Muhammad Yulis Hamidy1,2* , Fadil Oenzil3
, Fadil Oenzil3 , Yanwirasti4 and Yufri Aldi5
, Yanwirasti4 and Yufri Aldi5
1Biomedical Programme, Faculty of Medicine, Andalas University, Padang, Indonesia.
2Department of Pharmacology, Faculty of Medicine, Universitas Riau, Pekanbaru, Indonesia.
3Department of Biochemistry, Faculty of Medicine, Andalas University, Padang, Indonesia.
4Department of Anatomy, Faculty of Medicine, Andalas University, Padang, Indonesia.
5Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Andalas University, Padang, Indonesia.
Corresponding Author E-mail: yulis.hamidy@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1745
Abstract
To evaluate the effect of andrographolide on monocyte chemoattractant protein-1 (MCP-1) expression at the initiation stage of atherosclerosis in rats induced by an atherogenic diet. The research was conducted on 27 rats divided into 3 groups (n=9). Group 1 was given a standard diet. Group 2 was given an atherogenic diet of vitamin D3 700.000 IU/kg on the first day followed by 5% goat fat, 2% cholesterol, 0.2% cholic acid and standard diet up to 100% for 2 days to induce atherosclerosis initiation stage. Group 3 was given an atherogenic diet and treated with andrographolide 40 mg/kg. An immunohistochemical examination was performed to determine the expression of MCP-1. Data analysis using one-way Anova followed by post hoc test. The results showed the expression of MCP-1 in group 1 was 6.61 + 1.90, in group 2 was 32.99 + 3.74 and in group 3 was 9.61 + 2.47. There was a significant difference between group 3 treated with andrographolide 40 mg/kg compared with group 2 (p<0.001). There was no significant difference between group 3 treated with andrographolide 40 mg/kg and group 1 (p>0.05). In conclusion, andrographolide may inhibit MCP-1 expression at the initiation stage of atherosclerosis in the andrographolide treated rats. Thus, andrographolide could be a potential anti-atherosclerosis drug.
Keywords
Andrographolide; Atherosclerosis; MCP-1; Atherosclerosis
Download this article as:| Copy the following to cite this article: Hamidy M .Y, Oenzil F, Yanwirasti Y, Aldi Y. Effect of Andrographolide on Monocyte Chemoattractant Protein-1 Expression at the Initiation Stage of Atherosclerosis in Atherogenic Diet-Fed Rats. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Hamidy M .Y, Oenzil F, Yanwirasti Y, Aldi Y. Effect of Andrographolide on Monocyte Chemoattractant Protein-1 Expression at the Initiation Stage of Atherosclerosis in Atherogenic Diet-Fed Rats. Biomed Pharmacol J 2019;12(3). Available from: http://biomedpharmajournal.org/?p=28367 |
Introduction
Atherosclerosis is a chronic inflammatory disease that occurs in the walls of blood vessels. Various processes are involved in the pathogenesis of atherosclerosis, including inflammatory cells infiltration, smooth muscle cells proliferation, increasing extracellular matrix, and formation of thrombus1. Inflammation has an important role and is the main mechanism underlying the pathogenesis of atherosclerosis2. This inflammation occurs through the interaction between monocytes and cells found in the walls of arteries such as endothelial cells and vascular smooth muscle cells3.
Monocytes play an essential role in the initiation stage of atherosclerosis4. In early atherosclerotic lesions, accumulation of monocytes/macrophages can be found in the intima of blood vessels. Macrophages accumulate cholesteryl esters resulting in foam cell formation1. Various existing studies show the important role of monocyte chemoattractant protein-1 (MCP-1) in the process of recruiting monocytes/macrophages5. Monocyte chemoattractant protein-1, a C-C chemokine family member, has a potent chemoattractant activity for monocytes/macrophages6. This chemokine is highly expressed in the macrophage-rich area of the atherosclerotic lesions in human as well as experimental animal models5. These findings suggest that monocyte chemoattractant protein-1 has an important role in the initiation stage of atherosclerosis.
