Ahlem Bargougui1, 2٭ , Hend Maarof Tag1,3
, Hend Maarof Tag1,3 , Mohamed Bouaziz4, and Saida Triki2
, Mohamed Bouaziz4, and Saida Triki2
1Department of Biology, Faculty of Sciences and Arts-Khulais, University of Jeddah, PO Box 355, ISIN Code 21-921, Jeddah, Saudi Arabia
2Laboratory of Biochemistry, Faculty of Science, University El Manar Campus PO Box 2092 Tunis, Tunisia
3Department of Zoology, Faculty of Sciences, Suez Canal University, PO Box 41522, Egypt.
4Laboratory of Electrochemistry and Environment, Higher Institute of Biotechnology of Sfax, University of Sfax, PO Box 1175, 3038 Sfax, Tunisia
Corresponding Authors E-mail: bargouguiahlem@yahoo.fr
DOI : https://dx.doi.org/10.13005/bpj/1764
Abstract
Opuntia ficus-indica is a cactus that is well adapted to harsh climatic conditions. It is an interesting source of food ingredients in the normal diet and of food industry. The present study aims at evaluating the some physical and morphological parameter cactus cultivars fruit, besides the phytochemical, antioxidant and antimicrobial activities of methanolic and ethyl acetate extract of four Opuntia ficus-indica cultivars. The present results revelead that the highest levels of phenol found in the ethyl acetate extract of Sanguinea and methanolic extract of Ain Jemaa. While ethyl acetate extract Ain Jemaa displayed significant elevated concentrations of total flavonoids and flavonols as compared with other cultivars. The findings of this study show that methanolic crude extracts exhibited a considerably broader antimicrobial activity compared to ethyl acetate extracts. The Moroccan cultivar has displayed the best antioxidant and antibacterial activity. The antioxidant activity found in juice extract of Maroco and Algeria cultivars are the highest, and is mainly due to beta-carotene, as demonstrated by principal component analysis, allowing the use of these fractions as sources of natural antioxidants.
Keywords
Antimicrobial Activity; Antioxidant Activity; Juice; Opuntia Ficus-Indica; Phenolic Compounds; Sugar
Download this article as:| Copy the following to cite this article: Bargougui A, Tag H. M, Bouaziz M, Triki S. Antimicrobial, Antioxidant, Total Phenols and Flavonoids Content of Four Cactus (Opuntia ficus-indica) Cultivars. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Bargougui A, Tag H. M, Bouaziz M, Triki S. Antimicrobial, Antioxidant, Total Phenols and Flavonoids Content of Four Cactus (Opuntia ficus-indica) Cultivars. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/2mq6ZqR |
Introduction
The cactus belongs to the Cactaceae family which is native to arid and semi-arid regions (Shetty, Rana, and Preetham 2012). About 1500 species of cactus are in the genus Opuntia and are distributed in Europe, Mediterranean countries, Africa, southwestern United States, northern Mexico and other areas. Because cactus pear can withstand prolonged drought, it is considered as a potential alternative crop for dry regions (Shetty, Rana, and Preetham 2012; Duru & Turker, 2005).
Cactus pear fruit is a many-seeded berry with a thick peel, enclosing a flavored seedy pulp. Although used as forage, cactus pear fruit is primarily consumed as a fresh commodity. Many species of Opuntia produce edible and highly flavored fruits. Cactus pear (Opuntia ficus-indica) fruit can be considered as a nutraceutical and functional food of high importance thanks to its high content of beneficial chemicals. Cactus is also known for its diuretic and relaxant effects on the renal tract (Sáenz, Estévez, Sepúlveda, & Mecklenburg, 1998; Piga 2004; Vieira et al., 2008). Moreover, in many arid areas, farmers use cactus extensively not only as forage to prevent the disastrous consequences of droughts, but also as a source of water for livestock (Gebremariam, Melaku, and Yami 2006). There is increasing evidence that fruits and vegetables may protect against numerous diseases.
Their protective effect has generally been attributed to their antioxidant constituents, phenolic compounds, as well as to some minor components found in seed-oil and seed-protein contents. The nutritional importance of cactus pear fruit emanates mainly from the content of ascorbic acid, fibers and free amino acids (Stintzing, Schieber, and Carle 2001). Furthermore, (Ramadon & Morsel 2003) reported that cactus seed oil contains essentially unsaturated fatty acids (ca. 76%) and an appreciable level of fat-soluble vitamins. In many countries, cactus fruit and cactus pear juice is consumed at home or in restaurants. The processing of cactus pear fruit to obtain juices and other kinds of processed foods in order to increase the shelf-life of the fresh fruit has been studied in recent years (Sáenz et al., 1996).
