Syeda Nishat Fathima*1 and S. Vasudeva Murthy2
and S. Vasudeva Murthy2
1Department of Pharmacy, Shri Jagdishprasad Jhabarmal Tibrewala University, Rajasthan, India.
2Department of Pharmacology, Jayamukhi College of Pharmacy, Warangal, Telangana, India.
Corresponding Author E-mail: syeda.nishat.fathima85@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1744
Abstract
The present study is designed to evaluate the cardioprotective effect of Rosa damascena petals called rose on isoproterenol-induced myocardial infarction in experimental rats. The experimental rats were divided into 4 groups, each consisting of 6 animals. Group 1 rats received standard diet and drinking water ad libitum for 45 days and will serve as a control group. Group 2 received a standard diet and drinking water ad libitum for 45 days. Group 3 Rats received metoprolol succinate via oral route at a daily dosage of 2.5 mg/Kg body weight for a period of 45 days. Group 4 Rats received Rosa damascena extract via oral route at a daily dosage of 500 mg/Kg body weight for a period of 45 days. All the groups except group 1 were then treated with isoproterenol in two doses (85 mg/kg body weight) by subcutaneous injection on 44 and 45th day at an interval of 24 hrs. At the end of the treatment, blood was collected from all the groups by puncturing the retro-orbital plexus for the estimation of biochemical parameters and the animals were sacrificed to remove the heart for histopathological studies. Serum cardiac marker enzymes such as creatine kinase (CK), creatine kinase muscle-brain (CK-MB), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total protein were estimated. Total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL) and very low density lipoprotein (VLDL) levels were also measured Antioxidant parameters like catalase (CAT), superoxide dismutase (SOD), glutathione (GSH), and malondialdehyde (MDA) levels were evaluated in heart tissue homogenate. Histopathological and ultrastructural studies were then carried out. The results of the present study indicated that ethanolic extract of Rosa damascena showed myocardial retrieval by restoring the cardiac marker enzymes and decreasing the level of plasma lipid profiles along with an increase in HDL. Additionally, level of myocardial antioxidants increased along with a lessening in the content of malondialdehyde. The cardioprotective effect was compared with Metoprolol which was used as the standard. Histopathological findings revealed a decrease in the degree of necrosis and inflammation following pretreatment with Rosa damascena. The present investigation indicates that Rosa damascena exerts cardioprotective activity against isoproterenol-induced cardiac damage in rats.
Keywords
Cardiac Markers; Isoproterenol; Myocardial Infarction
Download this article as:| Copy the following to cite this article: Fathima S. N, Murthy S. V. Cardioprotective Effects to Chronic Administration of Rosa Damascena Petals in Isoproterenol Induced Myocardial Infarction: Biochemical, Histopathological and Ultrastructural Studies. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Fathima S. N, Murthy S. V. Cardioprotective Effects to Chronic Administration of Rosa Damascena Petals in Isoproterenol Induced Myocardial Infarction: Biochemical, Histopathological and Ultrastructural Studies. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/2zc3Eis |
Introduction
In the 21st century, there is a constant effort to incorporate complementary and alternative medications into the practice of conventional medicine, for the management of cardiovascular diseases which are the foremost cause of morbidity and mortality in the world.1 Myocardial infarction (also known as heart attack) is defined pathologically as myocardial cell death due to prolonged ischemia due to decreased oxygen supply or increased oxygen utilization. Reduced cellular glycogen, and relaxed myofibrils and sarcolemmal disruption, are the first ultrastructural changes of myocardial infarction followed by mitochondrial abnormalities. The latest clinical definition of myocardial infarction denotes the presence of acute myocardial injury identified by abnormal cardiac biomarkers in the setting of evidence of acute myocardial ischemia.2
Isoproterenol also known as Isoprenaline is a synthetic derivative of catecholamine used for the management of bradycardia, heart block, and sometimes for asthma. It belongs to the class of non-selective Beta adrenoreceptor agonist as well as Trace amine-associated receptor 1 agonist which is the isopropylaminomethyl analog of adrenaline, which is a significant controller of myocardial contractility and metabolism, thus serving as a standard model for the screening of potentially valuable effects of numerous drugs on cardiac function.3 Isoproterenol induces cardiac necrosis by numerous mechanisms such as enhanced oxygen intake, functional hypoxia leading to ischemia, coronary insufficiency, poor oxygen consumption, increased calcium overload, accumulation of calcium and magnesium in myocardial cells, altered cardiac cell metabolism, increased myocardial cyclic adenosine monophosphate levels, reduced level of high-energy rich phosphate stores, deranged electrolyte ambiance, changed membrane permeability, intracellular myocardial acidosis, raised oxidative stress, and raised levels of myocardial lipid peroxides formation. Isoproterenol induces myocardial infarction by causing changes in hematological, biochemical and histopathological and ultrastructural parameters.4
