Nova Sylviana1,3, Christina Natalia1, Hanna Goenawan1,3, Yuni Susanti Pratiwi1,3, Iwan Setiawan1,3, Vita Murniati Tarawan1,3, Titing Nurhayati1,3, Andri Rezano3, Juliati Juliati1,3, Ambrosius Purba1,3,Unang Supratman3,4 and Ronny Lesmana1,3*
1Physiology Division, Department of Biomedical Science, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
2Cell Biology Division, Department of Biomedical Science, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
3Physiology Molecular Laboratory, Biology Activity Division, Central Laboratory, Universitas Padjadjaran, Bandung, Indonesia.
4Departement of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Bandung, Indonesia.
Corresponding Author E-mail: ronny@unpad.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1759
Abstract
Endurance exercise induces specific skeletal muscle adaptation by increasing mitochondrial oxidative phosphorylation eficiency and mitochondrial biogenesis. Many previous studies suggesting both PGC-1a and COX IV as a potential biomarker of skeletal muscle adaptation induced by exercise. But most of them only studied the effect of long-term endurance exercise, whereas the effect of short-term exercise remains unclear. To investigate short-term physiological adaptation induced by endurance exercise on expression of COX IV and PGC-1a mRNA in rat skeletal muscle. Twenty healthy male Wistar rats (Rattus norvegicus) aged 10-11 weeks old were used in this experiment. Rats were randomly assigned into 4 groups based on the time period of exercise: 1) control (C; n=5), 2) three days of exercise (E3; n=5), 3) six days of exercise (E6; n=5), 4) fifteen days of exercise (E15; n=5). The exercise groups were run at 20m/s for 30 minutes on the rat treadmill and the stationary control group was only placed inside treadmill with the machines turned off. On the last day of exercise, the rats were sacrificed then RNA from skeletal muscle was extracted. COX IV and PGC-1a mRNA expressions were measured by Reverse Transcriptase PCR. The results showed that there were statistically significant differences of PGC-1a mRNA expression levels in both soleus (F(3,16)=3.740, ps=0.033) and gastrocnemius (F(3,16)=3.969, pg=0.027) muscles. The COX IV mRNA expression levels in soleus (F(3,16)=3.801, ps=0.031) and gastrocnemius (F(3,16)=5.429, ps=0.009) muscles were also significantly increased. There were significant increases of PGC-1a and COX IV expressions in fifteen days of exercise group compared to control group in both muscles. Short-term endurance exercise induced mitochondrial biogenesis marker and mitochondrial activity marker by increasing the PGC-1a and COX IV mRNA expression levels in rat skeletal muscle significantly following the time periods of exercise.
Keywords
Biogenesis; Biomarkers; Endurance Exercise; Exercise, Mitochondria; Rats; Skeletal Muscle
Download this article as:| Copy the following to cite this article: Sylviana N, Natalia C, Goenawan H, Pratiwi Y. S, Setiawan I, Tarawan V. M, Nurhayati T, Rezano A, Juliati J, Purba A, Supratman U, Lesmana R. Effect of Short-Term Endurance Exercise on COX IV and PGC-1 mRNA Expression Levels in Rat Skeletal Muscle. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Sylviana N, Natalia C, Goenawan H, Pratiwi Y. S, Setiawan I, Tarawan V. M, Nurhayati T, Rezano A, Juliati J, Purba A, Supratman U, Lesmana R. Effect of Short-Term Endurance Exercise on COX IV and PGC-1 mRNA Expression Levels in Rat Skeletal Muscle. Biomed Pharmacol J 2019;12(3). Available from: http://biomedpharmajournal.org/?p=28503 |
Introduction
Regular physical activity or exercise has been known to improve the health status and well being of a person (1-3). On the other hand, lack of physical activity is the fourth highest risk factor that increases the risk of death. (1-3) Exercise intensity promotes an adaptation in the body that will enhance its physiological functions especially most active tissue during exercise is skeletal muscle.
