Nema S. Shaban*1 , Abeer M. Radi1, Alsadek H. Bogzil2, H. A. El-Banna3, Elham Ahmed Mobarez4 and A. A. M. El-Gendy1
, Abeer M. Radi1, Alsadek H. Bogzil2, H. A. El-Banna3, Elham Ahmed Mobarez4 and A. A. M. El-Gendy1
1Department of Pharmacology, Faculty of Veterinary Medicine, Beni-Suef University, 62511, Egypt.
2Department of Pharmacology, toxicology and forensic medicine, Faculty of Veterinary Medicine, Omar Al Mukhtar University, Al Bayda, Libya.
3Department of Pharmacology, Faculty of Veterinary Medicine, Cairo University, 12211, Giza, Egypt.
4Department of Pharmacology, Animal Health Research Institute, Dokki, Giza, Egypt.
Corresponding Author E-mail: nsh.pharma@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1738
Abstract
Concurrent administration of drugs may alter their pharmacokinetic parameters, so; investigation to what extent bromhexine hydrochloride affects the pharmacokinetic behavior of tilmicosin was our aim of this work. Ten broiler chickens were classified into two groups as follow, the first one (tilmicosin group) was given single oral dose of tilmicosin (20 mg/kg.b.wt.) while the 2nd (pre-treated group) was given single oral dose of bromhexine hydrochloride (1 mg/kg.b.wt.) followed by single oral dose of tilmicosin (20 mg/kg.b.wt.) one hour later. The serum concentration of tilmicosin was measured using High Pressure Liquid Chromatography (HPLC) method. The results revealed that the mean serum concentrations of tilmicosin were significantly lower in pre-treated group when compared with tilmicosin alone group at the corresponding time intervals. Pharmacokinetic parameters were significantly differed (p<0.001) between both groups. The maximum serum concentration were (Cmax0.70±0.02, 0.81±0.04µg/ml), achieved at Tmax of (tmax 0.89±0.16, and 2.10±0.06h), absorption half-life (t0.5ab) of 0.16±0.08, and 0.37±0.01 hour, area under curve (AUC) of 12.96±0.42 and 16.73±0.42µg.h/ml) in tilmicosin-bromhexine and tilmicosin alone groups respectively. In conclusion, based on the obtained pharmacokinetic parameters, these findings showed that bromhexine accelerates the tilmicosin penetration into body tissues, achieving higher and faster concentrations than when given tilmicosin alone.
Keywords
Broilers; Bromhexine; HPLC; Pharmacokinetic; Tilmicosin
Download this article as:| Copy the following to cite this article: Shaban N. S, Radi A. M, Bogzil A. H, El-Banna H. A, Mobarez E. A, El-Gendy A. A. M. Effect of Bromhexine on the Pharmacokinetic of Tilmicosin in Broiler Chickens. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Shaban N. S, Radi A. M, Bogzil A. H, El-Banna H. A, Mobarez E. A, El-Gendy A. A. M. Effect of Bromhexine on the Pharmacokinetic of Tilmicosin in Broiler Chickens. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/2TQv2f8 |
Introduction
Broilers production is considered as one of the largest and fastest growing industries in the world for providing the opportunity of animal protein needs for humans. However, poultry production has been facing the critical problems that require great efforts by the research institutions and the different studies to be explored and solved1 which encouraged us to choose broilers chickens in this research work.
Macrolides antibiotics are composed of macrocyclic lactone rings to which one or more sugar residues are attached by glycosidic linkages2. The kinetic behavior of macrolides is characterized by high volume of distribution enabling them to reach a high concentration in the target tissue even after administration of a small dose3. Tilmicosin is one of the most important broad-spectrum macrolides developed for veterinary use especially for treatment of respiratory infections in cattle and poultry because of its extensive accumulation in pulmonary tissues. Tilmicosin is a semi synthetic macrolide antibiotic of tylosin derivatives commonly used by veterinaries, has been shown to reveal beneficial pharmacological activities. It suppressed bacterial protein synthesis by penetrating the cell membrane of sensitive microbes and binding to the 50s ribosomal subunit, Moreover, the translocation of immature peptide chains between the 50s and 30s ribosomal subunits is interfered leading to early detachment and synthesis of incomplete peptide chains4. It inhibits Gram-positive bacteria, such as Corynebacterium and Listeria species, some Gram-negative bacteria, such as Pasteurella and Haemophilus species, as well as atypical bacteria as Mycoplasma species3.
