Manuscript accepted on :12-June-2019
Published online on: 21-08-2019
Plagiarism Check: Yes
Reviewed by: Mahmudul Hasan
Second Review by: Md. Sarwar Hossain
Final Approval by: Dr. H Fai Poon
Vijaybabu K1 and Punnagai K2
1Department of Pharmacology Annapoorna Medical College and Hospital Salem 636302, Tamil Nadu, India.
2Department of Pharmacology, Sri Ramachandra Medical College and Hospital Sri Ramachandra Institute of Higher education and Research (Deemed to be University) Porur Chennai Tamil Nadu, India.
Corresponding Author E-mail: punnagaiguna@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1742
Abstract
Squamous cell carcinoma is the second largest among skin cancer diseases. The aim of the present study is to reveal the antiproliferative property of vanilla leaf extract against A431 cells. Antiproliferative property was assessed by MTT assay. Apoptotic property was assessed by DNA fragmentation assay. Antiproliferative property of extract was revealed in a dose dependent manner. IC50 of the extract against A431 cells was revealed at 31.2µg/ml. This study revealed the cancer suppression capability of vanilla leaf extract in skin cancer cell lines.
Keywords
A431 Cell Lines; DNA Fragmentation; MTT Assay; Vanilla Leaf Extract
Download this article as:| Copy the following to cite this article: Vijaybabu K, Punnagai K. In-Vitro Anti-Proliferative Effects of Ethanolic Extract of Vanilla Planifolia Leaf Extract Against A431 Human Epidermoid Carcinoma Cells. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Vijaybabu K, Punnagai K. In-Vitro Anti-Proliferative Effects of Ethanolic Extract of Vanilla Planifolia Leaf Extract Against A431 Human Epidermoid Carcinoma Cells. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/2ZeZxg5 |
Introduction
Cancer is the major and common among various diseases worldwide. Among various types of cancer, skin cancer is the common type. Squamous cell carcinoma is the second largest among skin cancer diseases.1-3 The treatment for this disease include surgical management and medical management including topical and systemic drug therapies.4 Current available medical management include doxorubicin, vincristine, cisplatin, 5-fluorouracil and bleomycin. But these drugs cause more side effects due to lack of specificity in drug action.5 Many herbal extracts have been shown to possess anticancerous effects with less side effects against skin cancer in the recent researches.6-8 Vanilla planifolia (vanilla)is a well known plant which is extensively used as flavouring agent in various food and medicinal products. Because of growing interest for natural products or phytochemicals and their medicinal values, recent studies had shown many medicinal properties of V. planifolia. The flavouring agent was obtained majorly from beans of vanilla. This included vanillin, vanillic acid which comprised 250 compounds altogether.9,10 Another study revealed that out of 200 compounds, 26 compounds were at a concentration of 1mg.11-13 Vanilla was first discovered by Aztecs who were Mexicans during 1300’s. Aztecs were the first to use it as flavouring agent in drinks. This crop was native to Mexico, due to absence of natural pollinator outside Mexico as stated by the botanist Charles Morren (1836) and this led to the discovery of vanilla’s artificial pollination.14 With this it has explored to other parts of world. Use of vanilla plant as health food agent had ethnical variation. Aztecs used for its stimulant, carminative and aphrodisiac properties. In Venezuela it is used for treating fever and spasm. Argentinian used it for spasms and sexual dysfunctions. Palauans used this plant for treating dysmenorrhoea, fever and hysteria.15 Recently Sophie et al. 2003 has revealed its protective properties against free radicals in skin.16 The leaf extracts of vanilla species were revealed to possess compounds that have mosquito larvicidal properties at a dose of 0.1-0.2mg/ml.17 A431 cells were reported to have tumor forming cells similar to squamous cell carcinoma and they were profoundly used in skin cancer research.18 Till date vanilla plant extract has never been tried to explore its anticancerous effects in these cell lines. This study aimed to investigate the anticancer effects of vanilla leaf extract against these cell lines which may benefit the patients with skin cancer.