The development of anti-atherosclerosis drugs can be done through the study on anti-inflammatory drugs that act more specifically by influencing pathogenesis of atherosclerosis. One of the ingredients that has anti-inflammatory effect and potentially as anti-atherosclerosis is andrographolide, the main active ingredient contained by Andrographis paniculata. Studies that have been carried out prove that andrographolide has anti-inflammatory effect7,8,9 and potential to be developed as anti-atherosclerosis10.
This study aimed to evaluate the andrographolide effect on MCP-1 expression at the initiation/early stage of atherosclerosis in rats induced by an atherogenic diet.
Materials and Methods
Experimental animals
Twenty-seven male Wistar rats, 10 weeks of age (150-200 g) were obtained from School of Pharmacy, University of Riau. Rats were placed in cages in a well-ventilated room and allowed for food and water ad libitum. The room temperature was 20–26 ̊C and room lighting was regulated light and dark for 12 hours, alternately. Rat health was well monitored and rat cages were cleaned every day. Acclimatization of rats was carried out for one week before being used in this study. The treatment of animals was subjected to the declaration of Helsinki. Ethical approval was obtained from the Ethical Review Board for Medicine & Health Research, Faculty of Medicine, University of Riau (No:457/UN.19.5.1.1.8/UEPKK/2017).
Experimental design
Rats were randomly divided into 3 groups (n=9), Group 1 (normal control) was given a standard diet. Group 2 (atherogenic control) was given an atherogenic diet of vitamin D3 (cholecalciferol) 700.000 IU/kg (Sigma, St. Louis, MO, USA) on the first day followed by 5% goat fat, 2% cholesterol, 0.2% cholic acid (Sigma, St. Louis, MO, USA) and 92.8% standard diet for 2 days to induce atherosclerosis initiation stage11,12. Group 3 (treated group) was given an atherogenic diet and treated with andrographolide 40 mg/kg (Andalas Sitawa Fitolab, Padang, Indonesia). Andrographolide was given orally once a day using a gastric tube.
Evaluation of initiation stage of atherosclerosis
The initiation stage of atherosclerosis was assessed based on foam cell formation. After the treatment was completed, ether anesthesia was used to sacrifice the rats. Abdominal aortic tissues were excised rapidly and fixed in 10% neutralized formaldehyde in 0.1 M phosphate buffer. The aortic sample was embedded in paraffin. We performed a hematoxylin and eosin (H&E) staining to measure the number of foam cells in the rat abdominal aortic. Foam cells were calculated with ×400 magnification at 9 fields of view. The images were obtained using a light microscope equipped with a camera (Leica, Wetzlar, Germany) and connected to a computer monitor.
Immunohistochemical staining
MCP-1 in rat abdominal aortic were identified using MCP-1 polyclonal antibody (Bioss Antibodies Inc., Massachusetts, USA). Paraffin embedded mouse kidney with positive IHC for MCP-1 was used as positive control. Slides were observed using light microscope at ×400 magnification. Images were taken using microscope camera (Leica, Wetzlar, Germany) as many as 10 pictures each slide. MCP-1 expression in abdominal aortic was assessed by Photoshop image analysis software to calculate the percentage of area12. The percentage of area presented the comparison area containing MCP-1 with a whole cross section of the aorta, which is assumed to be width of expression. Two independent pathologists were invited to examine the slides.
Statistical analysis
Data are presented as mean ± SEM. One-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test was used to analyze the results. P<0.05 was considered statistically significant.
Results
Effect of atherogenic diet on foam cell formation at the initiation stage of atherosclerosis
The H&E staining showed that in Group 1 (normal control) and Group 3 (treated group) demonstrated few foam cells (Figures 1A and 1C), while in Group 2 (atherogenic control) showed abundance of foam cells in the aortic lesion (Figure 1B). These results demonstrated that the administration of an atherogenic diet induced foam cell formation in rats and andrographolide treatment reduced the accumulation of these cells in aortic lesion
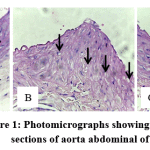 |
Figure 1: Photomicrographs showing histological sections of aorta abdominal of rats. |
(A) Group 1 (normal control) was given a standard diet, (B) Group 2 (atherogenic control) was given an atherogenic diet, (C) Group 3 (treated group) was given an atherogenic diet and treated with andrographolide 40 mg/kg, at magnification of ×400 stained with H&E. The arrow indicated foam cell.