In skeletal muscle, exercise induces adaptations that are specific to the type of exercise.(4) Aerobic or endurance exercise promotes muscle fibers transformation into type I fibers, increased fatty acid oxidation, increased mitochondrial oxidative phosphorylation efficiency, and mitochondrial biogenesis. These adaptations allow prolonged strenuous endurance activities and high resistance against fatigue which will improve the exercise capacity and individual performance.(5-7)
During training, skeletal muscle adaptation involves complex signaling pathways which will activate several genes involved in mitochondrial biogenesis and mitochondrial oxidative enzyme genes. One of the most important components is peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α).(8, 9) There is a significant increase of PGC-1a expression following acute single bout and long-term endurance exercise in rats and human. (10-13) PGC-1α is a transcription coactivator which function is to mediate gene transcription by binding to transcription factor. It belongs to nuclear receptor proliferator-activated receptor (PPAR) family and known as a master regulator of mitochondrial biogenesis (9). PGC-1α regulates the expression of both nuclear- and mitochondrial-encoded genes in mitochondrial biogenesis. It stimulates nuclear-encoded mitochondrial genes expression by interacting with nuclear transcription factors, it will also activate mitochondrial transcription factor A (TFAM) which is involved in mtDNA transcription. (8, 9)
Previous studies also showed increase in mitochondrial activities and its protein levels such as the electron transport chain enzymes, cytochrome c oxidase (COX), in skeletal muscle of exercised rat compared to sedentary rat.(14, 15) Subunit COX I-III are encoded by mitochondrial DNA (mtDNA) and subunit IV-VIII are encoded by nuclear DNA.(16) Its forth subunit or COX IV has been considered as one of key markers of mitochondrial oxidative capacities in skeletal muscle. Its mRNA and protein expression are used as reflection to assess changes in mitochondrial oxidative activity and content induced by exercise.(15, 17) Both acute and long-term endurance exercise induce the upregulation of COX IV expression.(10, 14, 15, 18)
Thus, many previous studies suggesting both COX IV and PGC-1a as a potential biomarker of skeletal muscle adaptation induced by exercise. Increase in its expression indicates enhanced mitochondrial biogenesis and aerobic capacity. But most of them only studied the effect of acute single bout or long-term endurance exercise to skeletal muscle adaptation. The adaptation process of skeletal muscle in short-term exercise remains uncertainty. The aim of this present study, to investigate the effect of short-term endurance exercise in skeletal muscle of rat. We examined the expression of COXIV and PGC1a mRNAs in soleus and gastrocnemius muscle of rats in three different periods of endurance exercise.
Method
Materials
Modified rat treadmill machine (IDEAS, Bandung, Indonesia) was used for rat’s training in the experiment. The treadmill machine has five lanes in which rats were placed inside each lane (one lane for one rat) for training experiment.
Animals
The subjects of this animal experimental study were 20 healthy male Wistar rats (Rattus norvegicus) which were purchased from PT Biofarma, Bandung. All rats aged 10-11 weeks old with body weight between 250-300 g. They were housed three-four per cage (size : 50cm x 47cm x 45cm) and placed in a room with 12/12 h light/dark cycle, adequate air circulation and controlled temperature (21–23°C). They were fed Rat Bio and given water ad libitum. This study was approved by Health Research Ethics Committee Faculty of Medicine Universitas Padjadjaran No. 936/UN6.KEP/EC/2018. Animals were cared for according to the Guide for the Care and Use of Laboratory Animals.(19).
Treadmill Exercise Protocol
Rats were randomly assigned into 4 groups based on the exercise periods : 1 control group (C; n=6) and 3 exercise groups : 1) three days of exercise (E3; n=5), 3) six days of exercise (E6; n=5), 4) fifteen days of exercise (E15; n=5). The exercise group ran at 20m/s for 30 minutes on the rat treadmill with 0o inclination. This intensity has previously been demonstrated to be at 50-70% of rats V̇O2max.(20) First, second, and third exercise groups ran for 3,6, and 15 days consecutively. The rats exercised for 5 days/week, with rest on Saturday and Sunday. Prior to the beginning of the experiment, the rats were habituated to treadmill exercise for 2 days.(12) The control group was not forced to run and only placed inside the lanes for 30 minutes.
Muscle isolation, RNA extraction and Reverse Transcriptase PCR
On the last day of exercise, the rats were sacrificed in 1-3 hours after exercise. We isolated soleus and gastrocnemius muscle. The muscle was stored inside eppendorf tubes in liquid nitrogen and then -80o C refrigerator until the analyses were performed. We extracted the RNA from the soleus and gastrocnemius muscles. Total RNA were extracted from the skeletal muscle using TRIsure reagent (Bioline, London., UK). Reverse Transcriptase PCR was performed using the PCR Kitv(Bioline, London., UK) and electrophoresis using agarose gel 1,2%. The DNA band of PCR products from agarose gel was visualized by Bluepads detection system (Genetic Science, Indonesia) and quantified using ImageJ Software (NIH). Average intensity of relative gene expression of the samples was normalized by GAPDH mRNA levels. Primer sequences and PCR condition of each gene were provided in Table 1.