Rosa damascena also known as Damask rose is a perennial bushy shrub which is the most renowned ornamental plant and the holy ancient herb with novel applications belonging to the family Rosaceae. Its vernacular names include Gulab in Hindi, Gulaabi poovvu in Telugu and Mahakumari or Satapatri in Sanskrit. The chemical composition had shown the presence of Citronellol, nerol, geraniol, phenyl ethyl alcohol, nonadecane, eicosane, nonadecene, geranyl acetate, heneicosane, tricosane, a-guaiene, geranyl acetate, and eugenol. The utmost valuable effects in ancient medicine of Rosa damascena includes management of menstrual bleeding, digestive problems, abdominal pain, chest pain, strengthening of the heart, reduction of inflammation, especially of the neck, cough tonic for children, mild laxative as well as Rose oil cures allergies, depression, nervous stress, grief, tension, headaches, and migraine. Some of the proved pharmacological properties include anti-HIV, antibacterial, antidiabetic, antioxidant, antitussive, hypnotic, and relaxant effect on tracheal.5-6 Rosa damascena petals are used in different Unani formulations for strengthening the heart. The present study has been carried out to discover the cardioprotective effect of Rosa damascena petals as a phytomedicine for the treatment of myocardial infarction.
Materials and Methods
Collection and Authentication of Plant Material
The fresh flowers of the deciduous shrub of Rosa damascena were collected in bulk from the gardens in and around Warangal Urban, Telangana, India. The flowers were authenticated by Dr. P. Veera Reddy, Professor, Anantha Lakshmi Government Ayurvedic Medical College, Telangana. The petals were separated from the sepals and shadow dried.
Drugs and Chemicals
Omeprazole was procured from Ranbaxy Research Laboratories, India and Tween 80 was obtained from S.D Fine Chemicals, India. All other reagents used for the experiments were of high analytical grade.
Preparation of Extract
The ethanolic extract of Rosa damascena petals was prepared by soxhlation extraction method in which 500 grams of petal powder was extracted with 2000 ml. of ethanol. Dry extract was obtained from vacuum distillation, evaporation followed by vacuum drying. The percentage yield was found to be 17.24% and the extract was stored in between 2 and 8°C for further studies. Phytochemical exploration of ethanolic extract revealed the presence of alkaloids, glycosides, carbohydrates, proteins and amino acids, tannins, flavonoids, sterols, and cardiac glycosides.
Experimental Animals
Wistar rats of either sex weighing between 180 to 200 grams were used for the screening of cardioprotective effect. Animals were obtained from the animal house of Jayamukhi College of Pharmacy. All the animals were accustomed to laboratory condition for 1 week before the commencement of experiment and had free access to a standard pellet diet and water ad libitum. The experimental procedures were appraised and approved by Institutional animal ethical committee (IAEC Approval Number of JJTU 29117030/JCP/IAEC/2017, dated 19th August 2017) and experiments conducted according to CPCSEA.
Drugs and Chemicals
Analyzing kits like alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT), total proteins, creatine kinase (CK), triglycerides and cholesterol were obtained from Excel Diagnostics Pvt. Ltd, Hyderabad, India. Creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) kits were obtained from Adi Diagnostics Pvt.Ltd, Telangana, India. All other chemicals which are used for the biochemical parameters assessment were of analytical grade.
Acute Toxicity Studies
The acute oral toxicity test on ethanolic extract of Rosa damascena petals was performed using methods described in guide 423 of the Organization for Economic Cooperation and Development in mice. The ethanolic extract of Rosa damascena petals was found to be safe at 2000mg/kg body weight by Oral route. Even after 14 days, mice were found to be well tolerated with no mortality and no signs of toxicity. The extract is classified in class 5 (a substance with LD50 higher than 2000 mg kg-1 and less than 5000 mg/ kg) and are being deliberated as having low toxicity. Hence the dose of 500 mg/kg was selected for carrying out the evaluation of the cardioprotective activity.
Screening of Cardioprotective Potential by Isoproterenol-Induced Myocardial Infarction in Rats
Treatment Protocol
The experimental rats were divided into 6 groups, each consisting of 6 rats in each group.
