Numlil Khaira Rusdi1, 2 and Jeanne Adiwinata Pawitan3, 4, 5*
1Doctoral Program for Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
2Faculty of Pharmacy and Science, University Muhammadiyah Prof. DR. Hamka, Jakarta, Indonesia
3Department of Histology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
4Stem Cell Medical Technology Integrated Service Unit, Dr. Cipto Mangunkusumo General Hospital/Faculty of Medicine Universitas Indonesia, Indonesia
5Stem Cell and Tissue Engineering Research Center, Indonesia Medical Education and Research Institute (IMERI), Faculty of Medicine Universitas Indonesia, Indonesia
Corresponding Author’s E-mail: jeanneadiwip@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1789
Abstract
Immunotherapy for cancer treatment continues to be developed and various strategies have been carried out including bioengineering. This endeavour requires development of technology, and efforts to find specific and sensitive tools to monitor immune responses during and after therapy. The purpose of this mini-review was to discuss cancer immunotherapy using T cell and immune checkpoint blockade therapy, as well as immunotherapy monitoring methods using flow cytometry (FCM). Bioengineering of T lymphocytes for immunotherapy and immune checkpoint blockades can be combined with nanoparticles as drug delivery carrier against cancer to increase drug distribution to tumor cells, as well as T cell stimulation regulation to reduce autoimmune effects. In addition, T cell engineering can also prevent Host versus Graft alloreactivity in chimeric antigen receptor (CAR) T cell administration. FCM is a monitoring method that is widely used in pre-clinical and clinical cancer immunotherapy studies.
Keywords
Bioengineering; Cancer Immunotherapy; Immune Checkpoint; Flow Cytometry; T Cells
Download this article as:| Copy the following to cite this article: Rusdi N. K, Pawitan J. A. Cancer Immunotherapy and Flow Cytometry in Immunotherapy Monitoring. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Rusdi N. K, Pawitan J. A. Cancer Immunotherapy and Flow Cytometry in Immunotherapy Monitoring. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/2navyZS |
Introduction
Immunotherapy is a method that is continuously developed in the treatment of cancer.1, 2 Immunotherapy has a great potential to treat and prevent cancer recurrence by activating the immune system to recognize and kill cancer cells.3 Clinical studies using chimeric antigen receptor (CAR) T cells and immune checkpoint blockade are examples of the success in cancer immunotherapy.4, 5 Adoptive cell therapy (ACT) using tumor infiltrating lymphocytes (TIL) has also been used successfully in treating patients with metastatic melanoma.6
Various immunotherapy strategies continue to be developed including cancer and dendritic cell vaccines, T cell engineering, and immune checkpoint blocking.1,3 Although it is believed that the immune system can provide an immune response to the growth of malignant tumors, in fact, many malignant tumors still grow in immunocompetent individuals.7, 8 Therefore, research in immunotherapy also needs to be conducted to understand how and why certain cancers fail to respond to immunotherapy, and also to develop appropriate immunotherapy strategies.3
This endeavour requires development of technology, including rapid methods to characterize the immune microenvironment of tumors, to find specific and sensitive tools to assess patient therapeutic outcomes, and to make it possible to monitor immune responses, tumor growth, and spread of tumors during and after therapy.3 One method to overcome these problems is the use of immunoengineering in cancer therapy.9 In addition, methods for immunotherapy monitoring are needed, and one of these methods that are widely used is flow cytometry.10, 11
Flow cytometry (FCM) is a multi-parameter analytic test for the characterization of single cells and has been widely used in preclinical as well as in clinical cancer immunotherapy studies.12, 13 The aim of this mini-review was to discuss cancer immunotherapy using T cell and immune checkpoint blockade therapy, as well as immunotherapy monitoring methods using FCM.
