 0.00001 |
Histology of testis
Microphotographs of testes suggesting the reduced diameter of seminiferous tubule and increased intertubular space in all stress received groups than control [Table – 2, Fig- 6]. Group 1, 2 and 3 showed normal and complete spermatogenesis, group 3 showed mild incomplete spermatogenesis but group 4 showed less than 25 % complete spermatogenesis, remaining 75 % tubules showed incomplete, only primary spermatocyte and sertoli cells were observed. Sever maturation arrest and calcification of capillary wall was observed [Fig- 6].
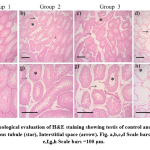 |
Figure 6: Morphological evaluation of H&E staining showing testis of control and stress received rats. Seminiferous tubule (star), Interstitial space (arrow). Fig. a,b,c,d Scale bars =200 μm. Fig. e,f,g,h Scale bars =100 μm. |
Discussion
Stressors activate the sympathetic nervous system and the hypothalamic pituitary adrenal (HPA) axis to assist the individual to deal with the situation. The initial one is short which turn-on the fight or flight response. The second mechanism is a slow and more extended hormonal reaction mediated by the HPA axis, this is initiated by neurons of the paraventricular nucleus of the hypothalamus, they secrete corticotropin-releasing hormone (CRH), which will give signals to the hypophyseal gland to release adrenocorticotropic hormone (ACTH), which in turn activates the suprarenal glands to produce glucocorticoids (i.e., cortisol in human beings and corticosterone in most of the rodent species).
After the withdrawal of stress, the glucocorticoids act though negative feedback mechanism on the hypophyseal gland and various parts of the brain, such as the hypothalamus, hippocampus, and prefrontal cortex, finally ending the reaction by decreasing the further secretion and release of CRH and ACTH [45]. So, in the context of the Hypothalamo – hypophysial axis to stress, the brain is both the activator and target of the glucocorticoid response. The glucocorticoids are accountable for many of the adaptive physiological and behavioral responses to stressors namely mobilization of energy provisions, increasing the immune response, and intensify learning and memory abilities. Though, continuous exposure to these hormones can result in several defective transformation outcomes, such as metabolic problems and defective immune response and cognitive functions. Stress significantly raises the plasma corticosterone, frequent activation with a lower potentiality stressor leads to habituation of the HPA axis, but the lower frequency and/or higher potentiality will induce more elevation of response [46]. The role of the hippocampus is in the control of various vegetative functions, such as ACTH secretion, and provides confirmation that repeated exposure of immobilization stress can leads to atrophy of hippocampus [47]. Taking into consideration the impending role of the hippocampus in glucocorticoid production, dendritic atrophy because of stress may have contributed to high corticosterone levels after continuous restraint stress. In the current study results conclude that stress can cause a considerable rise in the serum corticosterone levels due to activation of the hypothalamic-pituitary-adrenal axis.
Most of the studies have engaged with one stressor to check the consequences of stress, which may cause habituation to that stressor and an increase in the intensity of the same stressor may cause pain. In the present study, five different stressors have used at the rate of one per the day, so that animals do not get habituated. Serum corticosterone is one of the stress markers in animals, results of this study support that stress can cause significant elevation of serum corticosterone levels.
Stress is also one of the risk factor for obesity [48] stress might elevates the incidence of obesity [49, 50], stress causes reduced appetite in the beginning, but chronic stress actually increases hunger and stressed people intended to take more saturated fat and carbohydrate [51, 52]. Our results suggest that variable stress received animals show significantly higher body weight than control, long-term stress can induce more obesity than short-term stress.
Chronic restraint stress causes hypoactivity in Wister albino rats which might represent a reduced input to interact with the environment which might justify the diminished searching behavior [2]. In the present study variable stress as one per day also causes decreased motor coordination, locomotor activity, and muscle strength and gripping. Interestingly animals in the pubertal age receiving stress didn’t show any significant loss of motor activity due to age factor and weight of the body.