Bromhexine is a mucolytic expectorant used in the treatment of respiratory disorders alone or in combination with other antimicrobials because it has ability to disturb the muco-polysaccharide of bronchial secretion enhancing the penetration power of antimicrobials. In addition, it produces an increase in immunoglobulin levels in airway secretions. Besides, it was recently recommended as a new drug for pathological states, such as alcoholic chronic pancreatitis where there is an increased pancreatic secretion5.
In veterinary medicine, co-administration of bromhexine hydrochloride and antibiotics can increase antibiotic concentrations in lung tissue6, nasal mucus7 and sputum8. It promotes intra-tracheal mucus and stimulates secretion of pulmonary surfactant particles9 to enhance their efficiency in the treatment of respiratory infections10. Based on above data, the present study was planned to explore the effect of bromhexine hydrochloride on the disposition kinetic of tilmicosin after single oral administration in normal healthy broilers.
Materials and Method
Drugs
Tilmicosin phosphate was kindly provided by Pharma-sweede pharmaceutical company, Egypt as a white powder (80 %) with good solubility in water. It was used at a dose level of 20 mg kg-1 b.wt. Bromhexine hydrochloride was kindly provided by Pharma- sweede pharmaceutical company, Egypt as a white powder (98%) with poor solubility in water but soluble in N-methyl pyridine/ propylene glycol (NMP/PG) (50%: 50%) solvent.
Animals and Experimental Design
The study was carried out on broiler chickens of both sexes with an average body weight from 2.5 to 3 kg. b.wt. and 45 days old. These birds were obtained from a special poultry farm at Beni-suef Governorate. The birds were kept on balanced commercial ration and water ad-libitum. They were kept under good hygienic conditions and left without treatment for two weeks before the experiment for acclimatization and ensuring complete clearance of any antibacterial agents. The experimental protocol was designed according to Ethical Committee of the Faculty of Veterinary Medicine, Beni-suef University, in accordance with the Guide for the Care and Use of Laboratory Animals. Feed was withheld 12 hours before giving drugs. They divided into two groups each of 5 chickens. the first one (tilmicosin group) was given single oral dose of tilmicosin (20 mg/kg.b.wt.) while the 2nd (pre-treated group) was given single oral dose of bromhexine hydrochloride (1 mg/kg.b.wt.) followed by single oral dose of tilmicosin (20 mg/kg.b.wt.) one hour later.
Blood samples (1-1.5 mL) were collected from wing vein into test tubes at 15, 30 minutes, 1, 2, 4, 8, 12, 24, 48 and 72 hours post administration. All blood samples were left to clot for 30 minutes, centrifuged at 3000 r.p.m for 15 minutes and the obtained clear sera were transferred to eppendorff’s tubes and kept in deep freeze (-20 Co) till assayed by High Pressure Liquid Chromatography (HPLC).
Analytical Procedure
Chemicals and Reagents
Reagent grade methanol, acetonitrile, n- hexane (Merck, Nogent–Sur–Marne, France), de-Ionized water or HPLC grade water, Ammonium acetate, di-potassium hydrogen phosphate (Merck) and calcium chloride (Sigma, USA). Trifluoroacetic acid: – UV grade (Merck). The solvents used during the chromatographic analysis of the drug were HPLC grade.
Chromatographic Condition
Serum tilmicosin concentrations were measured using HPLC method. The HPLC system12 (in Animal Health Research Institute, Dokki, Giza, Egypt) which is consisted of: Agilent series 1200 quaternary gradient pump ,Series 1200 auto sampler, Series 1260 UV Vis detector, HPLC 32D Chemstation software (Hewlett-Packard, Les Ulis, France), Analytical column: the chromatographic column was a reversed-phase column (Extend-C18, Zorbax (5µm, 250mm x 4.6mm) column (Agilent Company), Acrodises (syringe filters), Millex HV13 filters (0.45 µm (tilmicosin), 13 mm id) (Millipore, Saint Quentin Yvelines, France).
Sample Preparation
Plasma protein in each collected sample was precipitated by adding acetonitrile to chicken plasma or a standard sample (1:1). The mixture was mixed using the vortex for 30 seconds, and then centrifuged for 5 minutes at 1000xg. The clear supernatant was evaporated using nitrogen evaporator (0.5ml). The dried residue was dissolved in equivalent volume of dipotassium hydrogen phosphate buffer (0.5ml). The sample was injected directly into HPLC system after filtration with a fit acrodisc 0.45 μm.