The aim of this work is to determine some characteristics of such fruit and juice and to study the antioxidant, antibacterial and antifungal activities of four Opuntia ficus-indica fruit cultivars growing in the same region. This evaluation will be important to select the best cultivar for the producers and certainly for the consumers.
Materials and Methods
Chemicals
Solvents were purchased from Riedel-de-Haën™, Bucharest, Romania. Beta-carotene, Catechin, Gallic acid, Rutin, Tween, and linoleic acid were obtained from Fluka, Bucharest, Romania.
Collection of Plant Materials
The fruit of four Opuntia cultivars were harvested randomly from at least three trees from FAO collection, Kairouan (35° 40′ North 10° 05′ East of Tunisia), the average temperature ranges between 5 and 21° C in winter and between 25 and 42° C in summer. The annual rainfall is between 250 and 400 mm. The cultivars were: Sanguinea (Italy), Lengissima (Algeria), Ain Jemaa-(Morocco) and Ain Amara (Tunisia).
Some Physical Fruit Properties of Cactus Pear cultivars
The fruit diameter and length were determined using calipers. The fruits were washed; prickles and glochids on the peel surface were removed under running tap water by scrubbing. After water drained from the fruits, their weight were recorded. Fruits were cut longitudinally, the flesh peel were segregated from pulp, and the weights of these parts were recorded then the precentage of pulp wieght to the whole fruit were calculated. Seeds were washed under running water several times on a sieve (700 micron) to separate the seeds from the pulp. They were dried at room temperature and weighed. The dried seeds number were counted. The pulp weight was calculated by subtracting the fruit wieght from wet-seed weight and skin weight. Aslo the percentage of seeds to pulp were calculated.
Determination of total sugars in different parts of Cactus Pear cultivars
The sugar content for fruit juice were tested using the colorimetric method described by Miller (1959) using dinitrosalicylic acid as a reagent. Then sugar content were calculated per fruit.
Extraction Procedures
The Opuntia ficus-indica extract was prepared as described before by Azwanida (2015) with minor modifications. Briefly, for extraction, a hundred millilitres (100 ml) of juices filtrates were extracted using 300 mL of 100 % methanol or 300 mL of 100% ethyl acetate for 48 h to obtain the extract. The extract was then filtered and evaporated to dryness at 40°C under reduced pressure nearly to dryness (gummy residue) on a rotary evaporator (Yamato, Rotary Evaporator, model-RE 801). The yield (%, w/w) of crude methanol or ethyl acetate extracts (table 1) of Opuntia ficus-indica juice extract were calculated as Yield (%) = (W1 * 100)/W2 where W1 is the weight of the extract after evaporation of solvents, and W2 is the weight of juice.
Table 1: Percentage extract yield of four Opuntia ficus-indica cultivars
| Solvent | Ain Amara (Tunisia) |
Lengissima (Algeria) |
Ain Jemaa (Moraco) |
Sanguinea (Italy) |
| Ethyl acetate | 0.2 | 0.31 | 0.30 | 0.04 |
| Methanol | 15.01 | 1.40 | 4.55 | 6.13 |
Estimation of Total Phenolic, Total Flavonoid and Total Flavonol Contents in extracts
The total polyphenols were determined by the Folin-Ciocalteau procedure, using gallic acid as standard. The absorbance was measured at 765nm according to Li et al., (2007). The amount of the total flavonoids in the extracts was measured using the method described by (Djeridane et al., 2006). This method is based on the formation of a complex flavonoid_aluminium, having the maximum absorbance at 510 nm. Catechin was used as standard for the calibration curve. The flavonols content were determined as reported by (Miliauskasa and Venskutonisa, 2004). The absorbance was measured at 440nm and the rutin was used as standard for the calibration curve.
HPLC- (UV/SPD-10Avp) Quantitative Analysis of Cactus Pear cultivars extracts
HPLC chromatography was used for the separation of phenols from fruit extracts as described by Bouaziz et al., (2008). The device (Shimadzu) consists of a pump (LC-10ATvp) and a UV detector (SPD-10Avp). The column used is a column C-18 alkyl silica (4.6 μm x 250 mm) Shim-pack VP-ODS. Each sample is filtered on 0.2 microns and then driven under pressure in the column filled with a stationary phase of small particle size, by a high-pressure liquid (mobile phase), which reduces the time required to separate the components present in the stationary phase; these components thus have less time to diffuse into the column which produces narrower peaks and therefore better selectivity and sensitivity. The mobile phase used is 0.1% phosphoric acid (PA) (Prolabo, France) in water versus 70% acetonitrile (ACT) (Dharmadrug, GmbH, Germany) in water for total duration of 50 min. The elution conditions applied for the phenolic compounds are: 0-25 min, 10-25% B; 25-35 min, 25-80% ACT; 35-37 min, 80-100% ACT; 37-40 min, 100% ACT, and finally washing and equilibration of the column require (40-50 min) with linear gradient 100-10% ACT. The flow rate is 0.6 ml / min and the injection volume of 50 µl. The eluates were detected at 280 nm and the temperature is maintained at 40°C.
