Ridwan Ridwan1, Rosdiana Natzir2 , Haerani Rasyid3,7, Ilhamjaya Patellongi4
, Haerani Rasyid3,7, Ilhamjaya Patellongi4 , Mochammad Hatta5
, Mochammad Hatta5 , Elmiana Bongga Linggi6, Agussalim Bukhari7 and Uleng Bahrun8
, Elmiana Bongga Linggi6, Agussalim Bukhari7 and Uleng Bahrun8
¹Mappa Oudang Nursing Academy, Makassar, Indonesia.
2Department of Biochemistry, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
3Division of Nephrology-Hypertension, Department of Internal Medicine, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
4Department of Physiology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
5*Molecular Biology and Immunology Laboratory, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
6Nursing Science Study Program, Stella Maris School of Health Sciences of Higher Education, Makassar, Indonesia.
7Department of Nutritional Science, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
8Department of Clinical Pathology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
Corresponding Author E-mail: hattaram@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1756
Abstract
Researches on the effects of High Fat Diet (HFD) on decreased renal function with cystatin C (cysC) serum levels biomarker are few and show different findings. Renin Angiotensin System (RAS) plays a key role in controlling renal function and one of the integral components of the RAS is Angiotensin Converting Enzyme 2 (ACE2). Research on the relationship between plasma ACE2 levels with serum cysC levels in animals induced by HFD has not been done. We hypothesize that administration of HFD can cause a decline in early stage renal function through the role of ACE2. 30 male wistar rats aged 10-12 weeks (body weight between 170-220 grams) were randomly divided into 5 groups (6 rats/group): baseline, normal diet for 8 weeks (ND8), ND for 16 weeks (ND16), HFD for 8 weeks (HFD8) and HFD for 16 weeks (HFD16). Body weight and naso-anal length were measured to get the index value of obesity and body fat percentage. Obesity index measured are lee index, rohrer index and TM index. Blood samples obtained by intracardiac for examination of plasma ACE2 levels and serum cysC levels. After 8 and 16 weeks, HFD increases body weight, obesity index and body fat percentage. HFD also increases plasma ACE2 levels and serum cysC levels. Body weight, obesity index and body fat percentage have a positive correlation with plasma ACE2 levels. Plasma ACE2 levels were positively correlated with serum cysC levels. HFD causes a decrease of early stage renal function as evidenced by the increase in serum cysC levels. Plasma ACE2 levels play a role in the pathogenesis of the decline in early stage renal function induced by HFD.
Keywords
ACE2; Cystatin C;High Fat Diet; Renal Function
Download this article as:| Copy the following to cite this article: Ridwan R, Natzir R, Rasyid H, Patellongi I, Hatta M, Linggi E. B, Bukhari A, Bahrun U. Decreased Renal Function Induced by High-Fat Diet in Wistar Rat: The Role of Plasma Angiotensin Converting Enzyme 2 (ACE2). Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Ridwan R, Natzir R, Rasyid H, Patellongi I, Hatta M, Linggi E. B, Bukhari A, Bahrun U. Decreased Renal Function Induced by High-Fat Diet in Wistar Rat: The Role of Plasma Angiotensin Converting Enzyme 2 (ACE2). Biomed Pharmacol J 2019;12(3). Available from: http://biomedpharmajournal.org/?p=28495 |
Introduction
In recent years, HFD has increasingly been seen as a significant risk factor that can lead to disease. However, the mechanism of HFD that has a negative impact on health is still difficult to understand. Current research related to HFD focuses on the impact of HFD on medical conditions, the underlying mechanisms and the development of therapeutic strategies1.
HFD is a risk factor for kidney disorders2. In HFD induced obesity it causes damage to kidney structure, inflammation3,4 and oxidative stress5. Decreased renal function is characterized by increased urinary albumin excretion or albuminuria6,7, increased plasma creatinine levels3 and lower creatinine clearance8. However research on the effects of HFD on renal function with biomarkers of cysC levels was very small and showe different findings. HFD causes an increase in serum cysC levels which implies glomerular and proximal tubular changes7. Crinigan et al (2015) found that although HFD increased serum cysC levels, it was not statistically significant9.