T Cell Immunotherapy For Cancer
Engineering technology has been developed to improve cancer therapy using T cells. One of them is the use of nanocarriers as drug delivery vehicle (carrier) that are incorporated on the surface of T cells for cancer therapy. Nanocarriers are often referred to as nanoparticles, nanomaterials, or nanosomes. The use of nanoparticles as a drug delivery carrier in cancer therapy aims to increase the bioavailability and payload (distribution) of drugs, and to improve drug release to tumor cells, as well as to improve T cell function in infiltrating tumors and to minimize autoimmune toxicity.14, 15
To avoid recognition by the immune system, cancer cells will activate Shp 1/2 phosphatase in TIL to stimulate T cell inhibitory receptors such as PD-1, and CTLA-4. Moreover, Shp1/2 phosphatases migrate to the immunological synapse (IS), deactivate molecular signals of proliferation, and down-regulate T cell activation.14 Stephan et al.,14 engineered T lymphocytes by conjugating a synthetic nanocarrier NSC-87877 to the surface of T lymphocytes. NSC-87877 is a phosphatase inhibitor, which inhibit Shp1/2 phosphatase. Therefore conjugation of NSC-87877 synthetic nanocarrier on the surface of T lymphocytes can activate T cells, increase immunologic synapses, and facilitate molecular interaction between T cells and antigen presenting cells (APCs). This interaction causes activation of tumor antigen recognition by APC cells, increases T cell (TIL) function in tumors, and minimizes autoimmune toxicity. Engineered T cell-synthetic nanocarrier NSC-87877 was injected to prostate cancer mice models through the tail vein, and the distribution was observed using bioluminescent imaging. This study proved the expansion of T cells in tumor sites resulting in an increase in the survival of experimental animals with prostate tumors. In addition, T cells conjugated with synthetic nanocarrier NSC-87877 could activate immunological synapses, and concentrate NSC-87877 in the contact zone of T-cell/tumor cell based on “demand”, so that it could regulate T cell stimulation and reduce autoimmune reactions.14
Other studies also proved that T lymphocytes could be engineered as carriers of topoisomerase-1 SN-38 that were effectively transported to the cancer site.16 Topoisomerase-1 SN-38 is a systemic chemotherapy, which is an active form of camptothecin derivative of Irinotecan. This drug has poor pharmacokinetics. To increase its biodistribution and effectiveness, researchers engineered polyclonal autologous T lymphocytes as carriers of topoisomerase 1 SN-38 in the form of controlled release lipid nanocapsules (NCs), namely SN-38 NC-T cells. As lymphocytes migrate throughout the tissue to look for antigens, polyclonal T cells, which express lymph node receptors but do not specifically recognize tumor cell antigens, can be used as an effective agent to deliver chemotherapy drugs for lymphoma. SN-38 NC-T cells were tested on Burkitt’s lymphoma animal model, and their effectiveness and toxicity were observed. The results showed that SN-38 NC-T cell therapy significantly increased the efficacy of SN-38 without increasing the drug side effects.16
The application of T cell immunotherapy was also developed by Valton, et al.,17 where engineering was performed to prevent host versus graft alloreactivity in the administration of chimeric antigen receptor (CAR) T cell therapy. CAR T-cell therapy was obtained by taking T cells’patients with leukapheresis procedures.18 T cells taken were then engineered in the laboratory. The engineered T cell receptors could recognize specific antigens in cancer cells, which would destroy cancer cells specifically. This modified T cell was called CAR T-cell. T cells that had been modified were then reinfused into the patient’s body.19
Giving infusion of CAR T-cell might cause alloreactivity in patients. To prevent this reaction, Valton, et al.,17 engineered T cells by inactivating TCRαβ and purine nucleotide analogs on the surface of T cells to prevent alloreactivity and to prevent damage of lymphocytes through host versus graft (HvG) reactions. T-cell engineering includes lentiviral transduction for CAR expression followed by inactivation “TCRαβ within CAR-T cell genome”, through inactivation of deoxycytidine kinase (dCK), and coelectroporation with mRNA that coded TCR α constant region (TRAC) transcription activator-like effector nuclease (TALEN). DCK is responsible for TCRαβ expression and toxicity of purine nucleotide analogs (PNA), which can damage the immune system. PNA becomes toxic after being metabolized by dCK. Therefore dCK inactivation prevents PNA metabolism and toxicity, thus protect the immune system. Inactivation of dCK was done by designing TALEN that targeted the second exon of dCK gene, and TALEN mRNA was used to electroporate primary T cells. This approach was reported to be efficient in inactivating the TCRα gene and preventing the expression of TCRαβ.17