Table 1: Primer sequences
| Gene | Sequences | Tm (oC) | Product (bp) |
| PGC-1a | F: CGCACAACTCAGCAAGTCCTC
R: CCTTGCTGGCCTCCAAAGTCTC |
62 | 263 |
| COX IV | F: CTCCCATCTTATGTTGATCG
R: GTACAATTGGACTTTCTCATCC |
53 | 144 |
| GAPDH | F: GTTACCAGGGCTGCCTTCTC
R : GATGGTGATGGGTTTCCCGT |
61 | 177
|
Abbrevations : PGC-1a, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; COX IV, cytochrome c oxidase IV; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; mRNA, messenger ribonucleic acid.
Statistical analysis
All statistics were computed using IBMâ SPSS version 20.0 software for Windows. Quantitative data were expressed as the means ± standard error of mean (means ± SEM). Data were statistically analyzed with one way ANOVA followed by post hoc comparisons using Tukey. The p < 0.05 were considered to be significant.
Result
In present study, we examined the effect short-term endurance exercise at three different periods on mitochondrial biogenesis and its oxidative activities in soleus and gastrocnemius muscle of rats. The PGC-1a and COX IV mRNA expression levels were measured using Reverse Transcriptase PCR. The results are showed in Table 2.
Table 2: Genes expression levels
| Groups
|
PGC-1a mRNA expressions | COX IV mRNA expressions | ||
| Soleus | Gastrocnemius | Soleus | Gastrocnemius | |
| C | 1.005±0.005 | 0.790±0.016 | 0.654±0.020 | 0.946±0.009 |
| E3 | 1.020±0.028 | 0.829±0.029 | 0.715±0.035 | 0.972±0.027 |
| E6 | 1.052±0.033 | 0.856±0.026 | 0.745±0.034 | 0.988±0.013 |
| E15 | 1.121±0.030 | 0.902±0.022 | 0.780±0.016 | 1.043±0.016 |
| p | 0.033 | 0.027 | 0.031 | 0.009 |
Abbreviations : C, control group; E3, three days-exercise group; E6, six days-exercise group; E15, fifteen days-exercise group; PGC-1a, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; COX IV, cytochrome c oxidase IV. Values are presented as means ± SEM. (P < 0.05)
PGC-1a mRNA Expression Levels
There were significant differences of PGC-1a mRNA expression levels in both soleus (F(3,16)=3.740, ps=0.033) and gastrocnemius (F(3,16)=3.969, pg=0.027) muscles on day 14 (Figure 1). The PGC-1a expressions in soleus and gastrocnemius muscle were increased following the periods of exercise (Figure 1). Tukey post hoc test revealed that there is significant increase of PGC-1a expressions in third exercise group (E15s =1.121±0.030, p=0.033) compared to control group (Cs = 1.005±0.005) in soleus muscle. In gastrocnemius muscle, the PGC-1a expression in third exercise group (E15g = 0.902±0.022, p=0.019) was also significantly increased compared to control group (Cg = 0.790±0.016). However, we observed that the differences between other groups were insignificant.
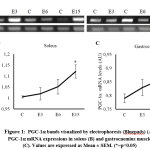 |
Figure 1: PGC-1a bands visualized by electrophoresis (Bluepads) (A), PGC-1a mRNA expressions in soleus (B) and gastrocnemius muscle(C). Values are expressed as Mean ± SEM. (*=p<0.05) |
COX IV mRNA Expressions Levels
We observed that short-term endurance exercise was significantly increased COX IV mRNA expression levels in soleus (F(3,16)=3.801, pS=0.031) and gastrocnemius (F(3,16)=5.429, pG=0.009) muscle. COX IV mRNA levels was increased following the period of exercise (Figure 2). Post hoc comparisons using the Tukey test showed a significant increase of COX IV expressions in third exercise group (E15S = 0.780±0.016, p=0.023) compared to control group (CS = 0.654±0.020) in soleus muscle. The increase of COX IV expressions was also significant between third exercise group (E15G = 1.043±0.016, p=0.006) compared to control group (CG = 0.946±0.009) in gastrocnemius muscle. COX IV mRNA expression levels was not significantly differ between other groups.