CysC is a marker for measuring Glomerolus Filtration Rate (GFR)10,11 and more appropriately used to diagnose kidney damage with a decrease in GFR compared to creatinine clearance10. CysC is a low molecular weight protein (13kD) which is an endogenous cysteine proteinase inhibitors produced by all nucleating cells in the human body at a fairly constant level. CysC is filtered freely by the glomerulus and is not secreted and is almost completely absorbed back in the proximal tubule11. This characteristic makes cysC useful for detecting early stage renal dysfunction. CysC not influenced by age, gender, muscle mass and ethnicity12.
One of the pathophysiological mechanisms that also plays a central role in the development of kidney disease is the activation of RAS13. RAS plays a key role in controlling kidney function14,15. One of the integral components of the RAS is ACE216.
ACE2 is a monocarboxypeptidase that degrades angiotensin (Ang) II to Ang (1-7)13 and convert angiotensin (Ang) I to Ang (1-9)17. Imbalance between increasing levels of Ang II and ACE2/Ang (1-7)/Mas receptor axis contribute to kidney injury13. ACE2 is widely expressed in the kidneys especially in proximal tubular epithelial cells14 and visceral glomerolus18. ACE2 protein and mRNA levels have been shown to change in diabetic kidney disease, hypertensive kidney disease and various injurious renal models14.
Li et al (2015) examined the effect of giving HFD to various RAS components. However, they did not examine the effect on ACE2 which is also one of the components of the RAS19. The findings is encouraging research on the effects of HFD to the activation of RAS components through the role of ACE2. Very few data is available about the effects of giving HFD to plasma ACE2 levels. Not even found data related to the relationship between plasma ACE2 levels with serum cysC levels in animals induced by HFD. We hypothesize that administration of HFD can cause a decline in early stage renal function through the role of ACE2.
Materials and Methods
Diets
Preparation of animal diet was conducted in Animal Food and Nutrition Division, Faculty of Animal Husbandry, Hasanuddin University, Makassar, Indonesia. The animal diet was arranged with normal diet (ND) composition consisting of 3.1% fat, 16.1% protein, 3.9% fiber and 5.1% ash/ mineral. Composition of the high fat diet (HFD) consisted of 21.4% fat, 17.5% protein, 50% carbohydrate, 3.5% fiber and 4.1% ash/mineral20 (table 1). To get rations/feed according to the composition that has been prepared, analysis of the feed samples using the proximate method was carried out. The analysis was done twice to get accurate results (table 2).
Table 1: The Composition of Animal Diet
| HFD | ND | |||||||||
| Composition | CH (%) | Protein (%) | Fat (%) | Fiber
(%) |
Ash
(%) |
CH (%) | Protein (%) | Fat
(%) |
Fiber (%) | Ash
(%) |
| Corn
Bran MBM Premix Fish Flour Soybean meal Tallow Vegetable oil |
38,11
6,32
5,76
|
4,50
2,29 1,29
1,16 7,54 0,41 |
2,02
1,87 0,33
0,17 0,53 16,16 0,41 |
0,74
1,06 0,07
0,08 0,44
|
0,01
1,28
0,22 1,18 0,02 |
2,20
48,30
5,40 6,32
|
4,72
7,07 2,29 1,74 |
0,09
0,56
0,50 1,87 0,26 |
0,94
0,41 1,06 0,12 |
0,20
1,11 1,28 0,32 |
| Total | 50,19 | 17,19 | 21,49 | 2,39 | 2,71 | 62,22 | 15,82 | 3,28 | 2,53 | 2,91 |
CH: Carbohydrate; MBM: Meat Bone Meal; ND: Normal Diet; HFD: High Fat Diet
Table 2: Proximate Analysis Results
| No | Sample | Composition | |||||
| Water | Protein | Fat | Fiber | CH | Ash | ||
| 1 | ND1 | 13,04 | 20,41 | 4,67 | 3,70 | 67,54 | 3,68 |
| 2 | ND2 | 13,32 | 20,52 | 5,34 | 4,39 | 66,51 | 3,24 |
| 3 | HFD1 | 10,32 | 19,85 | 23,53 | 3,59 | 49,02 | 4,00 |
| 4 | HFD2 | 10,35 | 19,26 | 24,71 | 3,81 | 47,69 | 4,52 |
Except for water, all fractions are expressed in dry matter. ND1 and HFD1: results of 1st analysis; ND2 and HFD2: results of the 2nd analysis. CH: Carbohydrate; ND: Normal Diet; HFD: High Fat Diet.