Materials and Method
Cell Line and Culture
A431 cells were bought from National Centre for Cell Sciences, Pune. The cultured cells were maintained in cell culture media, Minimal Essential Medium (MEM) composed with antibiotics like streptomycin (100 μg/ml) and penicillin (100 U/ml), 10% fetal bovine serum (FBS) in a suitable temperature (37°C) and atmosphere(5% CO2).
Reagents
FBS and MEM were purchased from Cistron laboratories (Chennai, India) and Hi Media Laboratories (Mumbai, India) respectively. Dimethyl sulfoxide (DMSO), methyl thiazolyl diphenyl- tetrazolium bromide (MTT) and Trypsin were obtained from Sisco research laboratory chemicals (Mumbai, India). Other reagents and chemicals were provided by Sigma Aldrich (Mumbai).
Extract Procedure
Vanilla leaves were collected from yercaud and authenticated by Dr. A. Balasubramanian, Botanist and Siddha Research Consultant (AYUSH). The leaves were shade dried and powdered. The powdered was further treated with ethanol to obtain ethanolic extract. The procedure followed Sun et al. 2001, with slight modifications.17 Two hundred grams of powdered extract was treated with 500 ml of ethyl alcohol (98%) and allowed to stand for 2 days. Then the procedure was repeated twice with the precipitate. The obtained alcohol was freezed and filtered. The obtained solution was lyophilized to produce powdered extract of vanilla leaves.
Cytotoxicity Assay
The cytotoxicity assay included 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl–tetrazolium bromide (MTT) assay. Culture media specific to this assay was prepared as suggested by previous literature.19 A431 cells were treated with MTT and sample with a dose concentration of 1000µg/ml to 7.8µg/ml dilution. This was compared with control cells. The reaction absorbance was observed by spectrophotometer at 570nm. The cell viability was calculated as per standard formula.20
Table 1: Anticancer effect of Vanilla planifolia leaf extract on A431 cells by MTT Assay.
|
S.No |
Concentration (µg/ml) | Dilutions | Absorbance (O.D) | Cell viability (%) |
| 1 | 1000 | Neat | 0.184 | 13.77 |
| 2 | 500 | 1:1 | 0.273 | 20.43 |
| 3 | 250 | 1:2 | 0.388 | 29.04 |
| 4 | 125 | 1:4 | 0.516 | 38.62 |
| 5 | 62.5 | 1:8 | 0.592 | 44.31 |
| 6 | 31.2 | 1:16 | 0.668 | 50.00 |
| 7 | 15.6 | 1:32 | 0.783 | 58.60 |
| 8 | 7.8 | 1:64 | 0.927 | 69.38 |
| 9 | Cell control | – | 1.336 |
100 |
DNA fragmentation Assay
The assay method was performed as described by Wyllie et al. (1980).21 UV transilluminator (Uvitec, England) was used for analysing the DNA fragmentation. The A431 cells were plated and incubated. Then DNA was extracted using manufacturer’s protocol. The final DNA sample was mixed with Tris-Taps-EDTA buffer and processed for electrophoresis. DNA was visualized using UV Transilluminator.
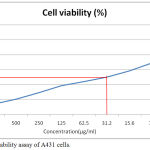 |
Figure 1: Cell viability assay of A431 cells.
|
Results
Cytotoxicity of Vanilla Leaf Extract on A431 Cells
Vanilla leaf extract has shown cytotoxicity to cancer cells in a dose dependent manner. With increase in dose of the extract from 7.8µg/ml, 15.6, 31.2, 62.5, 125, 250, 500, 1000µg/ml there was significant decrease in the percentage of cell viability of A431 cells in the culture starting from (69.38%, 58.60, 50.00, 44.31, 38.62, 29.04, 20.43, 13.77% respectively). The IC50 of the plant extract was calculated to be 31.2µg/ml.
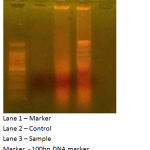 |
Figure 2: Apoptotic effects of vanilla leaf extract on A431 cells.
|
Apoptotic Effects of Vanilla Leaf Extract on A431 Cells
The DNA fragmentation assay with gel electrophoresis method has revealed the DNA fragmentation which was not seen in control A431 cells. But in the cell line (A431) treated with vanilla leaves, the DNA is damaged because of the activity of the sample, the DNA was fragmented.