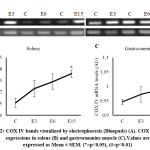 |
Figure 2: COX IV bands visualized by electrophoresis (Bluepads) (A). COX IV mRNA expressions in soleus (B) and gastrocnemius muscle (C). Values are expressed as Mean ± SEM. (*=p<0.05), (#=p<0.01) |
Discussion
Effect of short-term endurance exercise to mitochondrial biogenesis in rats skeletal muscles
Skeletal muscle has a great plasticity and capability to elicit adaptations in response to contractile activity such as during exercise. Those adaptations are necessary to improves physical performance and also health status of person.(7, 21) Exercise-induced adaptations in skeletal muscle are specific depend on the type of exercise.(4) Endurance exercise which is characterized by lower energy ouput with longer duration and high frequency, induce muscle fibers transformation, increased fatty acid oxidation, and decrease lactic acid accumulation rate.(4, 22) Other important exercise-induced adaptation in skeletal muscle are mitochondrial quantitative changes by mitochondrial biogenesis,(23) and qualitative changes by enhancing mitochondrial oxidative phosphorylation capacity(24). Those changes increase the ability to perform prolonged strenuous exercise, for which will increase the performance.(6, 21)
In this study, we examined the mitochondrial biogenesis in skeletal muscle by measuring the PGC-1a mRNA expression level. PGC-1a is widely known as mitochondrial biogenesis master regulator by coactivating transcription factors that control mitochondrial protein genes expressions.(9) Many studies found that long-term or chronic endurance exercise induce mitochondrial biogenesis in skeletal muscle of rats as indicated by increased of PGC-1a protein or mRNA expressions significantly.(11, 14) Prior studies conducted by Strobel et al. (2011) and Huang et al. (2016) observed the exercise-induced mitochondrial biogenesis in rats muscle by measuring PGC-1a mRNA expression after long-term endurance exercise, 14 and 12 weeks of exercise respectively.(11, 25)
In our present study, we found that mitochondrial biogenesis was also increased even in short-term endurance exercise. Increase of mitochondrial biogenesis as indicated by increase of PGC-1a mRNA expression level was significant in both soleus and gastrocnemius muscle of exercised groups, with the most significant effect was seen between control group and fifteen-days exercise group. Similar findings also observed in Suwa et al. (2008) study that PGC-1a expression was increased after 14 days of endurance exercise in rats soleus muscle.(26) These findings indicate that skeletal muscle adaptation is already started in earlier period of exercise. Other study by Vainshtein et al. (2015) found that even acute single bout of endurance exercise also increased PGC-1a mRNA expression significantly, however our findings showed that the increase in first and second exercise group was not significant in this study.(12) Means of PGC-1a expression level in soleus muscle was higher than in gastrocnemius muscle of exercised rats. It was in accordance with previous study by Suwa et al. (2008), which found that PGC-1α protein content in the red oxidative muscles such as soleus muscle was higher than that in the white glycolytic muscles. Although gastrocnemius has red and white portion, PGC-1a expression in red portion of gastrocnemius was still lower than in soleus muscle.(26)
Effect of short-term endurance exercise to mitochondria oxidative capacities in rats muscles
Qualitative changes in exercise-induced adaptation can be observed by assessing mitochondrial enzyme activity. Cytcohrome c oxidase or COX is the last enzyme complex in electron transport chain of mitochondria. Its forth subunit, COX IV has been commonly used as a biomarker for mitochondrial oxidative activity and contents in skeletal muscle.(27) Both acute and chronic endurance exercise has been known to increase mitochondria oxidative capacities as shown by increasing expression levels of COX mRNA or protein. Previous study conducted by Sun et al. (2015) provided evidence of increasing COX IV mRNA expressions in skeletal muscle after 3 weeks of chronic endurance exercise.(15) Acute single bout of endurance exercise was also increased COX IV mRNA expression in skeletal muscle of rats.(12)
In present study, short-term endurance exercise significantly increased COX IV mRNA expressions in soleus and gastrocnemius muscle. In both muscles, the expressions were increased following the periods of exercise. There is significant increase of COX IV expression observed between control group and fifteen days of exercise in both muscle (p=0.030). Increase of COX IV indicated increase of mitochondrial oxidative capacities and its contents. This finding suggests that short-term endurance exercise also increase mitochondrial oxidative as qualitative adaptation in soleus and gastrocnemius muscle of rats. Increase of mitochondrial oxidative phosphorylation and its contents improve muscle aerobic capacities, which will enhance performance in exercise.