Animals
30 male wistar rats aged 10-12 weeks (body weight between 170-220 grams) were maintenance in Molecular Biology and Immunology Laboratory, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia21,22,23. After acclimatization for 2 weeks (in the room with sufficient air circulation, room temperature 28±2⁰C, humidity 50±10% and room lights arranged in a 12-h light and dark cycle), wistar rats were randomly divided into 5 groups (6 rats/group). Group I was the group 0 weeks (baseline), group II (ND8) was the controls group given ND for 8 weeks, group III (ND16) was a control group given ND for 16 weeks, group IV (HFD8) was the treatment group given HFD for 8 weeks and group V (HFD 16) was the treatment group given HFD for 16 weeks. All wistar rats have free access to food and drink (ad libitum). Blood samples were taken through intracardiac at week 0 (after acclimatization) for the baseline group, week 8 for the HFD 8 and ND8 groups and week 16 for the HFD16 and ND16 groups for examination of plasma ACE2 levels and serum cysC levels. All of these procedures were carried out at the Laboratory of Molecular Biology and Immunology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
Measurement of body weight, body fat percentage and obesity index
The body weight and length of the naso-anal were measured to obtain the obesity index value. The obesity index measured was lee index, rohrer index, and TM index. Rats were declared obese if Lee index value is >0.3, Rohrer index >30, and TM index >50. Body fat percentage is calculated based on TM index24. The formula used is:
Rohrer index = {body weight (gram)/naso-anal length (cm)3}×103
formula
TM index = body weight (gram)/naso-anal length (cm)2.383×103
Body fat percentage = 0.581 × TM index – 22.03
Collection of blood samples, Examination of Plasma ACE2 Levels and Serum CysC Levels
Rats were restrained to control head and body movements. After intraperitonial anesthesia using ketamine anesthetic agents (100 mg/kg) and xylasine (10 mg/kg), blood was taken as much as 2 ml intracardiac using a needle 19-21. Blood was inserted into the sample tube. Blood was centrifuged to obtain plasma/serum and stored at -80°C before examination. Plasma ACE2 levels were measured by elisa method using ACE2 reagents (Rat ACE2/ACE-2 Elisa Kit LS-F33783, LifeSpan BioScience, Inc.). Serum CysC levels were measured by the Elisa method using a cystatin C reagent (Rat CST3/Cystatin C Elisa Kit LS-F21524, LifeSpan BioScience, Inc.). Plasma ACE2 levels and serum CysC were read using Elisa Reader 270 (Biomeriaux, France) with a wavelength of 450 nm for 30 minutes in units of ng/ml. Each the experiment were done in duplicate.
Statistical Analysis
The data obtained was processed using SPSS version 24 for Windows then analyzed with a significance level of <0.05. Before testing the difference hypothesis and correlation, the data normality test was first carried out. The difference test conducted was a one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. Correlation test performed using Pearson correlation test.
Results
Effects of HFD on Body Weight, Obesity Index and Body Fat Percentage
As expected, there were differences in body weight, obesity index and body fat percentage between ND and HFD groups (table 3). The body weight in the HFD8 group was higher than of ND8 group (p< 0.001), as well as between groups given HFD16 and ND16 (p<0.001), even between ND8 and ND16 groups (p<0.05). Body weight in the HFD8 group was higher than HFD16 group.
Obesity index measured was Lee index, Rohrer index and TM index. Lee index in the HFD8 group was higher than ND8 group (p<0.001). Likewise between HFD 16 group and ND16 group (p<0.001). The duration of administration of HFD did not affect the Lee index as evidenced by not finding differences between groups given HFD 8 and HFD16. Interestingly, Lee index after administration of ND or HFD did not show obesity. Rohrer index and TM index were also higher in the HFD group than ND for both between ND8 and HFD8 and between ND16 and HFD16 (respectively, p<0.001 and p<0.001). The interesting thing was also the Rohrer index and TM index in all groups showed good value of obesity after given ND or HFD.