Group 1 (Normal control): Rats will receive a standard diet and drinking water ad libitum for 45 days and will serve as negative control group.
Group 2 (Positive Control): Animals will receive a standard diet and drinking water ad libitum for a duration of 45 days.
Group 3 (Active Control): Rats will receive metoprolol succinate via oral route at a daily dosage of 10 mg/Kg body weight for a duration of 45 days.
Group 4 (EERD): Rats will receive Rosa damascena extract via oral route at a daily dosage of 500 mg/Kg body weight for a duration of 45 days.
All the groups except group 1 will be then treated with isoproterenol in two doses (85 mg/kg body weight) by subcutaneous injection on the 44th and 45th day at an interval of 24 hrs.7-8 After the treatment duration, by penetrating the retro-orbital plexus, blood will be collected from all the groups and was allowed to clot at room temperature. The serum was then separated by centrifugation of blood at 2500 rpm for 10 min. The separated serum was used for the assessment of serum and cardiac markers such as total proteins, alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT), triglycerides, cholesterol, high density lipoproteins (HDL),low density lipoproteins (LDL), very low density lipoproteins (VLDL), creatine kinase (CK), creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) was carried out using standard kits. The animals were sacrificed and the heart was isolated for histopathological and Ultrastructural studies. Apart from that heart homogenate was prepared and analyzed for biochemical parameters such as total cholesterol, phospholipids, cardiac glycogen, glutathione, catalase, superoxide dismutase, lipid peroxidation and myocardial membrane-bound enzymes.
Statistical Analysis
Values were expressed as Mean ± Standard Deviation. The Significance of differences among the group was assessed using one-way analysis of variance (ANOVA). The test followed by student’s t-test of significance. p values less than 0.05 were considered as statistically significant.
Results
Effect of Extract on Heart weight
In the present study, there was a significant increase in heart weight of the positive control group when compared to the normal group. A significant decrease in heart weight was seen in Rosa damascena and standard treatment groups (Table 1). The changes in the relative heart weight (Heart weight/Bodyweight Ratio (%)) in the experimental groups was noted with the highest relative heart weight being in the isoproterenol-induced group, which was significantly raised, compared to that of the normal control group. This elevation, however, decreased significantly in the standard metoprolol, and Rosa damascena extracts treated groups.
Table 1: Effect of EERD on heart weight and relative heart weight.
| Body Weight on last day (gm) | Heart Weight (gm) | Heart weight/Bodyweight Ratio (%) | % Change in activity | |
| Group-1 | 227.36±6.47 | 0.814±0.054 | 0.358±0.018 | – |
| Group-2 | 211.54±8.22 | 1.128±0.046 | 0.533±0.016 | 51.42 |
| Group-3 | 229.83±4.64 | 0.847±0.071** | 0.369±0.024** | 4.83** |
| Group-4 | 221.64±7.14 | 1.066±0.027** | 0.498±0.011** | 41.47* |
Values represents mean ± SD. (n=6) One way ANOVA followed by Student’s t-test. *P < 0.05 and **P < 0.01 when compared to Group-2
Effect of Extract on Serum Marker Enzymes
The total protein levels in the serum of the isoproterenol-induced myocardial infarcted rats were significantly lower when compared to that of rats of the normal control group. In rats pretreated with standard metoprolol and EERD significantly increased the reduced protein levels were noted. (Figure 1).
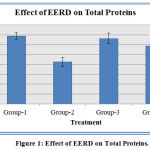 |
Figure 1: Effect of EERD on Total Proteins.
|
Data represented as mean ± SD. n = 6; When compared with group 2 P value is less than 0.001 in group 3 and group 4.
Myocardial damage induced by isoproterenol caused a significant increase in ALP as compared to normal group animals. The treatment with isoproterenol and EERD showed a significant decrease in the levels of enzymes compared to that of the positive control group. The activities of aspartate transaminase and alanine transaminase in serum were significantly higher in Isoproterenol treated positive control when compared with normal control rats. Animals pretreated with EERD showed decreased activities of AST and ALT in serum. The activities of these enzymes were significantly lower in standard (metoprolol), treated rats compared to isoproterenol induced myocardial infarcted rats. (Figure 2).
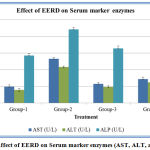 |
Figure 2: Effect of EERD on Serum marker enzymes (AST, ALT, and ALP).
|
Data represented as mean ± SD. n = 6; When compared with group 2, the P value is less than 0.001 in group 3 and group 4.