Discussion
A431 cells has provided a better match to squamous cell carcinoma cells for research in skin cancer.18 A431 cells were characterized by many studies. Epidermal growth factor (EGF) was related to proliferation of A431 cells and their presence was reported by many studies. Kawamoto et al. (1983) showed that high affinity EGF receptors were involved in proliferation of A431 cells.22 The same was revealed by Xu et al. (1984).23 Parker et al. (1984) revealed A431 cells as immunogens which can produce anti-receptor monoclonal antibodies that are expressed only in this cells.24 Walker et al. (1988) revealed that stimulation of EGF was related to phosphotidylinositol kinase.25 Using this highly characterized cancer cells, we studied the anticancerous screening of vanilla leaf extract. The results of this study revealed the benefits of vanilla leaf extracts against skin cancer type. DNA fragmentation assay using gel electrophoresis method demonstrated the apoptotic effects of the vanilla leaf extract. The anti-proliferative properties of the vanilla leaf extract was shown in this study using cytotoxicity assay. MTT assay showed effective anti-cancerous activity of extract against A431 cells. The assay detects the reduction of MTT salt to blue formazan product by mitochondrial dehydrogenase, which indicates the cell viability.26 The cell viability of A431 cells decreased with increase of extract dose confirming the anti-cancerous property of the extract. Many studies have revealed the anticancerous properties of various plant extracts using A431 cells.27-30 Wu et al. (2011) revealed the reduction of migration and invasiveness of A431 cells through inhibition of ezrin by Bacalein. Ezrin protein was reported to enhance tumor metastasis and this protein is highly expressed in skin cancer.31-37 Rabdosia rubescens plant extract was shown to possess apoptotic activity through tyrosine kinase pathway in A431 cells. Oridonin was revealed as the active compound in Rabdosia rubesecens.38-42 The results of the present study may be helpful to characterize the vanilla leaf extract for screening anticancerous property against skin cancer in future studies.
 |
Figure 3: Graphical Abstract.
|
Conclusion
The present study concludes the antiproliferative and apoptotic effects of vanilla leaf extracts against A431 cells [Figure 3]. The antiproliferative effects of vanilla leaf extract was shown in a dose dependent manner. Vanilla leaf extract might contain the lead molecule which may be developed as chemotherapeutic agent for treating skin cancer of squamous cell carcinoma type. Further research is required to characterize and understand the molecular aspects of the anticancerous effects of this extract.
Conflict of Interest
There is no conflict of interest.
Acknowledgements
The author(s) received no specific funding for this work.
References
- Karia P.S, Han J, Schmults C.D. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States 2012. J Am Acad Dermatol., 2013; 68(6): 957–66.
- Christenson L.J, Borrowman T.A, Vachon C.M, Tollefson M.M, Otley C.C, Weaver A.L, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA., 2005; 294(6): 681– 90.
- Basra M.K, Shahrukh M. Burden of skin diseases. Expert Rev Pharmacoecon Outcomes Res., 2009; 9(3): 271–83.
- Rudnick E.W, Thareja S, Cherpelis B. Oral therapy for non melanoma skin cancer in patients with advanced disease and large tumor burden: a review of the literature with focus on a new generation of targeted therapies. Int J Dermatol., 2016; 55(3): 249–58.
- Soura E, Chasapi V, Stratigos A.J. Pharmacologic treatment options for advanced epithelial skin cancer. Expert Opin Pharmacother., 2015; 16(10): 1479–93.
- dela Cruz J.F, Kim Y.S, Lumbera W.M, Hwang S.G. Viscum Album Var Hot Water Extract Mediates Anti-cancer Effects through G1 Phase Cell Cycle Arrest in SK-Hep1HumanHepatocarcinoma cells. Asian Pac J Cancer Prev., 2015; 16(15): 6417–21.