Body fat percentage is calculated based on the TM obesity index value . Body fat percentage was also higher in the HFD group than ND for both between ND8 and HFD8 and between ND16 and HFD16 (respectively, p<0.001 and p<0.01).
This finding means that administration of HFD increased body weight, body fat percentage and obesity index in wistar rats. The longer the HFD administration, the higher the Rohrer index, TM index and body fat percentage.
Table 3: The Effect of HFD on Body Weight, Obesity Index and Body Fat Percentage
| Parameter | Diet Groups | ||||
| Baseline | ND8 | ND16 | HFD8 | HFD16 | |
| Body Weight (gr) | 185.17 ± 6.75 | 204 ± 14.77 | 220.17 ± 5.98d*** | 339.83 ± 14.77a* | 334.33 ± 10.94b* |
| Lee Index | 0.22 ± 0.01 | 0.24 ± 0.00 | 0.25 ± 0.00 | 0.30 ± 0.00a* | 0.30 ± 0.00b*c* |
| Rohrer Index | 29.10 ± 2.81 | 34.03 ± 1.44 | 33.85 ± 1.00 | 51.58 ± 2.20a* | 54.21 ± 2.82b* |
| TM Index | 49.35 ± 4.62 | 56.67 ± 0.00 | 58.66 ± 2.80 | 59.81 ± 5.35a* | 92.86 ± 6.27b* |
| Body fat percentage (%) | 6.44 ± 2.59 | 10.89 ± 2.30 | 12.03 ± 1.62 | 29.95 ± 3.09 a* | 31.07 ± 5.56 b** |
Data are presented in the mean ± SD form (n=6). Differences between groups were analyzed using the one-way ANOVA test followed by Bonferroni’s test. Significant differences are indicated by superscript: a between ND8 and HFD8, b between ND16 and HFD16, c between HFD8 and HFD16 and d between ND8 and ND16. *p<0.001, **p<0.01, ***p<0.05.
Effects of HFD on Plasma ACE2 Levels
Plasma ACE2 levels in the HFD group were higher than in the ND group for both between ND8 and HFD8 and between ND16 and HFD16 (respectively, p<0.001 and p<0.001). Plasma ACE2 levels in the HFD16 group were higher than HFD8 group (p<0.001). The longer the HFD is given, the higher the plasma ACE2 levels. Administration of HFD increases plasma ACE2 levels (table 4).
Effects of HFD on Serum CysC Levels
Serum cysC levels in the HFD group were higher than in the ND group between ND8 and HFD8 and between ND16 and HFD16 (respectively, p<0.001 and p<0.001). Although statistically it did not show a significant difference, serum cysC levels in the HFD16 group were higher than those of HFD8 group. Giving HFD increases serum cysC levels (table 4).
Table 4: Effects of HFD on Plasma ACE2 Levels and Serum Cystatin C Levels
| Biomarker | Diet Groups | ||||
| Baseline | ND8 | ND16 | HFD8 | HFD16 | |
| Plasma ACE2 Level (ng/ml) | 1.63 ± 0.07 | 1.92 ± 0.07 | 2.05 ±0.06 | 2.20 ±0.06a* | 2.37 ±0.07b*c* |
| Serum Cystatin C Level (ng/ml) | 0.99 ± 0.07 | 1.22 ± 0.08 | 1.32 ±0.06 | 1.46 ±0.07a** | 1.57 ±0.05b* |
Data are presented in the mean ± SD form (n = 6). Differences between groups were analyzed using the one-way ANOVA test followed by Bonferroni’s test. Significant differences are shown by superscript: a between ND8 and HFD8, b between ND16 and HFD16, c between HFD8 and HFD16 and d between ND8 and ND16. *p<0.001, **p<0.01, ***p<0.05.