There are numerous factors like environment, Certain drugs namely, supplemental testosterone, anabolic steroids, tobacco, marijuana, narcotics, 5-alpha-reductase inhibitors, PDE5 inhibitors, ketoconazole, and some ecological and lifestyle factors like sedentary life, exposure to heat, varicocele, industrial chemicals (e.g. benzenes, toluene), pesticides and heavy metals, processed meats, soy (contains isoflavones which mimic estrogen, a primary female sex hormone), Smoking, Alcohol, heat and radiation from mobile phones, radiotherapy, Obesity, Reactive Oxygen Species, Skipping sex for too long, untreated infections like epididymitis, orchitis and some sexually transmitted infections (STI), including HIV and gonorrhea [53,54], can have a harmful consequences on male fertility. Interestingly Nonsteroidal Anti inflammatory drugs (NSAIDs) like nimesulide also diminish the motility of the sperm [55]. The reproductive act of men is estimated via semen analysis, by evaluating sperm concentration, motility, and morphology. These parameters give an idea about sperm quality [56, 57].
Effect of stress on human spermatogenesis was first researched by [58] on death-sentenced prisoners and has found a considerable decrease in spermatogenesis. Immobilization stress may cause hyposensitivity of interstitial cells of Leydig to gonadotropin [59], elevated glucocorticoids act through glucocorticoid receptors on interstitial cells of Leydig, hence decreasing the testicular response to gonadotropins [60]. Stress produces a deleterious effect on animal and human semen quality, [61,62] reduced spermatid production, the density of spermatozoon, testosterone concentration in plasma and amount of sustentacular cell of Sertoli in the cross-section of the seminiferous tubule. So, chances of alterations in the spermatogenesis are more, which might lead to reduced fertility rate in stressed males observed [63]. ACTH increases the serum corticosteroids levels, which will reduce the episodic secretion pattern of luteinizing hormone and testosterone and conjointly decreases the basal secretion rate of testosterone [28, 64]. Stress initiates the HPA axis, which inhibits the sperm production by reducing the hypothalamic pituitary- testicular axis [65]. Long term restraint stress elevates the rate of acrosome reaction of sperm and head abnormalities in a rat [66]. So mild to severe stress reduces the testosterone levels resulting in compromised spermatogenesis and quality. The changes in the histology of testis suggestive of degeneration such as immature giant spermatids and alterations in cytoearchitecture of testis intestitium are usually observed following administration of certain drugs especially NSAIDS [67]. Administration of Trazodone to treat depression and anxiety result in toxic effect on male reproduction [68]. Histopathological examination is usually employed as an essential biomarker in toxicity study [69] decreased sperm quality and quantity will be combined with Histopathological changes in the testes [70]. Our study results concluded that short-term stress causes reduced the seminiferous tubular diameter, reduced number of spermatogonial cells in the tubules and increased intertubular space.
Strengths, Limitations and future recommendations
The present study has used multiple stressors, one per a day to avoid the animal from getting acclimatized to a particular stress. Since multiple stressors have been applied it is difficult to estimate or identify the impact of every single stressor independently. The notion of changing stressors randomly is mainly to extrapolate the results in humans in future.
Further studies are necessary to estimate the amount of stress caused by each stressor independently and transgenerational epigenetic studies are necessary to analyze the impact of stress on their off springs.
Conclusion
To conclude stress induced at different age period of animals using multimodal stress leads to elevated serum corticosterone and body weight, decreased motor activity, sperm quality and degenerative changes in the testis. This may be due to the disturbance in the hypothalamic-pituitary-testicular axis as mentioned above.
Acknowledgement
The authors appreciatively acknowledge Chettinad Academy Research and Education for funding project and support.
Funding Source
Chettinad Academy Research and Education, Kelambakkam, Chennai, Tamil Nadu, India.
Conflict of interest
The authors declare no conflicts of interest
References
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994 Jan 1; 19(4):313-33.
- Stone EA, Manavalan SJ, Zhang Y, Quartermain D. Beta adrenoceptor blockade mimics effects of stress on motor activity in mice. Neuropsychopharmacology. 1995 Feb; 12(1):65.
- Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, Schlegel PN, Howards SS, Nehra A, Damewood MD, Overstreet JW. Best practice policies for male infertility. Fertility and sterility. 2002 May 1; 77(5):873-82.
- World Health Organization. Infertility definitions and terminology. [Online] Available from: who.int/reproductivehealth/topics/ infertility/definitions/en/ (accessed 24 January 2019).
- Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, Van der Poel S. The international committee for monitoring assisted reproductive technology (ICMART) and the world health organization (WHO) revised glossary on ART terminology, 2009. Human reproduction. 2009 Oct 4; 24(11):2683-7.
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Mathers CD, Stevens GA. Trends in primary and secondary infertility prevalence since 1990: a systematic analysis of demographic and reproductive health surveys. The Lancet. 2013 Jun 17; 381:S90.
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Human reproduction. 2007 Jun 1; 22(6):1506-12.
- Agarwal A, Samanta L, Bertolla RP, Durairajanayagam D, Intasqui P. Proteomics in Human Reproduction: Biomarkers for Millennials. Springer; 2016 Dec 9.
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reproductive Biology and Endocrinology. 2015 Dec; 13(1):37.
- Brugh VM, Lipshultz LI. Male factor infertility: evaluation and management. Medical Clinics. 2004 Mar 1; 88(2):367-85.
- Carlsen E, Giwercman A, Keiding N, Skakkebæk NE. Evidence for decreasing quality of semen during past 50 years. British Medical Journal. 1992 Sep 12; 305(6854):609-13.
- Jørgensen N, Andersen AG, Eustache F, Irvine DS, Suominen J, Petersen JH, Andersen AN, Auger J, Cawood EH, Horte A, Jensen TK. Regional differences in semen quality in Europe. Human reproduction. 2001 May 1; 16(5):1012-9.
- Aitken RJ. Falling sperm counts twenty years on: where are we now?. Asian journal of andrology. 2013 Mar; 15(2):204.
- Sengupta P, Borges Jr E, Dutta S, Krajewska-Kulak E. Decline in sperm count in European men during the past 50 years. Human & experimental toxicology. 2018 Mar; 37(3):247-55.
- Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nature medicine. 2008 Nov; 14(11):1197.
- World Health Organization. Infecundity, infertility, and childlessness in developing countries. DHS Comparative Reports. 2004; 9.
- Talwar PP, Go OP, Murali IN. Prevalence of infertility in different population groups in India and its determinants. Statistics and demography. New Delhi: National Institute of Health & Family Welfare & Indian Council of Medical Research. 1986.
- Unisa S. Childlessness in Andhra Pradesh, India: treatment-seeking and consequences. Reproductive Health Matters. 1999 Jan 1; 7(13):54-64.
- Zargar AH, Wani AI, Masoodi SR, Laway BA, Salahuddin M. Epidemiologic and etiologic aspects of primary infertility in the Kashmir region of India. Fertility and sterility. 1997 Oct 1; 68(4):637-43.
- Kumar TA. In vitro fertilization in India. Current Science. 2004 Jan 25; 86(2):254-6.
- Sadock BJ, Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: Behavioral sciences/clinical psychiatry. Lippincott Williams & Wilkins; 2011 Dec 26.
- Velu A, Prasad G. Epidemiologic aspects of male infertility. International Journal of Reproduction, Contraception, Obstetrics and Gynecology. 2017 Jul 26; 6(8):3362-5.
- Amelar, Richard D., and Lawrence Dubin. “Other factors affecting male fertility.” 1977: 69-101.
- Louis GM, Platt RW. Reproductive and perinatal epidemiology. Oxford University Press; 2011 Mar 23.
- Fenster L, Katz DF, Wyrobek AJ, Pieper C, Rempel DM, Oman D, Swan SH. Effects of psychological stress on human semen quality. Journal of Andrology. 1997 Mar 4; 18(2):194-202.
- Hjollund NH, Bonde JP, Henriksen TB, Giwercman A, Olsen J, Danish First Pregnancy Planner Study Team. Reproductive effects of male psychologic stress. Epidemiology. 2004 Jan 1:21-7.
- Pook M, Krause W, Röhrle B. Coping with infertility: distress and changes in sperm quality. Human Reproduction. 1999 Jun 1; 14(6):1487-92.
- Clarke RN, Klock SC, Geoghegan A, Travassos DE. Relationship between psychological stress and semen quality among in-vitro fertilization patients. Human Reproduction. 1999 Mar 1; 14(3):753-8.