- Esmaeilbeig M, Kouhpayeh SA, Amirghofran Z. AnInvestigation of the Growth Inhibitory Capacity of Several Medicinal Plants From Iran on Tumor Cell Lines. Iran J Cancer Prev. 2015; 8(5): e4032.
- Reinau D, Surber C, Jick S.S, Meier C.R. Nonsteroidal anti-inflammatory drugs and the risk of nonmelanoma skin cancer. Int J Cancer., 2015; 137(1): 144–53.
- Guarino P.A, Brown S.M. Liquid chromatographic determination of vanillin and related flavor compounds in vanilla extract: cooperative study. Assoc. Off. Anal. Chem., 1985; 68: 1198–1201.
- Dignum M.J.W, Kerker J, Verpoorte R. Vanilla production: technological, chemical, and biosynthetic aspects. Food Rev. Int., 2001; 17: 199–219.
- Westcott R.J, Cheetham P.S.J, Arraclough A.J.B. Use of organized viable vanilla plant aerial roots for the production of natural vanillin. Phytochemistry., 1994; 35: 135138.
- Bettazzi F, Palchetti I, Sisalli S, Mascini M. A disposable electrochemical sensor for vanillin detection. Anal Chim Acta., 2006; 555: 134138.
- Sharma A, Verma S.C, Saxena N, Chadda N, Singh N.P, Sinha A.K. Microwave and ultrasound assisted extraction of vanillin and its quantification by high-performance liquid chromatography in Vanilla planifolia. J Sep Sci., 2006; 29: 613-619.
- Reineccius G. 1997. Source book of flavours. 2nd ed. New Delhi: CBS Publishers and Distributors, # New York: Chapman & Hall, Inc., pp 351-360.
- Duke JA, Bogenschutz-Godwin MJ, duCellier J, Duke PK. 2003. CRC handbook of medicinal spice. Boca Raton, FL: CRC Press., pp 303-308.
- Sophie L, Francois M.J. 2003. Use of a composition containing a vanilla extract to protect the skin against superoxide radicals or to limit the appearance of sunburn cells. French patent FR 2837384.
- Sun R, Sacalis J.N, Chin C.K, Still C.C. Bioactive aromatic compounds from leaves and stems of Vanilla fragrans. Journal of agricultural and food chemistry., 2001; 49(11): 5161-4.
- Adhikary G, Grun D, Kerr C, et al. Identification of a population of epidermal squamous cell carcinoma cells with enhanced potential for tumor formation. PLoS One., 2013; 8(12): e84324.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods., 1983; 65: 55-63.
- Khodaie F, Khazaei-Poul Y, Moini-Zanjani T. Anti-Proliferative Effects of Piroxicam and Nimesulide on A431 Human Squamous Carcinoma Cell Line. International Journal of Cancer Management., 2017; 10(4).
- Wyllie AH. Cell death. In Cytology and Cell Physiology (Fourth Edition) 1987 (pp. 755-785).
- Kawamoto T, Sato J.D, Le A, Polikoff J, Sato G.H, Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proceedings of the National Academy of Sciences., 1983; 80(5): 1337-41.
- Xu YH, Richert N, Ito S, Merlino GT, Pastan I. Characterization of epidermal growth factor receptor gene expression in malignant and normal human cell lines. Proceedings of the National Academy of Sciences., 1984; 81(23): 7308-12.
- Parker P.J, Young S, Gullick W.J, Mayes E.L, Bennett P, Waterfield M.D. Monoclonal antibodies against the human epidermal growth factor receptor from A431 cells. Isolation, characterization, and use in the purification of active epidermal growth factor receptor. Journal of Biological Chemistry., 1984; 259(15): 9906-12.
- Walker D.H, Dougherty N, Pike L.J. Purification and characterization of a phosphatidylinositol kinase from A431 cells. Biochemistry., 1988; 27(17): 6504-11.
- Abbas Momtazi-borojeni A, Behbahani M, Sadeghi-aliabadi H. Antiproliferative activity and apoptosis induction of crude extract and fractions of avicennia marina. Iranian journal of basic medical sciences., 2013; 16(11): 1203.