Body Weight, Obesity Index, Body Fat Percentage and Plasma ACE2 Levels
Body weight correlated with plasma ACE2 levels , direction of positive correlation with very strong correlation strength (p<0.001, r=0.867). Obesity index of Lee, Rohrer and TM also correlated with plasma ACE2 levels, direction of positive correlation with very strong correlation strength (respectively p<0.001, r=0.882; p<0.001, r=0.866; p<0.001, r=0.870). The body fat percentage also correlated with plasma ACE2 levels, the direction of the positive correlation with a very strong correlation strength (p<0.001, r=0.862). The direction of positive correlation means that the higher body weight, obesity index and body fat percentage, the higher the plasma ACE2 levels (Figure 1).
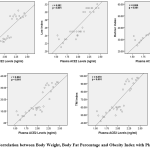 |
Figure 1: Correlation between Body Weight, Body Fat Percentage and Obesity Index with Plasma ACE2 Levels |
Plasma ACE2 Levels and Serum CysC Levels
Plasma ACE2 levels correlated with serum cysC levels (p<0.001). The direction of correlation was positive with a very strong correlation strength (r=0.918). A positive correlation direction means that the higher plasma ACE2 levels, the higher serum cysC levels (Figure 2).
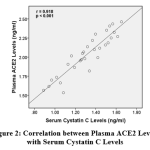 |
Figure 2: Correlation between Plasma ACE2 Levels with Serum Cystatin C Levels |
Discussion
Body weight and body fat percentage after the administration of HFD was higher than ND both for 8 weeks and 16 weeks. This finding is consistent with many studies that have been done before. There was an increase in adiposity (body weight, fat mass, percentage of fat and adipocyte size) after administration of HFD both for 8 weeks20 and for 16 weeks 25,26. Significant weight gain occurred during 4 weeks of diet and tended to persist until the end of the study24. Recent findings indicate that administration of HFD for 8 weeks adds 169% of adipose retroperitoneal tissue and 107% of epididymal tissue27. Other recent findings prove that tissue adipose visceral weight increases after 6 weeks of HFD administration even to 300% weigher after 24 weeks of HFD administration. Administration of HFD for 6 weeks is considered a short period. However, Crinigan et al (2015) reported that administration of HFD short-term (6 weeks) increases visceral adiposity9.
Increased body weight from several experimental animal studies given HFD varied. This is due to differences in research characteristics such as experimental animal clusters, intestinal microbiota conditions25, initial body weight, dietary fat composition, method of administration, experimental period and the amount of food intake consumed by experimental animals. Mice C57BL/6 and wistar rat strains are most widely used in research models of HFD administration.
The physiological mechanism by which a HFD can increase body weight is explained in many previous studies. HFD causes hypertriglyceridemia which causes leptin sensitivity. Leptin is a protein secreted by adipocytes, transported across the blood brain barrier and work in the central nervous system to regulate food and energy expenditure. Hypertriglyceridemia induced with HFD inhibits this mechanism. Because of limitations, this study did not measure triglyceride levels. In addition, energy from fat has a greater effect in increasing body weight than energy from non-fat. Fat has a very high efficiency in using nutrients compared to protein and carbohydrates. Signs of satiety that are weaker from fat than carbohydrates and proteins also play a role in the desire to consume a HFD28.
Obesity index after administration of HFD is also higher than ND both for 8 weeks and 16 weeks. The longer the diet is given, the higher the obesity index. The results of this study are consistent with previous research. Giving a high-fat diet for 8 weeks increased the Lee index, Rohrer index, and TM index and body fat percentage. The obesity index increases according to the duration of administration of HFD and the degree of obesity24. Giving HFD for 12 weeks increased the lee’s obesity index by 10.45% compared to the control group. Increasing adipocyte mass also increases body weight and obesity index29. In contrast, previous studies found that administration of HFD for 3 weeks did not increase obesity index30. This difference may be due to the duration of diet in their study which is too short. The macronutrient composition/percentage of fat in the diet given is not mentioned in their publications.
We were the first to research the effect of giving HFD to plasma ACE2 levels and we found plasma ACE2 levels in the HFD16 group were higher than in the HFD8 group. Administration of HFD increases plasma ACE2 levels. This shows the protective role of ACE231.