Table 1 shows the number of foam cells among different experimental groups. Atherogenic diet-fed animals exhibit increased number of foam cells in the aortic lesions compared to control rats (P<0.05). Andrographolide treatment significantly reduced the accumulation of foam cells in the aortic lesions (P<0.05).
Table 1: Number of foam cells among different experimental groups
|
Animal group |
Number of foam cells |
|
Group 1 |
5,33 + 1,73 |
|
Group 2 |
82,33 + 13,10a),b) |
|
Group 3 |
7,44 + 1,62 |
Group 1: normal control group; Group 2: atherogenic control group; Group 3: treated group.
Statistical analysis of the data was carried out using one-way analysis of variance (ANOVA) and Bonferroni’s post hoc test for average comparison on SPSS 17.0.
Values are presented as mean + SEM (N=9).
a)P<0.05 vs. Group 1. b)P<0.05 vs. Group 3.
Effect of andrographolide on MCP-1 expression
Immunohistochemical evaluation of MCP-1 in aorta section is shown in Figure 2 and Table 2. MCP-1 expression in atherogenic control group (Group 2) was markedly higher than normal control group (Group 1). However MCP-1 expression was reduced by andrographolide treatment (Group 3).
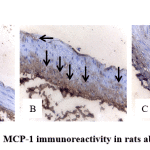 |
Figure 2: MCP-1 immunoreactivity in rats abdominal aorta. |
Table 2: MCP-1 expression among different experimental groups (by Photoshop)
|
Animal group |
Percentage area (%) |
|
Group 1 |
6,61 + 1,90 |
|
Group 2 |
32,99 + 3,74a),b) |
|
Group 3 |
9,61 + 2,47 |
Group 1: normal control group; Group 2: atherogenic control group; Group 3: treated group.
Statistical analysis of the data was carried out using one-way analysis of variance (ANOVA) and Bonferroni’s post hoc test for average comparison on SPSS 17.0.
Values are presented as mean + SEM (N=9).
a)P<0.05 vs. Group 1. b)P<0.05 vs. Group 3.
Discussion
The results of this study showed that andrographolide inhibits MCP-1 expression at the initiation stage of atherosclerosis in rats induced by an atherogenic diet. Andrographolide may reduce foam cell formation in the early stages of atherosclerosis.
Andrographolide was one of the main active ingredients contained by Andrographis paniculata. Recent studies were reported that andrographolide was effective in treating inflammatory diseases because it was a powerful NF-kB inhibitor13,14. Andrographolide has been shown to use anti-inflammatory effects in experimental models of respiratory inflammatory diseases including asthma15, acute lung injury (ALI)16, and idiopathic pulmonary fibrosis (IPF)17. Andrographolide has been shown to protect against stroke by inhibition of NADPH oxidase 2 (NOX2) and inducible-nitric oxide synthase (iNOS) production via inhibition of NF-kB18.
In order to develope andrographolide as an effective anti-inflammatory drug, various clinical trials have been carried out, including for the treatment of sinusitis, where the drug was reported to be effective in relieving inflammatory symptoms and well tolerated by patients19. Beneficial effects of A. paniculata have been shown in a phase 2 trial of rheumatoid arthritis20 and significantly reduced fatigue in multiple sclerosis patients21.
Toxicological study on andrographolide has been carried out both pre-clinical and clinical studies. In an acute toxicity study it was found that andrographolide up to 5 g/kg given orally daily for up to 14 days in rats had no observable adverse effect. The same results were also found in a sub-acute toxicity study which showed administration of oral dose of andrographolide up to 500 mg/kg given daily for up to 21 days in rats also had no observable adverse effect22. In a clinical trial, administration of 170 mg of A. paniculata purified extracts (about 85 mg andrographolide) given orally every 12 h for 12 months was found to be well tolerated in patients with relapsing-remitting multiple sclerosis. Only one patient received A. paniculata presented with a mild skin rash, which was attenuated with anti-histamine treatment21.