Effect of Extract on Serum Lipid Profile
Isoproterenol-induced group-2 rats showed a significant increase in the levels of serum triglycerides, cholesterol, low-density lipoproteins (LDL) and Very low-density lipoproteins (VLDL). The decrease in the levels of high-density lipoproteins (HDL) in serum was also observed. Oral pretreatment with the standard and EERD for a period of 45 days significantly decreased Triglycerides, cholesterol, LDL and VLDL, and increased HDL levels were seen. (Figure 3).
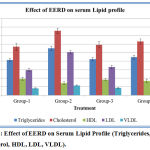 |
Figure 3: Effect of EERD on Serum Lipid Profile (Triglycerides, Cholesterol, HDL, LDL, VLDL).
|
Data represented as mean ± SD. n = 6; When compared with group 2, P value is less than 0.001 in group 3 and group 4.
Effect of Extracts on Cardiac Markers in Serum
The Creatine Kinase (CK) in the serum of Isoproterenol induced Myocardial Infarction rats was significantly higher in comparison to the normal control rats. Highly significant reduction in CK levels were observed in Myocardial Infarction rats that were pre-treated with EERD. Significant reduction in CK levels were also observed in Myocardial Infarction rats pre-treated with standard metoprolol. Isoproterenol administration produced a significant decrease in myocytes grievance indicator Creatine Kinase-MB (CK-MB) in serum compared to that of the normal control group. Pretreatment of EERD has significantly restored the levels of CK-MB as that of Metoprolol. Serum Lactate Dehydrogenase (LDH) content on estimation showed significant increase in the Isoproterenol treated rat, which were significantly brought down on treatment with EERD as well as standard metoprolol. (Figure 4).
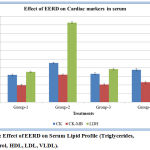 |
Figure 4: Effect of EERD on Serum Lipid Profile (Triglycerides, Cholesterol, HDL, LDL, VLDL).
|
Data represented as mean ± SD. n = 6; When compared with group 2, P value is less than 0.001 in group 3 and group 4.
Effect of Extract on biochemical parameters in heart homogenate:
Total cholesterol levels in heart homogenate were increased significantly in isoproterenol-induced positive control group when compared with the normal control group. EERD attenuated the increased total cholesterol levels to normal levels. EERD significantly decreased the total cholesterol in rats when compared to Isoproterenol-induced myocardial infarcted rats. The phospholipids levels of the Isoproterenol an induced myocardial infarcted rat was significantly lower compared to the normal control rats. Pretreatment with the EERD significantly brought the phospholipids level to the normal when compared to isoproterenol-induced positive control group. When compared with the normal control group cardiac glycogen content in isoproterenol positive group was significantly decreased. Pretreatment with EERD restored the cardiac glycogen levels towards the normal. (Figure 5).
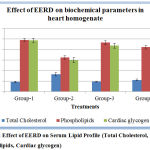 |
Figure 5: Effect of EERD on Serum Lipid Profile (Total Cholesterol, Phospholipids, Cardiac glycogen).
|
Data represented as mean ± SD. n = 6; When compared with group 2, P value is less than 0.001 in group 3 and group 4.
Effect of Extract on Antioxidant Parameters in Heart Homogenate
Glutathione (GSH), Catalase (CAT) and superoxide dismutase (SOD) content in the cardiac tissue on examination showed significant decrease in the positive control isoproterenol-treated rats, which was significantly elevated with EERD. Per se EERD however, did not show any significant changes when compared with control, which means that they manifested almost the same status as that of control. The malondialdehyde (MDA) content in the cardiac tissue showed significant increase in isoproterenol-treated rats when compared with the control and significant decrease in EERD treated rats, when compared with the isoproterenol positive control group. (Figure 5).
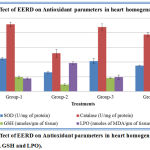 |
Figure 6: Effect of EERD on Antioxidant parameters in heart homogenate (SOD, CAT, GSH and LPO).
|
Data represented as mean ± SD. n = 6; When compared with group 2, P value is less than 0.001 in group 3 and group 4.
Effect of Extract on myocardial Membrane-bound enzymes in heart homogenate:
The activity of Na+/K+ ATPase was decreased significantly and the activity of Ca2+ ATPase were increased significantly in the heart of Isoproterenol induced positive control group when compared to normal control group. EERD pre‐treatment to isoproterenol-induced rats increased significantly the activity of Na+/K+ ATPase and decreased significantly the activity of Ca2+ ATPase in the heart when compared to isoproterenol-induced rats. (Figure 7).