The duration of giving a HFD influences the dynamic of plasma ACE2 levels both short and long term administration. Provision of a long-term diet is 8 weeks or more32. The longer the administration of HFD, the higher plasma ACE2 levels. We found that long-term administration of HFD increased plasma ACE2 levels. This increase is in response to compensation for renoprotective.
We also found levels serum cysC increased after administration of HFD compared to ND both for 8 and 16 weeks. The results of our study support that the administration of HFD causes a decrease in early stage kidney dysfunction seen in increasing cysC serum levels. This finding is not surprising because damage to renal structure and function caused by HFD has been known in many previous studies. The administration of 15 weeks of HFD in wistar rats caused a decrease in kidney function parameters characterized by urinary albumin excretion and increased plasma creatinine levels3. Rats given HFD for 6 weeks showed lower creatinine clearance8. Renal injury in rats given HFD for 16 weeks occurs due to lipid accumulation, infiltration of macrophages (inflammation) and oxidative stress in the nose that causes glomerulosclerosis, interstitial fibrosis and albuminuria5. Changes in kidney function and structure are also observed in C57BL/6 male mice given by HFD for 12 weeks showed albuminuria and lipids accumulation in the glomeruli and proximal tubules6. Changes in structure appear even worse in the renal cortex (glomerulus, tubules, interstitium and blood vessels) due to the administration of longer duration of HFD (18 months)4. A recent study found that giving HFD also causes a decrease in the diameter of the capsule of the tubules and the cell volume of bowman’s capsule27.
However, research on the effects of HFD on kidney function with biomarkers cysC levels is very little. In accordance with our findings in this study, recent studies in C56BL/6 male mice given HFD for 22 weeks proved to lead to increased excretion of urine albumin and cysC levels which implies glomerular and proximal functional changes in tubules. Increased urinary cysC excretion was accepted as a biomarker for tubular injury7. Other research found different results in which the provision of short term HFD for 6 weeks on srague-dawley rats did not cause early renal injury. CysC levels in rats given HFD were higher than ND, but not statistically significant. Creatinine and urine protein concentrations also show no difference. The possibility that the duration of HFD administration used in this study is not sufficient to cause a decline in kidney function9.
Plasma ACE2 levels are positively correlated with levels cysC serum. The direction of positive correlation means that the higher plasma ACE2 levels, the higher the serum cysC levels. We were the first to carry out a study related to the correlation between ACE2 and a decrease in early kidney function disorders using cysC biomarker. Previous studies have reported on the correlation of ACE2 with biomarker of different decrease on kidney function. The mRNA expression of urine ACE2 gene was positively correlated with kidney function parameters, namely the degree of proteinuria and creatinine serum and negatively correlated with eGFR33. In a rat model of CKD, ACE2 expression decreased significantly. Inhibition of ACE2 causes a decrease in cortical ACE2 activity, reduces 50% of FITC-inulin clearance and significantly increases urine albumin excretion34. The findings in this study indicate that plasma ACE2 levels play a role in decreasing early kidney function, as evidenced by an increase in serum cysC levels.
We also found that body weight, body fat percentage, and obesity index were positively correlated with plasma ACE2 levels with very strong correlation strength. The higher the body weight, the percentage of body fat, and the obesity index, the higher plasma ACE2 levels. Obesity is associated with overly active systemic and local RAS including adipose RAS. This proves that there is a relationship between RAS and obesity with special emphasis on the role of adipose tissue RAS in the pathogenesis of metabolic disorders in obesity35. Possible findings indicate that HFD-induced obesity activates RAS through the role of ACE2.
Conclusions
Administration of HFD has been proven to increase body weight, body fat percentage, and obesity index. Administration of HFD increases plasma ACE2 levels. Administration of HFD causes a decrease in early renal function as evidenced by the increase in serum cysC levels. Plasma ACE2 levels play a role in the occurrence of an decrease in early stage renal function induced by administration of HFD. Body weight, body fat percentage, and obesity index also play a role in increasing Plasma ACE2 levels.
Further research is needed on intervention variations in the form of conventional or herbal medicine which can inhibit the decline of early renal function due to HFD administration, research on the effects of HFD administration on the cysC gene and other RAS components, and research on the administration of HFD with a longer duration.