Atherosclerosis initiation stage was carried out in rats induced by atherogenic diet. We used rats in this study as experimental animal because these animals have several advantages including being practical, easy to obtain and are omnivorous diet animals, more like humans. Atherosclerosis can be induced in the rats by adding vitamin D3 (cholecalciferol) into high-cholesterol diet23. Rat model of atherosclerosis created by vitamin D3 and feeding with an atherogenic diet is similar to the human. The composition of the atherogenic diet given in this study consisted of 5% goat fat, 2% cholesterol, 0.2% colic acid and a standard diet up to 100%12. Atherogenic diet was given to produce hypercholesterolemia in experimental animals. Hypercholesterolemia causes LDL to infiltrate arteries, especially at the site of hemodynamic disturbance. Oxidative and enzymatic modifications will convert LDL to oxLDL24. oxLDL causes endothelial dysfunction so that it will cause recruitment of monocytes into the arterial wall25.
Monocyte infiltration into the arterial wall plays an important role in the initiation stage and development of atherosclerotic lesion. One factor that has an important role in the recruitment of monocytes is MCP-126. Various types of cells presented in the walls of blood vessels synthesize and secrete MCP-1. This chemokine has a strong chemotactic effect on monocytes/macrophages27. The chemotactic effect of MCP-1 on monocytes in circulating blood is mediated by a specific high-affinity receptor CCR24,5,26. Aiello et al demonstrated that MCP-1 can initiate atherosclerosis in mice with apolipoprotein-E deficiency28. Meanwhile, studies in mice with deletion of MCP-1 or CCR2 showed a decrease in monocyte accumulation in the arterial wall and prevent the formation of atherosclerotic lesions29,30.
These evidences prove that MCP-1 plays an important role in the atherosclerosis initiation stage and could be a potential target for atherosclerotic therapy.
The atherosclerosis initiation stage is characterized by the foam cells formation31. Foam cells found at the early atherosclerotic lesion originate from monocytes in the circulation32. Monocytes that have attached to vascular endothelial cells then migrate into the arterial intima layer mediated by MCP-124. These monocytes will then differentiate into macrophages in response to Macrophage-Colony Stimulating Factor (M-CSF) and other stimuli33. Macrophages express the scavenger receptor (SR) consisting of CD36, SR-A and lectin-like oxLDL receptors-1 (LOX-1) which facilitate oxLDL particle uptake by macrophages resulting in lipid-laden foam cells34.
We suggest that the mechanism of inhibition of MCP-1 by andrographolide occurs through inhibition of NF-kB activation. Previous studies have shown that andrographolide has anti-inflammatory effects through the inactivation of the NF-κB13. NF-kB is a transcription factor that has an important role in regulating the genes of various cytokines and chemokines involved in the inflammatory process35. NF-kB consists of: p105/p50 (NF-kB1), p100/p52 (NF-kB2), p65 (RelA), RelB dan c-Rel. The NF-kB pathway is activated by the IKK (IkB kinase) complex, which consists of the regulator subunit, IKKg which is also known as the NF-kB essential modifier (NEMO), and the catalytic subunit, IKKα and IKKb. As the downstream substrate is Ikbα. In normal circumstances the NF-kB p50/NF-kB p65 dimer is bound to Ikbα. In response to stimuli, IKKα and IKKb will be phosphorylated and cause Ikbα phosphorylation. The phosphorylated Ikbα will degrade through the ubiquitin proteasome pathway. Ikbα degradation causes NF-kB p50/NF-kB p65 to undergo phosphorylation and translocation which eventually results in gene transcription36.
Inhibition of NF-kB activity by andrographolide occurs through dephosphorylation of p65 by activating PP2A9,37. Dephosphorylation of p65 by andrographolide results in decreased translocation of NF-kB into the nucleus and reduces binding of NF-kB with DNA37. Through this mechanism, andrographolide inhibits MCP-1 expression so that it will inhibit monocyte recruitment into the arterial wall.