Liquid Chromatography Operating Conditions
Injection volume, 50µl: flow rate, 0.7 ml/min; wave length, 287 nm; column temperature, ambient; stop time, 20 min; post time, 5min; mobile phase A, 0.05% trifluoroacetic; mobile phase B, acetonitrile.
Liquid Chromatography Gradient Conditions
The gradient mobile phase consisted of (A): 0 min, acetonitrile –0.05% trifluoroacetic acid (22:78 v/v). (B): 6 min, acetonitrile –0.05% trifluoroacetic acid (45:55 v/v). (C): 10 min, acetonitrile –0.05% trifluoroacetic acid (22:78 v /v). The mobile phase was filtered using 0.45 µm membrane filter and degassed. The mobile phase was eluted at a flow rate of 0.7 ml/min with UV detection wave length of 287 nm.
Pharmacokinetic Analysis of Data Obtained
Serum concentration (log10) versus time curve were generated and best fitted by the aid of computer poly-exponential curve stripping program (R-strip, Micromath, Scientific software, USA). Data from each chicken were fitted individually and the pharmacokinetic variables were computed by the aid of the software program. The hybrid rate constants of the first order absorption and elimination rate constants(K ab and Kel ), absorption and elimination half-lives t0.5(ab), t0.5 (el), area under the curve from zero to infinite time (AUC 0-œ ), mean residence time (MRT), maximum serum concentration (C max) and time to be achieved (t max) were calculated. The results were expressed as Mean ± SE and the obtained data statistically analyzed using student T-test”.
Statistical Analysis
The results were expressed as mean ± standard error of mean (S.E). Statistical significance was determined by student (T-test) using SPSS (version 20.0) software (IBM SPSS Statistic 20.0, Armonk, NY, USA). The P values less than 0.05 were considered statistically significant13.
Results
Standard Curve of Tilmicosin
Tilmicosin standard concentrations of 0.03, 0.06, 0.15, 0.3, 0.6, 1.5 and 3 μg/ml and their corresponding peak responses are illustrated in Table (1) and Fig. (1), and Typical Chromatogram of tilmicosin are illustrated in Fig. (2). the calibration curve was calculated by linear regression equation method as y= 1538.5x + 21.755 where ‘y’ indicates the area under peak and ‘x’ indicates tilmicosin concentrations. Linearity existed within the range of 0.03 and 3 μg/ml with a correlation coefficient r2=0. 9994. The LOD for tilmicosin was 0.001 μg/ml, while, LOQ was 0.003 μg/ml.
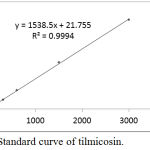 |
Figure 1: Standard curve of tilmicosin.
|
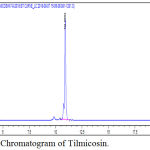 |
Figure 2: Typical Chromatogram of Tilmicosin.
|
Table 1: The concentrations of tilmicosin standard (μg/ml) and their corresponding peak response.
| Retention time | Level | Concentration (ug/ml) | Area |
| 10.88 | 1 | 0.03 | 46.344 |
| 2 | 0.06 | 97 | |
| 3 | 0.15 | 240.33 | |
| 4 | 0.3 | 476.91 | |
| 5 | 0.6 | 964.33 | |
| 6 | 1.5 | 2409.3 | |
| 7 | 3 | 4595.4 |
Single Oral Administration of Tilmicosin in Healthy Broiler Chickens
The mean serum concentrations of tilmicosin at different time intervals following single oral dose (20 mg.kg-1 body weights) in broiler chickens are tabulated in Table (2). The drug was firstly detected (0.19±0.01µg/ml) after 15 minutes and the peak serum concentration (0.85±0.02µg/ml) was reached at 2 hours post drug administration and the lowest drug concentration (0.03±0.002 µg/ml) was reached at 72 hours post drug administration.
Table 2: Mean Serum concentrations of tilmicosin and tilmicosin-bromhexine hydrochloride in healthy broiler chickens after single oral administration of 20 and 1 mg/kg. b.wt. respectively (n = 5).