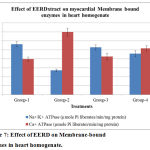 |
Figure 7: Effect of EERD on Membrane-bound enzymes in heart homogenate.
|
Data represented as mean ± SD. n = 6; When compared with group 2, the P value is less than 0.001 in group 3 and group 4.
Effect of Extract on histological and ultra-structural changes of the Myocardium:
Photomicrographs were used to evaluate the type of damage in the cardiac tissue (Figure 8).
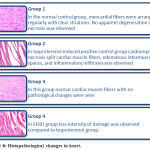 |
Figure 8: Histopathological changes in heart.
|
Ultrastructural analysis of the myocardial tissue showed the normal architecture of myocardial nucleus and mitochondria and myofibrils; in the Isoproterenol treated positive control group irreversible damage to myocardial tissue and damage to myofibrils were seen whereas pretreatment with EERD showed well-preserved mitochondria and less evidence of myocyte injury, as well as the preservation of the integrity of the mitochondrial membrane. (Figure 9).
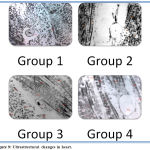 |
Figure 9: Ultrastructural changes in heart.
|
Discussion
Although total protein measurement is a non-specific measurement, as it can be influenced by many variables, it provides general information reflecting disease states in many organ systems. Among several important functions of proteins is the antioxidant scavenging capacity. Isoproterenol administration induces myocardial muscle damage by generation of free oxygen radicals. The decrease in serum total protein in myocardial infarction can also be due to, the increased degradation of plasma proteins by free oxygen radical, as protein sulfhydryls serve as a sacrificial antioxidant and prevent plasma lipid peroxidation as well as being targets for oxidative damage.9 The effects of the current study suggest that the increase in total serum protein may be due to the involvement of EERD in the defense against oxidative stress-related with acute myocardial infarction.
Alkaline phosphatase is present in all tissues throughout the whole body. The major function of alkaline phosphatase is transporting through the cell membranes. Injury to the tissues causes the release of ALP into the bloodstream. The higher ALP levels have been suggested, including vascular calcification, endothelial dysfunction, inactivation of inorganic pyrophosphates (a process that can increase vascular calcification) as well as inflammation and oxidative stress.10 In this study elevated ALP levels that were observed, due to the treatment of Isoproterenol were restored towards normalization in the standard and extract treated group.
AST is distributed in all tissues but primarily found in the liver, heart and skeletal muscles in high concentration. The elevated mitochondrial AST is seen in extensive tissue necrosis during myocardial infarction. Isoproterenol causes mitochondrial-related oxidative stress which causes injury to mitochondria that leads to cell necrosis and mitochondrial disintegration thereby releasing AST in the blood. ALT on the other hand increases due to endothelial dysfunction induced by atherosclerosis and inflammation.11 In the present study, marked elevations in the level of these enzymes in the serum of the positive control group suggest the occurrence of considerable membrane damage compared with the normal control group. Treatment with standard and EERD resulted in a significant reduction in the levels of these enzymes toward near-normal levels compared with the Isoproterenol induced positive control group.
Cardiovascular risk factors such as hypercholesterolemia and hypertriglyceridemia have extensively considered for gaining insight into pathophysiology; epidemiology and therapy of myocardial infarction. Excess lipids such as circulating cholesterol, triglycerides and its accumulation in the heart are associated with cardiovascular damage. Changed metabolism of lipids can change the cardiac function by altering the properties of the cardiac cell membrane, and these changes might contribute to the cell death. Cholesterol is also a foremost component of the atherosclerotic plaque that is associated with myocardial infarction. The increased cholesterol levels of lead to an increase in the myocardial membrane fluidity and regulates myocardial membrane permeability; alter internal viscosity of the myocardium, and also the internal chemical composition is changed. An increase in the VLDL, LDL, and decrease in HDL are associated with higher risk for myocardial infarction which has been established in this study. The reason for the surge in LDL in circulation is due to the hyperlipidemia caused by isoproterenol, which in turn leads to the buildup of harmful deposits of plaque in the arteries thus leading to coronary heart diseases. Isoproterenol administration also increases the uptake of LDL from the blood by myocardial membranes thus increasing the myocardial cholesterol levels. An increase in levels of LDL positively correlates with Isoproterenol, whereas HDL levels reveal a negative relationship. HDL is recognized to inhibit the uptake of LDL from the arterial wall and additionally helps the transport of cholesterol from peripheral tissues to the liver where it is catabolized and excreted from the body.12 Pre-treatment with EERD substantially minimized the changes in the levels of serum lipoproteins by increasing HDL and decreasing LDL and VLDL levels in isoproterenol initiated myocardial infarction. Increased levels of triglycerides are related with cardiovascular disturbances and Isoproterenol promotes lipolysis in the myocardium. This observed increase possibly be due to the impaired removal of VLDL from the serum. An increase in the synthesis of triglycerides in the heart tissue should be due to the accumulation of acyl CoA and an augmented production of glycerol by using accelerated glycolytic flux as indicated by previous study.13 Pre-treatment with EERD reduced the levels of triglycerides in myocardial infarcted rats by inhibiting lipolysis in the myocardium by exhibiting membrane stabilizing property of EERD.