Conclusion
The results of the present study suggest andrographolide has atheroprotective effects by inhibiting MCP-1 expression and eventually suppressing macrophage foam cell formation. Clinically, the results presented here provide insights into the potential use of andrographolide as an anti-atherosclerotic drug.
Acknowledgements
The authors gratefully acknowledge that the present research is supported by Ministry of Research and Technology and Higher Education Republic of Indonesia.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Funding Source
The present research is funded by Ministry of Research and Technology and Higher Education Republic of Indonesia (Grant No. 277/UN.19.5.1.3/PP/2018).
References
- Libby P, Ridker P.M, Maseri A. Inflammation and atherosclerosis. Circulation, 2002; 105: 1135–1143.
- Libby P, Okamoto Y, Rocha V.Z, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circulation Journal, 2010; 74: 213-220.
- Li Y, Guo Y, Chen Y, Wang Y, You Y, Yang Q, et al. Establishment of an interleukin‑1β‑induced inflammation‑activated endothelial cell‑smooth muscle cell‑mononuclear cell co‑culture model and evaluation of the anti‑inflammatory effects of tanshinone IIa on atherosclerosis. Molecular Medicine Reports, 2015; 12: 1665-1676.
- Namiki M, Kawashima S, Yamashita T, Ozaki M, Hirase T, Ishida T, et al. Local overexpression of monocyte chemoattractant protein-1 at vessel wall induces infiltration of macrophages and formation of atherosclerotic lesion: Synergism with hypercholesterolemia. Arterioscler Thromb Vasc Biol, 2002; 22: 115-120.
- Yla-Herttuala S, Lipton B.A, Rosenfeld M.E, Sarkioja T, Yoshimura T, Leonard E.J, et al. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci, 1991; 88: 5252–5255.
- Luster A.D. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med, 1998; 338: 436–445.
- Low M, Khoo C.S, Münch G, Govindaraghavan S, Sucher N.J. An in vitro study of anti-inflammatory activity of standardised Andrographis paniculata extracts and pure andrographolide. BMC Complementary and Alternative Medicine, 2015; 15: 18.
- Ren J, Liu Z, Wang Q, Giles J, Greenber J, Sheibani N, et al. Andrographolide ameliorates abdominal aortic aneurysm progression by inhibiting inflammatory cell infiltration through downregulation of cytokine and integrin expression. J Pharmacol Exp Ther, 2016; 356(1): 137-147.
- Zhu T, Wang D.X, Zhang W, Liao X.Q, Guan X, et al. Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-ĸB. Plos One, 2013; 8(2): e56407.
- Al Batran R, Al-Bayaty F, Al-Obaidi M.M.J, Hussain S.F, Mulok T.Z. Evaluation of the effect of andrographolide on atherosclerotic rabbits induced by Porphyromonas gingivalis. BioMed Research International, 2014; article ID 724718.
- Pang J, Xu Q, Xu X, Yin H, Xu R, Guo S, et al. Hexarelin suppresses high lipid diet and vitamin D3-induced atherosclerosis in the rat. Peptides, 2010; 31: 630-638.
- Ismawati, Oenzil F, Yanwirasti, Yerizel E. Changes in expression of proteasome in rats at different stages of atherosclerosis. Anat Cell Biol, 2016; 49: 99-106.
- Xia Y.F, Ye B.Q, Li Y.D, Wang J.G, He X.J, et al. Andrographolide attenuates inflammation by inhibition of NF-ĸB activation through covalent modification of reduced cysteine 62 of p50. J Immunol, 2004; 173: 4207–4217.
- Tan W.S.D, Liao W, Zhou S, Wong W.S.F. Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochem Pharmacol, 2017; 139: 71–81.
- Bao Z, Guan S, Cheng C, Wu S, Wong S.H, et al. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-ĸB pathway. Am J Respir Crit Care Med, 2009; 179: 657–665.
- Guan S.P, Tee W, Ng D.S.W, Chan T.K, Peh H.Y, Ho W.E, et al. Andrographolide protects against cigarette smoke-induced oxidative lung injury via augmentation of Nrf2 activity. Br J Pharmacol, 2013; 1707–1718.