Immune Checkpoint Blockade Therapy
Included in the immune checkpoint are cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1/PD ligand 1 (PD-1 / PDL-1), Human B7-H3 (CD276) and Lymphocyte-activation gene-3 (LAG-3, CD223).20 Immune checkpoint inhibitors that focus on CTLA-4 and PD-1 have been shown to have a potential of immunomodulators through their function as negative activators of T cells.5, 21, 22 CTLA-4 plays an important role in reducing activation of memory T cells and naive T cells. In contrast, PD-1 is mainly involved in modulating T cell activity in peripheral tissues through its interaction with PD-L1 and PD-L2.5
The discovery of a negative regulator of this immune response is very important in the development of immune checkpoint inhibitors. This shifts the focus of research from developing therapies that target immune system activation against cancer to immune checkpoint inhibitors, which aim to mediate the destruction of tumor cells through coinhibitory signal removal that blocks antitumor T cell responses.5, 23
One of the applications of immune checkpoint blocking therapy was the study by Zhang et al., 24 who performed genetic engineering of platelets from megakaryocyte (MK) progenitor cells to express PD-1 protein. This study aimed to prevent postoperative tumor recurrence.24 Genetically engineered platelets of MK cells that express PD-1/ PD-1 platelets and derivative microparticles accumulated in post-tumor surgery wounds, and restore CD8 + T cell state to lyse residual tumor cells. Furthermore, low-dose cyclophosphamide (CP) was inserted into PD-1 expression platelets to destroy regulatory T cells (Treg), as well as to increase CD8 + lymphocyte cells that were active, to directly prevent tumor recurrence.24
PD-1 expression platelets were produced from the L8057 cell line labeled with NHS-Cy5.5 in PBS. This labeled platelets were then infused into mice with 10% residual melanoma tumors after tumor removal. The study used 42 mice that were divided into 6 treatment groups, which were given IV PBS, free platelets, PD-1 platelets, cyclophosphamide, cyclophosphamide-loaded free platelets, or cyclophosphamide-loaded PD-1-expressing platelets. Tumor burden was monitored using bioluminescence. The parameters observed were the frequency of Treg (CD4 + Treg), CD8 + TIL, PD-L1, GzmB expression, and Ki67.
The results showed that the utilization of cyclophosphamide-loaded PD-1 expression platelets effectively disrupted PD-L1 immune blocking and reduced Treg, and significantly increased the frequency of CD8 + TIL in tumors, thereby reducing the rate of postremoval tumor recurrence. In addition, PD-1 expression platelets promoted the emergence of CD8 +, Ki67, and GzmB lymphocytes in the postremoval tumor microenvironment.24
Flow Cytometry in Monitoring Cancer Immunotherapy
The discovery of successful immunotherapy in cancer requires specific and sensitive tools to assess patient therapeutic outcomes and makes it possible to monitor immune response, tumor growth, and spread of tumors during and after therapy.3 Immune system monitoring becomes increasingly relevant to measure the effects of treatment on T lymphocytes and to explain the mechanism of successful treatment.25
Flow cytometry (FCM) is a multi-parameter test for the characterization of single cells, and has been widely used in pre-clinical and clinical cancer immunotherapy studies.25 FCM is a technique of measuring the number and characteristics of single cells that are flowing through a detector system, using a fluorochrome-labeled monoclonal antibody. Commonly used fluorochromes are fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridin-chlorophyll alpha complex (PerCP), allophycocyanin (APC), and propidium iodide (PI).26
The FCM application in monitoring cancer immune systems includes intracellular staining for effector cytokines, cytotoxicity evaluation, measurement of proliferation and evaluation of cells that regulate the immune system including regulatory T cells (T reg).25 FCM allows quantization of several parameters in many individual cells that combines various phenotypic and functional markers and has the potential to provide information about immune responses in cancer patients.10, 25