| Time | Mean ± S.E | |
| Tilmicosin group | Tilmicosin +bromhexine group | |
| 15 min | 0.19±0.01 | 0.28±0.01*** |
| 30 min | 0.34±0.01 | 0.60±0.05*** |
| 1h | 0.75±0.02 | 0.75±0.04 |
| 2 h | 0.85±0.02 | 0.60±0.02*** |
| 4 h | 0.73±0.02 | 0.54±0.02*** |
| 8 h | 0.55±0.01 | 0.44±0.003*** |
| 12 h | 0.45±0.01 | 0.35±0.002** |
| 24 h | 0.30±0.004 | 0.20±0.004*** |
| 48 h | 0.11±0.01 | 0.07±0.002** |
| 72 h | 0.03±0.002 | 0.015±0.003** |
** Significant at p ≤ 0.01, *** Significant at p ≤ 0.001.
The pharmacokinetic parameters of tilmicosin following its oral administration are tabulated in Table (3). The calculated value of maximum concentration (Cmax) was 0.81±0.02 µg/ml and the time (tmax) taken to reach the peak was 2.10±0.06 hours. The drug was rapidly absorbed from broilers gut with absorption half-life (t0.5ab) of 0.37±0.01hour but slowly eliminated with elimination half-life (t0.5el) of 13.49±0.54 hours, the area under curve (AUC) was 16.73±0.42 µg.h/ml and mean residence time (MRT) was 19.4±0.74 hours.
Single Oral Administration of Tilmicosin Pre-Treated with Bromhexine Hydrochloride in Control Healthy Broiler Chickens
The mean serum concentrations of tilmicosin (20 mg/kg b.wt.) pre-treated with bromhexine hydrochloride (1 mg/kg b.wt.) at different time intervals post single oral dose in five broiler chickens are tabulated in Table (2). The drug was firstly detected (0.28±0.01 µg/ml) after 15 minutes and the maximum serum concentration (0.75±0.04 µg/ml) was reached at 1 hour post drug administration and the lowest serum concentration (0.015±0.0003 µg/ml) was reached at 72 hours post drug administration.
The pharmacokinetic parameters of pre-treated group are tabulated in Table (3). The calculated value of maximum concentration (Cmax) was (0.70±0.01µg/ml) and the calculated value of (tmax) was 0.89 ± 0.16 hour. The drug was rapidly absorbed from healthy broilers gut with absorption half-life (t0.5ab) of 0.16±0.08 hour but slowly eliminated with elimination half-life (t0.5el) of 13.77±0.66 hours, the area under curve (AUC) was 12.96±0.42 µg.h/ml and mean residence time and (MRT) was (19.57±1.05 hours).
Comparison Pharmacokinetic Between Tilmicosin and Tilmicosin Pre-Treated Group After Single Oral Administration in Healthy Broiler Chickens
The mean serum concentrations of tilmicosin in control and pre-treated groups after single oral administration in healthy broiler chickens at different time intervals are shown in Table (2) and depicted in Fig. (3). The drug was firstly detected (0.28±0.01, 0.19±0.01µg/ml) at 15 minutes post single administration of tilmicosin pre-treated and tilmicosin alone respectively. The peak serum level (0.75±0.04 µg/ml) was higher in the first one hour then become lower in pre-treated group while the peak serum level of tilmicosin (0.85±0.02µg/ml) was reached at 2 hours post drug administration and the lowest concentration (0.015±0.0003, 0.03±0.02 µg/ml) were determined at 72 hours post single oral administration of tilmicosin in pre-treated and control groups respectively.
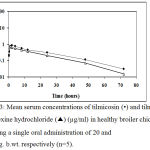 |
Figure 3: Mean serum concentrations of tilmicosin (•) and tilmicosin-bromhexine hydrochloride (▲) (µg/ml) in healthy broiler chickens following a single oral administration of 20 and 1 mg/kg. b.wt. respectively (n=5).
|
Pharmacokinetic parameters were significantly different (p<0.01) in both groups and recorded in Table (3). The maximum serum level (Cmax) was lower in pre-treated group (0.70±0.02, 0.81±0.04µg/ml), while calculated (Tmax) was shorter than control group (0.89±0.16, 2.10±0.06 hours) respectively, The drug was rapidly absorbed in pre-treated group with absorption half-life (tab) (0.16±0.08, 0.37±0.01hour), Area under the curve (AUC) (12.96±0.42, 16.73±0.42µg.h/ml) and Area under the maximum concentration curve (AUMC) (13.8 ± 0.13, 18.80±0.25 µg.h/ml2) in pre-treated and non- treated groups respectively.