- Yin J.N, Li Y.N, Gao Y, Li S.B, Li J.D. Andrographolide plays an important role in bleomycin-induced pulmonary fibrosis treatment. Int J Clin Exp Med, 2015; 8: 12374–12381.
- Chern C.M, Liou K.T, Wang Y.H, Liao J.F, Yen J.C, Shen Y.C. Andrographolide inhibits PI3K/AKT-dependent NOX2 and iNOS expression protecting mice against hypoxia/ischemia-induced oxidative brain injury. Planta Med, 2011; 77: 1669–1679.
- Gabrielian E.S, Shukarian A.K, Goukasova G.I, Chandanian G.L, Panossian A.G, Wikman G, et al. A double blind, placebo-controlled study of Andrographis paniculata fixed combination kan jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine, 2002; 9(7): 589–597.
- Burgos R.A, Hancke J.L, Bertoglio J.C, Aguirre V, Arriagada S, Calvo M, et al. Efficacy of an Andrographis paniculata composition for the relief of rheumatoid arthritis symptoms: a prospective randomized placebo controlled trial. Clin Rheumatol, 2009; 28: 931–946.
- Bertoglio J.C, Baumgartner M, Palma R, Ciampi E, Carcamo C, Caceres D.D, et al. Andrographis paniculata decreases fatigue in patients with relapsing-remitting multiple sclerosis: a 12-month double-blind placebo-controlled pilot study. BMC Neurol, 2016; 16: 77.
- Bothiraja C, Pawar A.P, Shende V.S, Joshi P.P. Acute and subacute toxicity study of andrographolide bioactive in rodents: evidence for the medicinal use as an alternative medicine. Comp Clin Pathol, 2012; 22: 1123–1128.
- Russell J.C, Proctor S.D. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol, 2006; 15: 318-330.
- Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med, 2005; 352:1685-1695.
- Tabas I, Williams K.J, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation, 2007; 116: 1832–1844.
- Libby P. Changing concepts of atherogenesis. J Intern Med, 2000; 247: 349–358.
- Gerszten R.E, Garcia-Zepeda E.A, Lim Y.C, Yoshida M, Ding H.A, Gimbrone M.A Jr, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature, 1999; 398: 718–723.
- Aiello R.J, Bourassa P.A, Lindsey S, Weng W, Natoli E, Rollins B.J, et al. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol, 1999; 19: 1518 – 1525.
- Gu L, Okada Y, Clinton S.K, Gerard C, Sukhova G.K, Libby P, Rollins B.J. Absence of monocyte chemoattractant protein 1 reduces atherosclerosis in low density lipoprotein deficient mice. Mol Cell, 1998; 2: 275-281.
- Guo J, Van Eck M, Twisk J, Maeda N, Benson G.M, Groot P.H.E, et al. Transplantation of monocyte CC-chemokine receptor 2-deficient bone marrow into ApoE3-Leiden mice inhibits atherogenesis. Arterioscler Thromb Vasc Biol, 2003; 23: 447– 453.
- Yu B.C, Hung C.R, Chen W.C, Cheng J.T. Antihyperglycemic effect of andrographolide in streptozotocin induced diabetic rats. Planta Med, 2003; 69: 1075-1079.
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature, 1993; 362: 801–809.
- Chistiakov D.A, Bobryshev Y.V, Orekhov A.N. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med, 2016; 20(1): 17-28.
- Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor J.L. CD36 and macrophages in atherosclerosis. Cardiovasc Res, 2007; 75: 468–477.
- Zhang X, Huang H, Yang T, Ye Y, Shan J, et al. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury, 2005; 36: 387–394.
- Li Q, Verma I.M. NF-kB regulation in the immune system. Nat Rev Immunol, 2002; 10: 725–734.
- Hsieh C.Y, Hsu M.J, Hsiao G, Wang Y.H, Huang C.W, Chen S.W, et al. Andrographolide enhances nuclear factor-kB subunit p65 ser536 dephosphorylation through activation of protein phosphatase 2A in vascular smooth muscle cells. The Journal of Biological Chemistry, 2011; 286(8): 5942–5955.