We showed that short-term endurance exercise induce upregulation of COX IV and PGC-1a in soleus and gastrocnemius muscles of rats, with most significant increase is between control group and third exercise group. Ju et al. (2016), also observed coincide upregulation of COX IV and PGC-1a mRNA in triceps muscle, but after 8 weeks of endurance exercise. Upregulation of COX IV in exercised rats could be influenced by PGC-1a activity. Prior study showed that PGC-1a induced the expression of nuclear-encoded mitochondrial gene, COX IV.(12) We believe that increase of COX IV expression levels was associated with PGC-1a expression and there is also other adaptation changes occurred in short-term endurance exercise.
Regular exercise has been convinced for its role to improve performance and health. Understanding of the skeletal muscle adaptation at molecular level will provides information to make a correct exercise prescription to enhance performance and therapeutic purpose against diseases. In this study we only observed the mitochondrial biogenesis and activity to assess exercise-induced adaptation in skeletal muscle at mRNA level. Whereas to have a complete understanding in adaptation mechanism in skeletal muscle, we also need to observe at protein level, measuring the aerobic capcity (VO2max) and assess other components such as citrate synthase (CS), or nuclear respiratory factor (NRF). However our study had found increases of PGC-1a and COX IV in skeletal muscle after short-term endurance exercise. These results suggest that PGC-1a and COX IV play important roles in exercise-induced adaptation. We suggest that this findings could be used as a foundation for further study and arranges exercise training program for an optimal performances.
Conclusion
Short term endurance exercise induced mitochondrial biogenesis and activity by increased the PGC-1a and COX IV mRNA expression in skeletal muscle significantly following the periods of exercise, with it most significant differences were between control and fifteen-days exercise group.
Acknowledgements
The authors thank Animal Physiology Laboratory, Faculty of Medicine, and Central Laboratory of Universitas Padjadjaran; Aziiz Mardanian Rosdianto as technical animal handling, Susianti, S.Si as technical assistance; and Central Laboratory, Universitas Padjadjaran that had supported this study
References
- Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241599979_eng.pdf?ua=1.
- Physical Activity and Health. Centers for Disease Control and Prevention; 2018 [updated February 13, 2018]; Available from: https://www.cdc.gov/physicalactivity/basics/pa-health/index.htm.
- Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization; 2011.
- Katch VL, McArdle WD, Katch FI, McArdle WD. Essentials of Exercise Physiology. Philadelphia: Lippincott Williams & Wilkins Health; 2011.
- Booth FW, Ruegsegger GN, Toedebusch RG, Yan Z. Endurance Exercise and the Regulation of Skeletal Muscle Metabolism. In: Bouchard C, editor. Progress in Molecular Biology and Translational Science: Academic Press; 2015. p. 129-51.
- Kim SH, Koh JH, Higashida K, Jung SR, Holloszy JO, Han D-H. PGC-1α mediates a rapid, exercise-induced downregulation of glycogenolysis in rat skeletal muscle. The Journal of Physiology. 2015;593(3):635-43. doi:doi:10.1113/jphysiol.2014.283820.
- Jacobs RA, Rasmussen P, Siebenmann C, Díaz V, Gassmann M, Pesta D, et al. Determinants of time trial performance and maximal incremental exercise in highly trained endurance athletes. Journal of Applied Physiology. 2011;111(5):1422-30. doi:10.1152/japplphysiol.00625.2011. [PubMed:21885805].
- Kang C, Li Ji L. Role of PGC-1α signaling in skeletal muscle health and disease. Annals of the New York Academy of Sciences. 2012;1271(1):110-7. doi:10.1111/j.1749-6632.2012.06738.x. [PubMed:23050972].
- Olesen J, Kiilerich K, Pilegaard H. PGC-1α-mediated adaptations in skeletal muscle. Pflügers Archiv – European Journal of Physiology. 2010;460(1):153–62. doi:https://doi.org/10.1007/s00424-010-0834-0.
- Konopka AR, Suer MK, Wolff CA, Harber MP. Markers of Human Skeletal Muscle Mitochondrial Biogenesis and Quality Control: Effects of Age and Aerobic Exercise Training. The Journals of Gerontology. 2014;69(4):371-8. doi:10.1093/gerona/glt107. [PubMed:23873965].