In FCM where the sample sources might come from peripheral blood, bone marrow, body fluids, urine, spleen cells or tissue, or cultured cell line, the cells should be process into cell suspension. The cell suspension is mixed with fluorochrome labeled antibodies, and injected into a fluidic system, where the flow pushes the cells to pass through a central channel to line up as single cells as they approach the laser. Lasers as light sources will excite the cells in the stream and optical filters direct the resulting light signals to the appropriate detectors. The detector that detects the light scatter in forward direction show the forward scatter (FSC) as the X-axis, and the detector that detects deflected light scatter show the side scatter (SSC) as the Y-axis. FSC correlates with cell size, while the SSC is related to the complexity of the cells, such as nucleus size, types and complexity of cytoplasmic granules.27
Interpretation of FCM results in the form of cell quantification and cell characteristics. The Scatter plot is a visualization of characteristics that is commonly used in FCM results. X-axis is as FCS and Y axis is as SSC. The X and Y axes can also be based on fluorochromes and surface markers on cells, for example: FITC-CD3 (CD3 surface markers labelled by FITC fluorochrome). Fluorochrome on the X and Y axes must be different. The results are translated into electronic signals and graphical displays. The signals are converted into digital numbers and shown on a histogram, 2-D or 3-D plot.
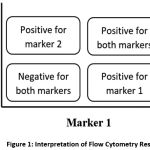 |
Figure 1: Interpretation of Flow Cytometry Result |
In a 2D plot, if the cell plot is in the upper right quadrant, then the cell is positive for marker 1 and marker 2, whereas if it is in the lower left quadrant, then the cell is negative for both markers. If the plot is in the lower right quadrant, the cell is positive for marker 1, and if the cell plot is in the upper left, the cell is positive for marker 2 (Figure 1).
The results of the FCM examination of cell quantification is in the form of a cell percentage, while to get an absolute number should be done by counting cells in a hemacytometer. FCM requires a lot of fluorochrome-labeled antibodies (according to parameters), which is a disadvantage. However it has some advantages, i.e.: (a) FCM has a high level of efficiency and sensitivity, and can obtain information simultaneously about various cellular processes, including expression of cell surface markers, intracellular cytokines, protein signals, and cell cycles, (b) the process is fast and enable quantitative measurements of many parameters simultaneously, (c) High specificity (can detect cells that are small in number) and (d) isolation of cells with high cell purity.13
Table 1: Cancer immunotherapy using T cells and blockade immune checkpoints
| No | Immuno therapy | Purpose of engineering | Type of engineering | Type of Study | Analytical Instrument |
| 1 | T cells Therapy
|
Using nanoparticles as a Drug Delivery vehicle (carrier) for cancer therapy to increase the payload (distribution) of drugs to tumor cells.
The use of T cells as the target delivery of the nanoparticle topoisomerase1-SN-38 drug Preventing Host versus Graft Alloreactivity in the administration of T cell infusion of chimeric antigen receptor (CAR) with T cell tissue engineering models
|
Surface engineering of T lymphocytes with synthetic nanocarrier NSC-87877
Engineering polyclonal autologous T cells conjugated with SN-38 topoisomerase 1 nanocapsules Engineering T cells by inactivating TCRαβ and purine nucleotide analogs on the surface of T cells, that can prevent alloreactivity and to prevent damage of lymphocyte cells through host versus graft reaction (HvG) |
Animal study
Animal study Clinical Study |
Flowcytometry
Confocal and videomicroscopy Bioluminescent imaging Flowcytometry IVIS imaging Bioluminescent imaging Flowcytometry Spectrophotometry (ELISA) Nested PCR Magnetic separation |
| 2 | Immune Checkpoint Blockade Therapy | Development of a drug delivery system that utilizes PD-1 expression platelets for the improvement of postoperative cancer immunotherapy. | PD-1 expression platelets was produced from the L8057 cell line labeled NHS-Cy5.5 In PD-1 expression platelets given a loading dose of cyclophosphamide. | Animal study | Flowcytometry
Western Blot Confocal microscopy, CSEM, TEM images Xenogen IVIS Lumina imaging |
In conclusion, engineering of T lymphocytes for cancer immunotherapy and immune checkpoint blockade can be combined with nanoparticles as a drug delivery carrier to increase drug distribution to tumor cells and reduce autoimmunity. In addition, T cell engineering was proven in preventing Host versus Graft alloreactivity in the administration of CAR T cells. FCM is a monitoring method widely used in cancer immunology in pre-clinical and clinical cancer immunotherapy studies.