- Yukio Nakamura MD. Apoptotic induction of skin cancer cell death by plant extracts. J Med Assoc Thai., 2013; 96(1): S60-4.
- Akindele A.J, Wani Z.A, Sharma S, Mahajan G, Satti N.K, Adeyemi O.O, Mondhe D.M, Saxena A.K. In vitro and in vivo anticancer activity of root extracts of Sansevieria liberica Gerome and Labroy (Agavaceae). Evidence-Based Complementary and Alternative Medicine., 2015.
- Mohansrinivasan V, Devi C, Deori M, Biswas A, Naine S. Exploring the anticancer activity of grape seed extract on skin cancer cell Lines A431. Brazilian Archives of Biology and Technology., 2015; 58(4): 540-6.
- Yajarla V.N, Nimmanapalli R.P, Parikapandla S, Gupta G, Karnati R. Anti-inflammatory and anti-proliferative properties of Chromolaena odorata leaf extracts in normal and skin-cancer cell lines. Journal of herbs, spices & medicinal plants., 2014; 20(4): 359-71.
- Wu B, Li J, Huang D, Wang W, Chen Y, Liao Y et al. Baicalein mediates inhibition of migration and invasiveness of skin carcinoma through Ezrin in A431 cells. Bmc Cancer., 2011; 11(1): 527.
- Palou J, Algaba F, Vera I, Rodriguez O, Villavicencio H, Sanchez-Carbayo M: Protein Expression Patterns of Ezrin Are Predictors of Progression in T1G3 Bladder Tumours Treated with Nonmaintenance Bacillus Calmette-Guerin. Eur Urol., 2009; 56(5): 829-836.
- Kocher H.M, Sandle J, Mirza T.A, Li N.F, Hart I.R: Ezrin interacts with Cortactin to form podosomal rosettes in pancreatic cancer cells. Gut., 2009; 58(2): 271-284.
- Wang G, Mao W, Zheng S: MicroRNA-183 regulates Ezrin expression in lung cancer cells. FEBS Lett., 2008; 582(25-26): 3663-3668.
- Elzagheid A, Korkeila E, Bendardaf R, Buhmeida A, Heikkila S, Vaheri A et al. Intense cytoplasmic ezrin immunoreactivity predicts poor survival in colorectal cancer. Hum Pathol., 2008; 39(12): 1739-1743.
- Li Q, Wu M, Wang H, Xu G, Zhu T, Zhang Y et al. Ezrin silencing by small hairpin RNA reverses metastatic behaviors of human breast cancer cells. Cancer Lett., 2008; 261(1): 55-63.
- Peng S, Fan S, Li X, Wang L, Liu H, Zhou M et al. The expression of ezrin in NPC and its interaction with NGX6, a novel candidate suppressor. Cancer Sci., 2007; 98(3): 341-349.
- Li D.A, Wu L.J, Tashiro S.I, Onodera S.A, Ikejima T.A. Oridonin induces human epidermoid carcinoma A431 cell apoptosis through tyrosine kinase and mitochondrial pathway. Journal of Asian natural products research.. 2008; 10(1): 77-87.
- L. Zhang, L.J. Wu, S.I. Tashiro, S. Onodera, T. Ikejima. Chin. Pharmacol. Bull., 2003; 19; 525.
- Osawa K, Yasuda H, Maruyama T, Morita H, Takeya K, Itokawa H. Antibacterial trichorabdal diterpenes from Rabdosia trichocarpa. Phytochemistry., 1994; 36(5): 1287-91.
- FuJi K, NoDE M, SAI M, FUJITA E, TAKEDA S, UNEMI N. Terpenoids. LIII.: Antitumor Activity of Trichorabdals and Related Compounds. Chemical and Pharmaceutical Bulletin., 1989; 37(6): 1472-6.
- Zhang CL, Wu LJ, Tashiro S, Onodera S, Ikejima T. Oridonin induces a caspase-independent but mitochondria- and MARK-dependent cell death in the murine fibrosarcoma cell line L929. Biol. Pharm. Bull., 2004; 27: 1527–1531.