- Huang C-C, Wang T, Tung Y-T, Lin W-T. Effect of Exercise Training on Skeletal Muscle SIRT1 and PGC-1α Expression Levels in Rats of Different Age. International journal of medical sciences. 2016;13(4):260-70. doi:10.7150/ijms.14586. [PubMed:27076782].
- Vainshtein A, Tryon LD, Pauly M, Hood DA. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. American journal of physiology Cell physiology. 2015;308(9):C710-C9. doi:10.1152/ajpcell.00380.2014. [PubMed:25673772].
- Wang L, Mascher H, Psilander N, Blomstrand E, Sahlin K. Resistance exercise enhances the molecular signaling of mitochondrial biogenesis induced by endurance exercise in human skeletal muscle. Journal of Applied Physiology. 2011;111(5):1335-44. doi:10.1152/japplphysiol.00086.2011. [PubMed:21836044].
- Zoladz JA, Koziel A, Woyda-Ploszczyca A, Celichowski J, Jarmuszkiewicz W. Endurance training increases the efficiency of rat skeletal muscle mitochondria. Pflugers Archiv : European journal of physiology. 2016;468(10):1709-24. doi:10.1007/s00424-016-1867-9. [PubMed:27568192].
- Sun Y, Qi Z, He Q, Cui D, Qian S, Ji L, et al. The effect of treadmill training and N-acetyl-l-cysteine intervention on biogenesis of cytochrome c oxidase (COX). Free Radical Biology and Medicine. 2015;87:326-35. doi:https://doi.org/10.1016/j.freeradbiomed.2015.06.035.
- Kadenbach B, Hüttemann M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion. 2015;24:64–76. doi:http://dx.doi.org/10.1016/j.mito.2015.07.002.
- Ju J-s, Jeon S-i, Park J-y, Lee J-y, Lee S-c, Cho K-j, et al. Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. The Journal of Physiological Sciences. 2016;66(5):417–30. doi:https://doi.org/10.1007/s12576-016-0440-9.
- Saleem A, Hood DA. Acute exercise induces tumour suppressor protein p53 translocation to the mitochondria and promotes a p53–Tfam–mitochondrial DNA complex in skeletal muscle. The Journal of Physiology. 2013;591(14):3625-36. doi:doi:10.1113/jphysiol.2013.252791.
- Guide for the Care and Use of Laboratory Animals 8th ed. Washington: The National Academies Press; 2011.
- Ito D, Cao P, Kakihana T, Sato E, Suda C, Muroya Y, et al. Chronic Running Exercise Alleviates Early Progression of Nephropathy with Upregulation of Nitric Oxide Synthases and Suppression of Glycation in Zucker Diabetic Rats. PLOS ONE. 2015;10(9):e0138037. doi:10.1371/journal.pone.0138037.
- Daussin FN, Zoll J, Ponsot E, Dufour SP, Doutreleau S, Lonsdorfer E, et al. Training at high exercise intensity promotes qualitative adaptations of mitochondrial function in human skeletal muscle. Journal of Applied Physiology. 2008;104(5):1436-41. doi:10.1152/japplphysiol.01135.2007. [PubMed:18292295].
- Ferraro E, Giammarioli AM, Chiandotto S, Spoletini I, Rosano G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: redox signaling and role of autophagy. Antioxidants & redox signaling. 2014;21(1):154-76. doi:10.1089/ars.2013.5773. [PubMed:24450966].
- Coffey VG, Hawley JA. The Molecular Bases of Training Adaptation. Sports Medicine. 2007;37(9):737-63. doi:10.2165/00007256-200737090-00001.
- Jacobs RA, Lundby C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. Journal of Applied Physiology. 2013;114(3):344-50. doi:10.1152/japplphysiol.01081.2012. [PubMed:23221957].
- Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant Supplementation Reduces Skeletal Muscle Mitochondrial Biogenesis. Medicine & Science in Sports & Exercise. 2011;43(6):1017-24. doi:10.1249/MSS.0b013e318203afa3. [PubMed:00005768-201106000-00013].
- Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor γ coactivator-1α protein expressions in rat skeletal muscle. Metabolism – Clinical and Experimental. 2008;57(7):986-98. doi:10.1016/j.metabol.2008.02.017.
- Lanza IR, Sreekumaran Nair K. Regulation of skeletal muscle mitochondrial function: genes to proteins. Acta physiologica (Oxford, England). 2010;199(4):529-47. doi:10.1111/j.1748-1716.2010.02124.x. [PubMed:20345409].