- Johnson BH, Welsh Jr TH, Juniewicz PE. Suppression of luteinizing hormone and testosterone secretion in bulls following adrenocorticotropin hormone treatment. Biology of reproduction. 1982 Mar 1; 26(2):305-10.
- Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiology & behavior. 2007 Jan 30; 90(1):29-35.
- Suarez M, Fiol de Cuneo M, Vincenti L, Ruiz RD. Changes in corticosterone levels and sperm functional activity by chronic stress in rats. Archives of physiology and biochemistry. 1996 Jan 1; 104(3):351-6.
- Short AK, Fennell KA, Perreau VM, Fox A, O’Bryan MK, Kim JH, Bredy TW, Pang TY, Hannan AJ. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Translational psychiatry. 2016 Jun; 6(6): e837.
- Jalali M, Saki G, Sarkaki AR, Karami K, Nasri S. Effect of noise stress on count, progressive and non-progressive sperm motility, body and genital organ weights of adult male rats. Journal of human reproductive sciences. 2012 Jan; 5(1):48.
- Saki G, Rahim F, Alizadeh K. Effect of forced swimming stress on count, motility and fertilization capacity of the sperm in adult rats. Journal of human reproductive sciences. 2009 Jul; 2(2):72.
- LI X, REN L, Weng Q, Trisomboon H, Yamamoto T, Pan L, Watanabe G, Taya K. Effects of acute restraint stress on sperm motility and secretion of pituitary, adrenocortical, and gonadal hormones in adult male rats. Journal of Veterinary Medical Science. 2010:1006220266.
- Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiology & behavior. 2007 May 16; 91(1):55-65.
- Bhosale UA, Yegnanarayan R, Pophale PD, Zambare MR, Somani RS. Study of central nervous system depressant and behavioral activity of an ethanol extract of Achyranthes aspera (Agadha) in different animal models. International Journal of Applied and Basic Medical Research. 2011 Jul; 1(2):104.
- Milovanović M, Milosavljević M, Đorđe MS, Trailović S, Vučinić M, Trailović JN, Marković M, Đurđević D. The effect of carvacrol on inflammatory pain and motor coordination in rats. Acta Veterinaria. 2016 Dec 1; 66(4):478-88.
- Deacon RM. Measuring motor coordination in mice. Journal of visualized experiments: JoVE. 2013(75).
- Deacon RM. Measuring the strength of mice. Journal of visualized experiments: JoVE. 2013(76).
- Akusu MO, Akpokodje JU, Ogwnegbu SO, Oke BO. Differences in morphology of bull spermatozoa from normal and pathological testis during epididymal transit. Nigerian Veterinary Journal. 1985; 14(2):30-3.
- Olugbenga OM, Oniovosa U, Oyeyemi M, Ubiogoro O. Spermiogram and morphological characteristics in testicular and epididymal spermatozoa of Large White Boar in Nigeria. International Journal of Morphology. 2005; 23(3):235-9.
- Suresh S, Prithiviraj E, Prakash S. Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. International journal of andrology. 2010 Feb; 33(1):22-32.
- Organizzazione mondiale della sanità. WHO laboratory manual for the examination and processing of human semen. World Health Organization; 2010.
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Brazilian journal of medical and biological research. 2012 Apr; 45(4):292-8.
- Pitman DL, Ottenweller JE, Natelson BH. Effect of stressor intensity on habituation and sensitization of glucocorticoid responses in rats. Behavioral Neuroscience. 1990 Feb; 104(1):28.
- McEWEN BS, Magarinos AM. Stress Effects on Morphology and Function of the Hippocampusa. Annals of the New York Academy of Sciences. 1997 Jun 1; 821(1):271-84.
- Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, Ruden D, Pietrobelli A, Barger JL, Fontaine K, Wang C. Putative contributors to the secular increase in obesity: exploring the roads less traveled. International journal of obesity. 2006 Nov; 30(11):1585.
- Rennie KL, Jebb SA. Prevalence of obesity in Great Britain. Obesity reviews. 2005 Feb; 6(1):11-2.
- Steptoe A, Kunz-Ebrecht SR, Brydon L, Wardle J. Central adiposity and cortisol responses to waking in middle-aged men and women. International Journal of Obesity. 2004 Sep; 28(9):1168.
- Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids—food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004 Jun 1; 145(6):2633-8.