Table 3: Pharmacokinetic parameters of tilmicosin and tilmicosin-bromhexine hydrochloride in healthy broiler chickens after single oral administration of 20 and 1 mg/kg. b.wt. respectively (n = 5).
| kinetic parameters | Unit | Tilmicosin | Tilmicosin+bromhexine |
| Kab | h-1 | 1.89±0.05 | 8.13 ± 2.94 |
| t0.5ab | h | 0.37±0.01 | 0.16 ± 0.08** |
| Kel | h-1 | 0.05 ± 0.002 | 0.05 ± 0.002 |
| t0.5el | h | 13.49±0.54 | 13.78 ± 0.65 |
| Cmax | µg/ml | 0.81±0.02 | 0.70 ± 0.01*** |
| tmax | h | 2.10±0.06 | 0.89 ± 0.16*** |
| AUC | µg.h-1.ml-1 | 16.73±0.42 | 12.96 ± 0.42*** |
| AUMC | µg.h2.ml-1 | 18.80±0.25 | 13.8 ± 0.13*** |
| MRT | h | 19.4±0.74 | 19.57 ± 1.05 |
** Significant at p ≤ 0.01, *** Significant at p ≤ 0.001.
Discussion
Tilmicosin is commonly used in veterinary field for treatment of respiratory diseases, so evaluation of the effect of bromhexine hydrochloride on the disposition kinetics of tilmicosin is our aim in this research. The adverse effects of tilmicosin including cardiovascular toxicity as well as deaths after intravenous administration in broiler chickens had been previously mentioned14. The pharmacokinetics of tilmicosin (20 mg/kg body weight) alone or pre-treated with bromhexine hydrochloride (1 mg/kg body weight) following a single oral administration were detected in this study. Tilmicosin was detected in serum 15 minutes post administration (0.19µg/ml) and increased gradually thereafter to reach its peak (0.81µg/ml) at 2.10 hours post administration then decreased gradually till reach its lower level (0.03µg/ml) at 72 hours in tilmicosin only group. Concerning of pharmacokinetic parameters, the result of Cmax0.81 µg/ml is consistent with that reported for azithromycin in broilers (0.95 µg/ml) 15, in calves (0.97 µg/ml) 16, and in cows (0.86 µg/ml) 17, but lower than that reported in sheep (1.29,1.19 μg/ml) 18, in goat (1.56 μg/ml) 19, in swine (2.03μg/ml) 20, in broilers for Pulmotil AC® at a single dose of 30 mg/kg (2.12 μg/ml) 14, and that reported in rabbits for Pulmotil® at a single dose of 12.5 mg/kg (1.31 µg/ml) 21. These differences might be attributed to dose, species and age variations, difference in formulations and/or the method used for assaying of the drug. On the other hand, time to peak serum level (tmax 2.10 hours) is similar to that reported in broiler chickens for azithromycin (1.9 hours) 15, also that reported for tylosin in chicken (2.36 h) 22 but lower than that recorded in broilers (5.82 hours) 14 for (Pulmotil AC®) at a single dose of 30 mg/kg, which might be the cause of variation while it waslonger than that detected in rabbits (0.66 h) 21, in calves and cows (1 h) 16, 17 which might be credit to species and dose variation, routes of drug administration and presence of food in the crop of chicken, that would affect the crop movements as well as the consistency of the feed might be affecting on the emptying of the crop. In addition; the presence of Lactobacillus flora in the crop which lead to inactivation of the macrolides may be attributed 23.
Tilmicosin was rapidly absorbed with an absorption half-life (t0.5ab) 0.37 h. Our finding is nearly similar to that reported for azithromycin in broiler chickens (t0.5ab 0.57 h) 15. Tilmicosin has been slowly eliminated with elimination half-life (t0.5el) of 13.49 h. This outcome is higher than that reported for erythromycin (1.9 h) 24 which may be attributed to that tilmicosin was detected in the serum till 72 h, but lower than that reported in sheep, swine and goat (29.3, 25.26 and 29.4 h)19, 20, 25. In this study, the calculated area under serum concentration-time curve (AUC) was 16.73 µg.h/ml which come in agree with that stated for tylosin in broilers (18.60 μg.h.ml-1) 26 while it is lower than that detected in chicken (21.82 μg.h.ml-1) 14 for tilmicosin but higher than that recoded in pigs (9.68 μg.h.ml-1) 27. These varieties might be credit to the species and dose variation.