The myocardium contains high concentrations of diagnostic markers of MI; when it is metabolically damaged, it releases its contents into the extracellular fluids. Of all the macromolecules that are leaked from the damaged tissue, myocardial enzymes are the best markers of tissue damage because of their tissue specificity and catalytic activity. When myocardial cells are damaged or destroyed due to a deficiency in the oxygen supply or deficiency in the availability of glucose, the cardiac membrane becomes permeable or may rupture entirely, resulting in the leakage of enzymes.14 Creatine kinase is an enzyme found in the skeletal muscle, brain, heart and other types of body tissues. It catalyzes creatine to phosphocreatine and adenosine diphosphate (ADP) exchange by consuming adenosine triphosphate (ATP). This enzyme reaction is a reversible process. The total creatine kinase is a very simple and cheap test. Increased amounts of creatine kinase are released into the circulation while there is muscle damage. The activity of total serum creatine kinase is found to be increased in isoproterenol induced myocardial infarcted rats.15 The increased CK activity in isoproterenol induced myocardial infarcted rats was attenuated by extract pretreatment.
The activity assay for CK-MB in serum is an important cardiac diagnostic marker due to its marked abundance of this enzyme in myocardial tissue and its virtual absence from most other tissues as well as its consequent sensitivity. CK-MB isoenzyme activity is useful as it is an index for the initial diagnosis of not only myocardial infarction, but also any type of myocardial injury. Leakage of CK-MB into the circulation may occur when cell membranes become more permeable or rupture due to injury or oxidative stress. The amounts of this cellular enzyme in the serum reflect the alterations in myocardial plasma membrane integrity and/or permeability. Furthermore, the amount of the enzymes appearing in serum is reported to be proportional to the number of necrotic cells, which also reflects a nonspecific alteration in the plasma membrane integrity and/or permeability as a response to β-adrenergic stimulation by Isoproterenol.16 In the present study, rats administered with Isoproterenol showed significant increases in the levels of CK-MB in serum, indicating Isoproterenol induced necrotic damage of the myocardium and leakiness of the plasma membrane. Pretreatment with EERD however, resulted in lowered activities of CK-MB in the serum, indicating that EERD helps in maintaining the membrane integrity, thereby restricting the leakage of these enzymes.
Lactate dehydrogenase enzyme is expressed extensively in body tissues, such as blood cells and heart muscle. Tissue breakdown releases LDH, and therefore, LDH can be measured as a surrogate for tissue breakdown that is caused due to myocardial injury. LDH oozes out of the damaged area into the circulation and gets raised during myocardial infarction which is seen with administration of isoproterenol.17 EERD showed a significant cardioprotective activity by lowering the levels of lactate dehydrogenase against myocardial infarction induced by isoproterenol.
Isoproterenol-induced elevation in cholesterol levels in heart homogenate could be due to an increase in biosynthesis and reduction in its utilization. Isoproterenol induces free radical formation, which may cause cellular cholesterol accumulation by accumulating cholesterol biosynthesis, by decreasing cholesterol ester hydrolysis and by reducing cholesterol efflux.18 Pretreatment with the plant extract restored the level of cholesterol to as that of normal control.
The increased phospholipids content in heart homogenate in isoproterenol-induced rats may be due to the damage/injury caused in the myocardial membrane. The membrane stabilizing the activity of the plant extract induced myocytes to synthesize more phospholipids which were necessary to repair the damaged membrane.19 The EERD ameliorated the phospholipids level in isoproterenol-induced myocardial infarction in rats. Myocardial infarction caused by Isoproterenol causes ischemia which depletes myocardium of glycogen stores. In myocardial infarction activation of Glycogen phosphorylase isoenzyme BB occurs, that leads to increases the glycogen degradation.20 Cardiac glycogen which was reduced in positive control isoproterenol group was significantly increased when treated with EERD and standard.