Ethical Approval
The research was carried out after obtaining recommendations for ethical approval from The Committee on Ethics of Medical Research, Faculty of Medicine, Hasanuddin University (Recommendation number: 366/H4.8.4.5.31/PP36-KOMETIK/2018) date May 14, 2018. Procedures for experimental animals were carried out in accordance with the principles of the Declaration of Helsinki.
Acknowledgments
A higher appreciation to Lembaga Pengelola Dana Pendidikan (LPDP) and all staff from the Laboratory of Molecular Biology and Immunology, Faculty of Medicine, University of Hasanuddin, Makassar.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Funding Sources
This research was funded by Lembaga Pengelola Dana Pendidikan (LPDP) Indonesia.
Abbreviations
ACE2: Angitensin Converting Enzyme 2; CH: Carbohydrate; CKD: Chronic Kidney Disease; CysC: Cystatin C; eGFR: Estimated Glomerular Filtration Rate; FITC-Inulin: Fluoresceinyl Isothiocyanate; HFD: High Fat Diet; mRNA: Messenger Ribonucleic Acid; ND: Normal Diet; RAS: Renin Angiotensin System.
References
- Duan Y, Zeng L, Zheng C, Song B, Li F, Kong X. Inflammatory links between high fat diets and diseases. Front Immunol. 2018;9(2649):1-10.
- Niu H, Li Y, Li H, et al. Matrix metalloproteinase 12 modulates high-fat-diet induced glomerular fibrogenesis and inflammation in a mouse model of obesity. Sci Rep. 2016;6(December 2015):1-14.
- Cao J, Inoue K, Sodhi K, Puri N, Peterson SJ, Abraham NG. High-fat diet exacerbates renal dysfunction in SHR: reversal by induction of HO-1–adiponectin axis. Obesity. 2017;20(5):945-953.
- Aguila MB, Mandarim-De-Lacerda CA. Effects of chronic high fat diets on renal function and cortical structure in rats. Exp Toxic Pathol. 2003;55(2-3):187-195.
- Kume S, Uzu T, Araki S, Sugimoto T, Isshiki K. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J Am Soc Nephrol. 2007;18(10):2715-2723.
- Deji N, Kume S, Araki S, et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Ren Physiol. 2018;296(1):118-126.
- Park J, Choi B, Ku S-K, et al. Amelioration of high fat diet-induced nephropathy by cilostazol and rosuvastatin. Arch Pharm Res. 2017;40(3):391-402.
- Lu J, Bankovic-calic N, Ogborn M, Saboorian MH, Aukema HM. Detrimental effects of a high fat diet in early renal injury are ameliorated by fish oil in han : SPRD- cy rats. Nutr Interact Toxic. 2003;133(1):180-186.
- Crinigan C, Calhoun M, Sweazea KL. Short-term high fat intake does not significantly alter markers of renal function or inflammation in young male sprague-dawley rats. J Nutr Metab. 2015:1-9.
- Schanz M, Pannes D, Dippon J, Wasser C, Alscher MD, Kimmel M. The Influence of thyroid function, inflammation, and obesity on risk prediction of acute kidney injury by cystatin c in the emergency department. Kidney Blood Press Res. 2016;41(5):604-613.
- Marwyne MNN, Loo CY, Halim AG, Norella K, Sulaiman T, Zaleha MI. Estimation of glomerular filtration rate using serum cystatin C in overweight and obese subjects. Med J Malaysia. 2011;66(4):313-317.
- Lafarge JC, Naour N, Clément K, Guerre-Millo M. Cathepsins and cystatin C in atherosclerosis and obesity. Biochimie. 2010;92(11):1580-1586.
- Rüster C, Wolf G. The Role of the Renin-angiotensin-aldosterone system in obesity-related renal diseases. Semin Nephrol. 2013;33(1):44-53.
- Soler MJ. ACE2 alterations in kidney disease. 2013;28(1):2687-697.
- Mizuiri S, Ohashi Y. ACE and ACE2 in kidney disease. World J Nephrol. 2015;4(1):74-82.
- Tikellis C, Thomas MC. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012:1-8.