- Freeman LM, Gil KM. Daily stress, coping, and dietary restraint in binge eating. International Journal of Eating Disorders. 2004 Sep; 36(2):204-12.
- Male Infertility: 10 Factors That Affect Sperm Count | IMI Hong Kong | Dr. Ardyce Yik (ND) | Fertility, Pregnancy, Childbirth – IMI [Internet]. Imi.com.hk. 2019 Available from: https://www.imi.com.hk/male-infertility-10-factors-that-affect-sperm-count.html. [Accessed 29 January 2019].
- Sharma A. Male Infertility; Evidences, Risk Factors, Causes, Diagnosis and Management in Human. Annals of Clinical and Laboratory Research. 2017; 5:188.
- Balaji T, Aruna S, Ramanathan M, Srinivasan M, Menon VP. Suppression of constitutively expressed cyclooxygenase-2 in the epididymis of mice by nimesulide decreases sperm motility. Journal of basic and clinical physiology and pharmacology. 2009; 20(4):357-76.
- Lamb DJ. Semen analysis in 21st century medicine: the need for sperm function testing. Asian journal of andrology. 2010 Jan; 12(1):64.
- Franken DR, Oehninger S. Semen analysis and sperm function testing. Asian journal of andrology. 2012 Jan; 14(1):6.
- Sellandi TM, Thakar AB, Baghel MS. Clinical study of Tribulus terrestris Linn. in Oligozoospermia: A double blind study. Ayu. 2012 Jul; 33(3):356.
- Charpenet G, Tache Y, Bernier M, Ducharme JR, Collu R. Stress-induced testicular hyposensitivity to gonadotropin in rats. Role of the pituitary gland. Biology of reproduction. 1982 Oct 1; 27(3):616-23.
- Orr TE, Mann DR. Role of glucocorticoids in the stress-induced suppression of testicular steroidogenesis in adult male rats. Hormones and Behavior. 1992 Sep 1; 26(3):350-63.
- Wallach E, Steinberger E. The etiology and pathophysiology of testicular dysfunction in man. Fertility and sterility. 1978 May 1; 29(5):481-91.
- Cui KH. The effect of stress on semen reduction in the marmoset monkey (Callithrix jacchus). Human reproduction. 1996 Mar 1; 11(3):568-73.
- Almeida SA, Petenusci SO, Anselmo-Franci JA, Rosa-e-Silva AA, Lamano-Carvalho TL. Decreased spermatogenic and androgenic testicular functions in adult rats submitted to immobilization-induced stress from prepuberty. Brazilian Journal of Medical and Biological Research. 1998 Nov; 31(11):1443-8.
- Kreuz ML, Rose RM, Jennings CJ. Suppression of plasma testosterone levels and psychological stress: A longitudinal study of young men in officer candidate school. Archives of General Psychiatry. 1972 May 1; 26(5):479-82.
- Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biology of reproduction. 1991 Oct 1; 45(4):523-32.
- Arun S, Burawat J, Sukhorum W, Sampannang A, Maneenin C, Iamsaard S. Chronic restraint stress induces sperm acrosome reaction and changes in testicular tyrosine phosphorylated proteins in rats. International Journal of Reproductive BioMedicine. 2016 Jul; 14(7):443.
- Balaji T, Ramanathan M, Menon VP. Localization of cyclooxygenase-2 in mice testis and assessment of its possible role through suppressing its expression using nimesulide: a preferential cyclooxygenase-2 inhibitor. Prostaglandins, leukotrienes and essential fatty acids. 2007 Jun 1; 76(6):341-8.
- Ilgın S, Aydoğan-Kılıç G, Baysal M, Kılıç V, Ardıç M, Uçarcan Ş, Atlı Ö. Toxic effects of trazodone on male reproductive system via disrupting hypothalamic-pituitary-testicular axis and inducing testicular oxidative stress. Oxidative medicine and cellular longevity. 2018 Jul 29; 2018:7196142.
- gov [Online]. Available from:https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM285297.pdf [accessed 29 January 2019].
- Hood RD, Parker RM. Reproductive and developmental toxicology. Preclinical Development Handbook. John Wiley; Hoboken, NJ. 2008 Mar 21:363-422.