Acknowledgement
This work was supported by a research grant from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia, Hibah Penelitian Pengembangan 2019, contract no.NKB-1804/UN2.R3.1/HKP.05.00/2019
Funding Source
This work was supported by a research grant from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia, Hibah Penelitian Pengembangan 2019, contract no.NKB-1804/UN2.R3.1/HKP.05.00/2019
Competing of Interest
The authors declare that they have no competing interest.
References
- Zhang H, and Chen J. Current status and future directions of cancer immunotherapy. J Cancer. 2018;9(10):1773–17
- Yu L.Y, Tang J, Zhang C.M, Zeng W.J, Yan H, Li M.P, and Chen X. New Immunotherapy Strategies in Breast Cancer. Int J Environ Res Public Health. 2017; 14(1). pii: E68.
- Jeanbart L, and Swartz M. Engineering opportunities in cancer immunotherapy.PNAS 100th Anniversary. 2015;112(47):14467–14472.
- Hou B, Tang Y, Li W, Zeng Q, and Chang D. Efficiency of CAR-T Therapy for Treatment of Solid Tumor in Clinical Trials : A Meta-Analysis. Dis Markers. 2019;2019:3425291 (11 pages).
- Webb E.S, Liu P, Baleeiro R, Lemoine N.R, Yuan M, and Wang Y. Immune checkpoint inhibitors in cancer therapy. J Biomed Res. 2018;32(5):317–3
- Rohaan M.W, van den Berg J.H, Kvistborg P, and Haanen J.B.A. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer. 2018;6(1):102.
- Sambi M, Bagheri L, and Szewczuk M. Current Challenges in Cancer Immunotherapy : Multimodal Approaches to Improve Efficacy and Patient Response Rates. J Oncol. 2019;2019:4508794 (12 pages).
- Zhu J, Powis de Tenbossche C.G, Cané S, Colau D, van Baren N, Lurquin C, Schmitt-Verhulst A.M, Liljeström P, Uyttenhove C, and Van den Eynde B. Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat Commun. 2017;8(1):1404.
- Lim W.A, and June C. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017;168(4):724-740.
- Danova M, Torchio M, Comolli G, Sbrana A, Antonuzzo A, and Mazzini G. The role of automated cytometry in the new era of cancer immunotherapy. Mol Clin Oncol. 2018 ;9(4):355-3
- Chargin A, Morgan R, Sundram U, Shults K, Tsay E.L, Ratti N, and Patterson B. Quantification of PD – L1 and PD – 1 expression on tumor and immune cells in non – small cell lung cancer ( NSCLC ) using non – enzymatic tissue dissociation and flow cytometry. Cancer Immunol Immunother. 2016 Nov;65(11):1317–1323.
- Gascue A, Merino J, and Paiva B. Flow Cytometry. Hematol Oncol Clin North Am. 2018;32(5):765-7
- Jahan-Tigh R.R, Ryan C, Obermoser G, and Schwarzenberger K. Flow Cytometry. J Invest Dermatol. 2012 ;132(10):1-6.
- Stephan M.T, Stephan S.B, Bak P, Chen J, and Irvine D. Synapse-directed delivery of immunomodulators using T-cell conjugated nanoparticles. Biomaterials. 2012;33(23):5776–5787.