- Williams VR, Scholey JW. Angiotensin-converting enzyme 2 and renal disease. Curr Opin Nephrol Hypertens. 2018;27(1):35-41.
- Lely A, Hamming I, van Goor H, Navis G. Renal ACE2 expression in human kidney disease. J Pathol. 2004;204(5):587-593.
- Li C, Culver SA, Quadri S, et al. High-fat diet amplifies renal renin angiotensin system expression , blood pressure elevation , and renal dysfunction caused by Ceacam1 null deletion. Am J Physiol Endocrinol Metab. 2015;309(9):802-810.
- Lozano I, Werf R Van Der, Bietiger W, et al. Disorders in rats : impact on diabetes risk, hepatic and vascular complications. Nutr Metab (Lond). 2016:1-13.
- Kamelia E, Islam AA, Hatta M, et al. The effect of administration of ethanol extract from musa paradisiaca L. (MPL) fruit on the caspase-3 mRNA gene expression in rat amyloid beta induced, an alzheimer’s disease model, Asian J Pharm and Clin Res. 2018; 11(4): 298–302.
- Simanjuntak TB, Hatta M, Tahir AM, Sirait RH, Karo MB, Tambaib T, Junita AR, Analysis of anti-toxoplasma immunoglobulin G and immunoglobulin M antibody levels after intervention with Curcuma Longa extract on early pregnant mice with acute toxoplasmosis. J Global Infect Dis. 2019; 11(1): 25-29.
- Kamelia E et al. Evaluation of caspase-3 mRNA gene expression activity in amyloid beta-induced alzheimer’s disease rats J Med Science. 2017;17(3): 117–125.
- Lee S, Kim J, Lee Y, et al. Anti-obesity effect of monascus pilosus mycelial extract in high fat diet-induced obese rats. J Appl Biol Chem. 2011;54(3):197-205.
- Cranford TL, Enos RT, Velasguez KT, et al. Role of MCP-1 on inflammatory processes and metabolic dysfunction following high-fat feedings in the FVB/N strain taryn. Int J Obes. 2016;40(5):844-851.
- Chodavarapu H, Chhabra KH, Xia H, Shenoy V, Yue X, Lazartigues E. High-fat diet-induced glucose dysregulation is independent of changes in islet ACE2 in mice. Am J Physiol Regul Integr Comp Physiol. 2018;311(51):1223-1233.
- José I, Garcia P, Cézar JS, et al. Effects of high fat diet on kidney lipid content and the Na , K-ATPase activity. Brazilian J Pharm Sci. 2018;54(1):1-13.
- Hariri N, Thibault L. High-fat diet-induced obesity in animal models. 2019;23(2):270-299.
- Miao Y, Kang H, Li J, et al. Effect of sheng-jiang powder on multiple-organ inflammatory injury in acute pancreatitis in rats fed a high-fat diet. World J Gastroenterol. 2019;25(6):683-695.
- Shabbir F, Khan S, Yousaf MJ, Khan MA, Rajput TA. Comparison of effect of high fat diet induced obesity and subsequent atorvastatin administration on different anthropometric measures in sprague dawley rats. Pak Armed Forces Med J. 2016;66(5):699-704.
- Liang B, Li Y, Han Z, et al. ACE2-Ang (1-7) axis is induced in pressure overloaded rat model. Int J Clin Exp Pathol. 2015;8(2):1443-1450.
- Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB. Central renin–angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin ii – elicited hypertension. Hypertension. 2015;67(1):1-8.
- Wang G, Lai FM, Lai K, Chow K. Urinary mRNA expression of ACE and ACE2 in human type 2 diabetic nephropathy. Diabetologia. 2008;51(6):1062-1067.
- Dilauro M, Zimpelmann J, Robertson SJ, Genest D, Burns KD. Effect of ACE2 and angiotensin-(1 – 7) in a mouse model of early chronic kidney disease. Am J Physiol Ren Physiol. 2010;298(6):1523-1532.
- Kalupahana NS. The renin-angiotensin system : a link between obesity, inflammation and insulin resistance. Obes Rev. 2011;13(2):136-149.