This study was planned to evaluate whether there is a pharmacokinetic connection amongst tilmicosin and bromhexine hydrochloride in broiler chickens after single oral administration, the mean serum concentrations of tilmicosin (Cmax) were significantly lower in bromhexine pre-treated (0.70±0.02 µg/ml) broilers contrasted with tilmicosin alone (0.81±0.04µg/ml). Similar finding indicated higher concentration of oxytetracycline within the secreted mucous when used in combination with bromhexine hydrochloride28. Also, patient given amoxicillin-bromhexine combination showed a significant reduction in symptoms such as cough frequency, cough discomfort, sputum volume and had favorable clinical response at the end of the course of treatment 29. Similar results revealed that the bioavailability of erythromycin and its concentration in bronchial fluid were increased after its administration as combined with bromhexine 30. Furthermore, injection of bromhexine with spirmycin resulted in an increase in concentration of spiramycin in bovine nasal secretion 31. The value of Cmax in both groups is higher than the minimum inhibitory concentrations (MICs) for Mycoplasma gallisepticum and Mycoplasma synoviae (0.0125-0.1 μg/ml) 32, Corynebacterium pyogenes in cattle (0.04 μg/ml) 33 and Ornithobacterium rhinotracheale (0.06–1 μg/ml) 34 but lower than the MICs for Clostridum perfringens strains isolated from commercial broiler farms32 as well as Pasteurella multocida and Mannheimia haemolytica (3.125 and 6.25 μg/ml) respectively 33. The National conference of constituency leaders (NCCLS) guidelines for tilmicosin susceptibility list a breakpoint of (≤8 μg/ml) 35. This revealed that the serum concentrations of tilmicosin are lower than the MICs for some susceptible bacteria. Nevertheless, previous studies have reported that administration of tilmicosin at the recommended dose is effective for control of respiratory diseases 36, 37 because of its prolonged duration in lung tissues at therapeutic level 38. Tilmicosin is rapidly absorbed when given in birds pretreated with bromhexine as appeared shorter t0.5ab (0.16±0.08 hour) compared to (0.37±0.01 hour). Tilmicosin concentration is rapidly reached to the peak in pre-treated group than control group as appeared shorter tmax (0.89±0.16) compared to 2.10±0.06 hours respectively. Similar finding was previously reported for enrofloxacin in sheep 39. They reported that, t0.5ab was found to be 0.53 ± 0.11h for enrofloxacin alone in sheep compared to 0.33 ± 0.09h, when enrofloxacin given in combination with bromhexine.
The data of our experiment reported that Cmax and AUC in pre-treated group are significantly lower than that for control group as reported that excipients are considered inert components of a drug formulation affecting only the physicochemical characters of the product (e.g. dissolution and drug stability) 40. However, there were previous studies revealed that some excipients are able to produce its own direct action for example mannitol which decreases gastrointestinal transit time via its osmotic activity 41, surfactants, which can change membrane characteristics 40, 42 and vitamin E which can change the activity of multi-drug resistance proteins thereby affecting drug bioavailability 43. Moreover the changes in the serum concentration and pharmacokinetic parameters induced by pre-treatment with bromhexine may be attributed to enhancing the absorption of tilmicosin and the distribution of tilmicosin to different tissues and body secretions by bromhexine. Similar results were reported previously for furaltadone into tracheobronchial secretions in broilers 44.
Conclusion
The obtained results explain that concurrent administration of tilmicosin and bromhexine altered serum concentration but improve pharmacokinetic parameters. Pre-treatment with bromhexine enhanced the absorption of tilmicosin and the distribution of tilmicosin to different tissues and body secretions by bromhexine, which reflects enhanced efficacy the combination of bromhexine as compared with tilmicosin alone.
Acknowledgements
The author(s) received no specific funding for this work.
Funding source
There is no funding source.
Conflict of Interest
There is no conflict of interest.
References
- Mengesha M. Biophysical and the socio-economics of chicken production. J. Agric. Res., 8(18): 1828-1836 (2013).
- Watteyn A, Plessers E, Wyns H, De Baere S, De Backer P. and Croubels S. Pharmacokinetics of gamithromycin after intravenous and subcutaneous administration in broiler chickens. Sci., 92(6): 1516-1522 (2013).
- El-Mahmoudy A. M, Gheith I. M, Elmajdoub A. A. and Awidat S. K. In-vivo assessment of the antipyretic activity of tilmicosin. J. Pharm. Pharmacol., 12(14): 176-182 (2018).
- Seiple I. B, Zhang Z, Jakubec P, Langlois-Mercier A, Wright P. M, Hog D. T, Yabu K, Allu S. R, Fukuzaki T. and Carlsen P. N. A platform for the discovery of new macrolide antibiotics. Nature, 533, 338-538 (2016).
- Tscimoto T, Takano M, Tsurano T, Hoppo K, Matsumura Y, Kuriyama S. and Fukui H. Mediastinal pancreatic pseudocyt caused by obstruction of the pancreatic duct was eliminated by bromhexine hydrochloride. Med., 43(11): 1034-1038 (2004).