- Artini I. Peranan Nanopartikel Dalam Penatalaksanaan Kanker di Era Targeting Therapy. Indones J Cancer. 2013;7(3):111–117.
- Huang B, Abraham W.D, Zheng Y, Bustamante López S.C, Luo S.S, and Irvine D. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci Transl Med. 2015 ;7(291): 291ra94.
- Valton J, Guyot V, Marechal A, Filhol J.M, Juillerat A, Duclert A, Duchateau P, and Poirot L. A Multidrug-resistant Engineered CAR T Cell for Allogeneic Combination Immunotherapy. Mol Ther. 2015 ;23(9):1507–15
- Juliano L, Eastwood G, Berard T, and Mathew A. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Insights 2018;4(4): 327–336.
- Wang X, and Rivière I. Clinical manufacturing of CAR T cells : foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:1–7.
- Galetto R, Lebuhotel C, Poirot L, Gouble A, Toribio M.L, Smith J, and Scharenberg A. Pre-TCR α supports CD3-dependent reactivation and expansion of TCR α -deficient primary human T-cells. Mol Ther Methods Clin Dev. 2014 ;1:1–9.
- Park J.A, and Cheung N. Limitations and opportunities for immune checkpoint inhibitors in pediatric malignancies. Cancer Treat Rev. 2017 ;58:22–33.
- Zhou Q, Munger M.E, Highfill S.L, Tolar J, Weigel B.J, Riddle M, Sharpe A.H, Vallera D.A, Azuma M, Levine B.L, June C.H, Murphy W.J, Munn D.H, andBlazar B. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood J. 2010;116(14):2484–2493.
- Dai F, Zhang F, Sun D, Zhang Z.H, Dong S.W, and Xu J. CTLA4 enhances the osteogenic differentiation of allogeneic human mesenchymal stem cells in a model of immune activation. Braz J Med Biol Res. 2015;48(7):629–636.
- Li Y, Li F, Jiang F, Lv X, Zhang R, Lu A, and Zhang G. A Mini-Review for Cancer Immunotherapy : Molecular Understanding of PD-1/PD-L1 Pathway & Translational Blockade of Immune Checkpoints. Int J Mol Sci. 2016;17(7):1–22.
- Zhang X, Wang J, Chen Z, Hu Q, Wang C, Yan J, Dotti G, Huang P, and Gu Z. Engineering PD-1-Presenting Platelets for Cancer Immunotherapy. Nano Lett. 2018 ;18(9):5716−57
- White S, Laske K, Welters M.J, Bidmon N, van der Burg S.H, Britten C.M, Enzor J, Staats J, Weinhold K.J, Gouttefangeas C, and Chan C. Managing Multi-center Flow Cytometry Data for Immune Monitoring. Cancer Inform. 2015;13(Suppl 7):111-1
- Adan A, Alizada G, Kiraz Y, Baran Y, and Nalbant A. Flow cytometry : basic principles and applications. Crit Rev Biotechnol. 2017;37(2):163-176.
Abbreviation
CAR= chimeric antigen receptor
ACT= adoptive cell therapy
TIL= tumor infiltrating lymphocytes
FCM= Flow cytometry
IS= immunological synapse
APC= antigen presenting cells
NCs=nannocapsules
HvG= host versus graft
dCK= deoxycytidine kinase
TCR α constant region= TRAC
TALEN= transcription activator-like effector nuclease
PNA= purine nucleotide analog
CTLA-4= cytotoxic T-lymphocyte-associated protein 4
PD-1= programmed cell death protein 1
PDL-1= PD ligand 1
LAG-3= lymphocyte-activation gene 3
MK= megakaryocyte
CP= cyclophosphamide
FITC= fluorescein isothiocyanate
PE= phycoerythrin
PerCP= peridin-chlorophyll alpha complex
APC= allophycocyanin
FSC= forward scatter
SSC= side scatter