Endogenous antioxidant enzymes such as SOD, CAT, and GSH are the first-line cellular and myocardial defense against oxidative stress, decomposing oxygen, and hydrogen peroxide before they interact to form the more reactive hydroxyl radical. The equilibrium between these enzymes is a significant process for the effective elimination of oxygen stress in intracellular organelles. SOD and CAT are important antioxidant enzymes in alleviating free radical-induced cell injury. A decrease in the activity of SOD and CAT can result in the decreased removal of superoxide ion and hydrogen peroxide radicals that brings about a number of reactions, which are harmful to the myocardium. Superoxide is inactivated by SOD, the only enzyme identified to use a free radical as a substrate. An increase in SOD activity is valuable in the event of an increased free radical generation. However, it has been stated that a rise in SOD activity, without a concomitant increase in the activity of CAT and/or GSH, may be detrimental because SOD generates hydrogen peroxide as a metabolite, which is more cytotoxic than oxygen radicals, and must be scavenged by CAT or GSH. Thus, a concurrent increase in CAT and/or GSH activity is essential for an overall beneficial effect of an increase in SOD activity [21]. In the present study, during isoproterenol-induced myocardial infarction, the levels of endogenous antioxidant enzymes (SOD, CAT, and GSH) in heart tissue were decreased significantly compared with normal control rats. In the treatment groups, EERD antagonized the decrease in endogenous antioxidant enzyme levels and produced beneficial effects.
Lipid peroxidation, a class of oxidative deterioration of polyunsaturated fatty acids has been associated with changed membrane structure and enzymes inactivation. Isoproterenol-induced myocardial infarction showed enlarged levels of heart lipid peroxidation products such as thiobarbituric acid reactive substances (TBARS). Enhanced lipid peroxidation appears to be the early stage of the tissue making it more susceptible to oxidative damage. This is responsible for the observed membrane damage as evidenced by the high level of lipid peroxidation in conditions of TBARS.22 Lipid peroxidation (MDA released) increases under mild stress and causes an alteration in the membrane structure, leading to its damage and enzyme inactivation. In the present study pretreatment with EERD significantly restored TBARS levels indicating maintained integrity of myocardium.
ATPases are closely associated with the plasma membrane and participate in the energy-requiring translocation of sodium, potassium and calcium ions. The inhibition of Na+, K+ ATPase will activate the Na+– Ca2+ exchange mechanism in the myocardium. This Na+– Ca2+ exchange mechanism plays a vital role in regulating the cellular calcium level. The decrease in the activity of Na+/K+ ATPase and increased activities of Ca2+ ATPase observed in isoproterenol-induced rats could be due to enhanced lipid peroxidation by free radicals, because the membrane‐bound enzymes are ‘SH’ group containing enzymes.23 Pre‐treatment with metoprolol and EERD to isoproterenol-induced rats increased the activity of Na+/K+ ATPase and decreased the activities of Ca2+ ATPase in the myocardium. Increased Na+/K+ ATPase activity due to metoprolol and EERD pretreatment could regulate the intracellular Ca2+ levels, thereby protecting the myocardium from excess damage by maintaining the membrane integrity.
The histopathological and ultra-structural improvement of cardiomyocytes further reinforced the Cardioprotective activity of chronic administration of ethanolic extract of Rosa damascena in isoproterenol-induced myocardial infarction.
Conclusion
The present study evaluated the cardioprotective effect of ethanolic extract of Rosa damascena petals used in traditional Unani medicine, on isoproterenol-induced myocardial infarction in rats. EERD ameliorated positively changes in relative heart weight, biochemical alterations in serum and heart homogenate, decreased hyperlipidemia, prevented oxidative stress in heart homogenate and histological and ultrastructural changes induced by isoproterenol. Therefore Rosa damascena petals have cardioprotective effects. Therapeutic success of Rosa damascena petals in traditional Unani medicine may be due to its antioxidant, anti-lipid peroxidative and anti-hyperlipidemic property thereby providing substantial evidence for the management of myocardial infarction.
Acknowledgements
Authors are sincerely thankful to Thankful to Sri TVRN Reddy, Joint Secretary, Jayamukhi Educational Society and Management of Jayamukhi College of Pharmacy, Warangal, India for providing necessary facilities, support for this research work and encouragement to carry out the research work successfully
Conflict of Interest
There is no conflict of interest.