- Spatola J, Poderoso J. J, Wiemeyer J. C. M, Ferna´ndez M, Guerreiro R. B. and Corazza C. Influence of ambroxol on lung tissue penetration of amoxicillin. Drug Res., 37(8): 965-966 (1987).
- Offermeier J, Miller R. and Brandt H. D. The effect of bromhexine on the concentration of oxytetracycline in nasal mucus. Afr. Med. J., 46(41): 1509-1511 (1972).
- Bach P. H. and Leary W. P. P. The effects of bromhexine on oxytetracycline entrance in sputum. Afr. Med. J., 46(41): 1512-1514 (1972).
- Ecker H, Lux M. and Lachmann B. The role of alveolar macrophages in surfactant turnover An experimental study with metabolite VIII of bromhexine. Lung, 161(4): 213- 218 (1983).
- Bergogne B, Berthelot G, Kafe H. P. and Doournovo P. Influence of a fluidifying agent (bromhexine) on the penetration of antibiotics into respiratory secretions. J. Clin. Pharmacol. Res., 5(5):341-344 (1985).
- Amer M. M, Hanafei A. El-H. A, EL-bayomi K. M. and Zohair G. A. Comparative study on the efficacy of antimycoplasma drugs on Performance of commercial broiler flocks from infected breeders. Global Vet.,3(2): 69-74 (2009).
- Juhel-Gaugain M, Anger B. and Laurentie M. Multiresidue chromatographic method for the determination of macrolide residues in muscle by high-performance liquid chromatography with UV detection. J AOAC int., 82(5): 1046-1053 (1999).
- Snedecor G. W. and Cochran W. G. Statistical Methods. 6th Edition, Iowa State University Press, Ames, USA, 593 (1982).
- Abu-basha E. A, Idkaidek N. M. and Al-shunnaq A. F. Pharmacokinetics of tilmicosin (Provitil powder and pulmotil liquid AC) oral formulations in chicken. Vet Res Commun., 31(4): 477-485 (2007).
- Abo-El-Sooud K, Fahmy E, Afifi N. A. and El-Aty A. M. A. Pharmacokinetics and bioavailability of azithromycin following intramuscular and oral administrations in broiler chickens. Rev. BioSci., 6(9): 264-270 (2012).
- Dimitrova D, Petkov P. and Tsoneva D. Pharmacokinetics of tilmicosin in calves after single subcutaneous application. Agricultural Science and Technology, 4(3): 211–214 (2012).
- Avci T. and Elmas M. Milk and blood pharmacokinetics of tylosin and tilmicosin following parenteral administrations to cows. Sci World J. 2014, (2014).
- Atef M, Abo El-Sooud K, Nahed E. and Tawfik M. Elimination of tilmicosin in lactating ewes. Dtsch Tierarztl Wochenschr, 106(7): 291-294 (1999).
- Ramadan A. Pharmacokinetics of tilmicosin in serum and milk of goats. Res Vet. Sci., 62(1): 48-50 (1997).
- Shen J, Li C, Jiang H, Zhang S, Guo P, Ding S. and Li X. Pharmacokinetics of tilmicosin after oral administration in swine. J. Vet. Res., 66(6): 1071–1074 (2005).
- Gallina G, Lucatello L, Drigo I, Cocchi M, Scandurra S, Agnoletti F. and Montesissa C. Kinetics and intrapulmonary disposition of tilmicosin after single and repeated oral bolus administrations to rabbits. Res. Commun., 34(1): 69-72 (2010).
- Abu-Basha E. A, Al-Shunnaq, A. F. and Gehring R. Comparative pharmacokinetics and bioavailability of two tylosin formulations in chickens after oral administration. J. Hellenic Vet. Med. Soc., 63(2): 159-166 (2012).
- Cerda R. O, Petruccelli M, Piscopo M, Origlia J. and Landoni M. Impact of the type of catheter on the absorption of tylvalosin (acetylvaleryltylosin) administered orally to broiler chickens. Vet. Pharmacol. Ther., 33(2): 202-203 (2010).
- Kowalski C. J. and Pomorskamol M. Evaluation of the bioequivalence of two erythromycin thiocyanate formulations after oral administration to broiler chickens. Vet. Inst. pulawy, 53, 247-250 (2009).
- Modric S, Webb A. I. and Derendorf H. Pharmacokinetics and pharmacodynamics of tilmicosin in sheep and cattle. Vet. Pharmacol. Ther., 21(6): 444-452 (1998).