References
- Kavita P, Varun S,Kandace L, Sarah M. J, Abhiram P and Amit S. Use of Complementary Therapies in Cardiovascular Disease. American Journal of Cardiology, 2013; 111(3): 339 – 345.
- Benjamin E. J, Blaha M. J, Chiuve S. E, Cushman M, Das S. R, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation, 2017;135, e146–e603.
- Kleinau G, Pratzka J, Nürnberg D, Grüters A, Führer-Sakel D, Krude H, Köhrle J., Schöneberg T and Biebermann H. Differential modulation of Beta-adrenergic receptor signaling by trace amine-associated receptor 1 agonists. 2011. PLoS ONE, 2011;6(10): e27073. doi:10.1371/journal.pone.0027073.
- Aman U, Hardik G and Balaraman R. (2011). Isoproterenol Induced Myocardial Infarction: Protective Role of Natural Products. Journal of Pharmacology and Toxicology, 2011; 6: 1-17.
- Mohaddese M. Rosa damascena as holy ancient herb with novel applications. Journal of Traditional and Complementary Medicine, 2016; 6(1): Pages 10-16.
- Boskabady M.H, Shafei M.N, Saberi Z, and Amini S. (2011). Pharmacological Effects of Rosa Damascena. Iranian journal of basic medical sciences. 2011; 14(4): 295-307.
- Vogel H.G. Chapter A: Cardiovascular activity. Drug Discovery and Evaluation-Pharmacological Assays. 2nd New York: Springer-Verlag Berlin Heidelberg, 2002; 180-200.
- Padma V.V and Devi C.S.S. Cardioprotective Effect Of Fish Oil On Isoproterenol-Induced Myocardial Infarction In Rats. Journal of Food Lipids, 2009; 16:19–32
- Lopez M.J.B, Garmendia L.J.R, Aguilar G.M.D, de Llano A.J.M, López A.C, Fernández A.J. Cardiovascular risk factors in the circadian rhythm of acute myocardial infarction. Rev Esp Cardiol. 2004; 57:850–858.
- Srivastava Tand Chosdol K. Clinical Biochemistry: Clinical enzymology and its applications. New Delhi: All India Institute of Medical Sciences, Ansari Nagar; 2007: 17.
- Shen J, Zhang J, Wen J, Ming Q, Zhang J and Xu Y. Correlation of serum alanine aminotransferase and aspartate aminotransferase with coronary heart disease. Int J Clin Exp Med. 2015; 8(3):4399-404.
- Nelson R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2012; 40(1):195-211.
- Talayero B.G, Sacks F.M. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011; 13(6):544-52.
- Bodor G.S. Biochemical Markers of Myocardial Damage. EJIFCC. 2016; 27(2):95-111.
- Mythili S, Malathi N. Diagnostic markers of acute myocardial infarction. Biomed Rep. 2015; 3(6):743-748.
- Khalil MI, Ahmmed I, Ahmed R, E. M. Tanvir E.M, Rizwana A, Sudip P, Siew H. G, and Nadia A. Amelioration of Isoproterenol-Induced Oxidative Damage in Rat Myocardium by Withania somnifera Leaf Extract. Biomed Res Int. 2015;2015:624159.
- Maghamiour N and Safaie N. High Creatine Kinase (CK)-MB and Lactate Dehydrogenase in the Absence of Myocardial Injury or Infarction: A Case Report. J Cardiovasc Thorac Res. 2014; 6(1):69-70.
- Daniels T.F, Killinger K.M, Michal J.J, Wright R.W and Jiang Z. Lipoproteins, cholesterol homeostasis and cardiac health. Int J Biol Sci. 2009; 5(5):474-88.
- Chien K.R, Han A, Sen A, Buja L.M and Willerson J.T. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ Res., 1984; 54(3):313-22.
- Johannes M. Glycogen phosphorylase isoenzyme BB to diagnose ischaemic myocardial damage. Clinica Chimica Acta. 1998; 272(1):79-86.
- Birben E, Sahiner UM, Sackesen C, Erzurum S and Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012; 5(1):9-19.
- Madhesh M and Vaiyapuri M. Luteolin promotes mitochondrial protection during acute and chronic periods of isoproterenol induced myocardial infarction in rats. The Egyptian Heart Journal. 2013; 65(4): 319-327.
- Shattock M.J, Ottolia M, Bers DM, et al. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J Physiol. 2015; 593(6):1361-82.