- Soliman A. M. and Sedek M. Pharmacokinetics and Tissue Residues of Tylosin in Broiler Chickens. Pharmacology & Pharmacy, 7, 36-42 (2016).
- Dimitrova D, Кatsarov V, Dimitrov D. and Tsoneva D. Pharmacokinetics of tilmicosin after oral application of Pulmotil G 200–premix in pigs. Agricultural science and technology, 3(4): 318-322 (2011).
- Martin G. P, Loveday B. E. and Mariot C. Bromhexine plus oxytetracycline: the effect of combined administration upon the rheological properties of mucus from the mini-pig., J Pharm Pharmacol., 45(2): 126-130 (1993).
- Roa C. C. Jr, and Dantes R. B. Clinical effectivness of combination of bromhexine and amoxicillin in lower respiratory tract infection. A randomized controlled trial. Arzneimittelforschung, 45(3): 267-72 (1993).
- Bergone-Berzin E, Pierre J. and Dournovo P. Effect of a secretolytic (bromhexine) on the penetration of erythromycin into bronchial secretions. Fortschr Med., 100(24): 1169-1171 (1982).
- Escoula L, Larrieu G. and Camguilhem R. Enhancement of spiramycin concentration by bromhexine in the bovine nasal secretions. Ann Tech Vet., 12(3): 317-320 (1981).
- Watkins K. L, Shryock T. R, Dearth R. N. and Saif Y. M. In-vitro antimicrobial susceptibility of Clostridium perfringens from commercial turkey and broiler chicken origin. Vet Microbiol., 54(2): 195-200 (1997).
- Ziv G, Shem-Tov M, Glickman A, Winkler M. and Saran A. Tilmicosin antibacterial activity and pharmacokinetics in cows. Vet. Pharmacol. Ther., 18(5): 340-345 (1995).
- Varga J, Fodor L. and Makrai L. Characterization of some Ornithobacterium rhinotracheale strains and examination of their transmission via eggs. Acta Veterinaria Hungarica., 49(2): 125-130 (2001).
- Watts J. L. “Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: approved standard”. (M31-A), NCCLS 19, 11 (1999).
- Vogel G. J, Laudert S. B, Zimmermann A, Guthrie C. A, Mechor G. D. and Moore G. M. Effects of tilmicosin on acute undifferentiated respiratory tract disease in newly arrived feedlot cattle. Am. Vet. Med. Asso., 212(12): 1919–1924 (1998).
- Christodoulopoulos G, Warnick L. D, Papaioannou N. and Fthenakis G. C. Tilmicosin administration to young lambs with respiratory infection: safety and efficacy considerations. Vet. Pharmacol. Ther., 25(5): 393-397 (2002).
- Papich M. G. and Riviere J. E. Chloramphenicol and derivatives, macrolides, lincosamides and miscellaneous antimicrobials. Vet. Pharmacol. Ther., 8th Edition (Iowa State Press, Ames, IA), 880-881 (2001).
- El-Banna H. A, Goudah A. and El-Zorba H. Comparative Bioequivalence Study of Three Formulations of Enrofloxacin in Sheep. Drug Metab. Lett., 5(2): 85-91 85 (2011).
- Lee V. H. L, Yamamoto A. and Kompella U. B. Mucosal penetration enhancers for facilitation of peptide and protein drug absorption. Rev. Ther. Drug Carrier Syst., 8(2): 91-192 (1991).
- Adkin D. A, Davis S. S, Sparrow R. A, Huckle P. D. and Wilding I. R. The effect of mannitol on the oral bioavailability of cimetidine. Pharm. Sci., 84(12): 1405-1409 (1995).
- Lee V. H. L. and Yamamoto A. Penetration and enzymatic barriers to peptide and protein absorption. Advances Drug Deliv. Rev., 4(2): 171-207 (1990).
- Yu L, Bridgers A, Polli J, Vickers A, Long S, Roy A, Winnike R. and Coffin M. Vitamin E-TPGS increases absorption flux of an HIV protease inhibitor by enhancing its solubility and permeability. Res., 16(12): 1812-1817 (1999).
- Sumano H, Gracia I, Capistran A, Meade G, Rivero A. and Ruiz-Ramirez L. Use of ambroxol and bromhexine as mucolytics for enhanced diffusion of furaltadone into tracheobronchial secretions in broilers. Br. Poult. Sci., 36(3): 503-507 (1995).