Gas chromatography/mass spectrometry (GC/MS) analysis
GC-MS analysis of Ain Amara extract (Tunisia) were performed to evaluate the concentration of the active compounds separated with HPLC; using a Trace gas chromatograph interfaced to a Polaris Q mass spectrometer (Thermo Finnigan, Hertfordshire, UK) as previously described Chien et al., (2009) at the Centre of Biotechnology of Sfax, Tunisia. For GC-MS detection, an electron ionization system with ionization energy of 70eV was used. Helieum gas (99.99%) was used as a carrier gas at a constant flow rate of 1.51ml/min. injector and mass transfer line temperature were set at 200 and 240°C respectively. The oven temperature was programmed from 70to 220°C at 10°C/min, held isothermal for 1min and finally raised to 300°C AT 10°C/min. 2µl of respective diluted samples was manually injected in the split less mode, with split ratio of 1:40 and with mass scan of 50-600 amu. Total running time of GC-MS is 35min. The relative percentage of the each extract constituents was expressed as percentage with peak area normalization. The identification of the components was based on the comparison of their mass spectra with spectrometer database (Wiley and NIST Libraries i.e library sources were used for matching the identified components from the plant material) and confirmed by determination of their Kovats indices, which were calculated relative to the retention times (Stein, 1990).
Evaluation of the antioxidant activity by β-carotene
The present work was carried out using glass equipment to minimize metal contamination. The antioxidant activity of different extracts was evaluated using a β-carotene linoleate model system as described by Bouaziz and Sayadi (2005). A β-carotene solution was prepared by dissolving 2 mg of β-carotene in 10 mL of chloroform. 1 mL of this solution was then pipetted into a round-bottom flask. After the removal of chloroform under vacuum, using a rotary evaporator at 40 °C, 20 mg of linoleic acid, 200 mg of Tween 80 emulsifier and 50 mL of oxygenated distilled water were added to the flask, which was shaken vigorously. The Aliquots (5 mL) of this prepared emulsion were transferred into a series of glass vials containing 200 ppm of each extract or 200 ppm of 2,6-di-tert-butyl-4-methylphenol (BHT), which were used as a positive control for comparative purposes. As soon as the emulsion was added to each vial, the zero time absorbance was read at 470 nm. Absorbance readings were then recorded at 15 min intervals until the control sample had changed color. The absorbance was then recorded at hourly intervals until the color of β-carotene in the experimental samples had disappeared. During the experiment, all samples were kept in a water bath at 50 °C. Absorbance decreased rapidly in the samples without an antioxidant, whereas in the presence of an antioxidant, they retained their color, and thus absorbance became longer.
DPPH radical-scavenging effect assay
The DPPH (1,1-diphenyl-2-picryl hydrazyl) radical scavenging effect was evaluated according to the method described by Bouaziz and Sayadi (2005). 4 ml of methanolic solution of varying sample concentration (25, 50, 100, and 150 µg/ml) was added to 10 ml of DPPH methanol solution (1.5 10-4 M). The mixture was shaken vigorously and placed for 30 min in the dark at room temperature. Then, the absorbance at 520 nm was measured using a Shimadzu UV-160 A spectrophotometer. BHT was used as the positive control. The antioxidant activities were expressed in terms of the required concentration, inhibiting 50% DPPH radical formation (IC50, µg/ml).
Antimicrobial activity of four Opuntia ficus-indica cultivars extracts using Agar diffusion method
The antibacterial activity of Opuntia ficus-indica juice extracts were tested against five strains of bacteria and fungi, kindly provided by Pr. Abdelhafidh Dhouib from the Tunisian microbial collection (CTM) of the Center of Biotechnology, Sfax-Tunisia: Staphylococcus aureus (CTM 50 257), Pseudomonas aeruginosa (CTM 50 258), Escherichia coli (CTM 50 259), Salmonella enterica (CTM 50 260) and Bacillus subtilis (CTM 50 261). The antifungal activity was tested using Aspergillus niger (CTM 10 099) and Candida albican (CTM 30 031).
Methanol and ethyl acetate extracts (100 µl) were dissolved in 100% dimethylsulfoxide (DMSO) (900 µl). The culture suspension (200 µl) of the tested microorganisms (106 colony-forming units (cfu/ml) of bacteria cells (estimated by absorbance at 600 nm) and 108 spores/ml of fungal strains (measured by Malassez blade) were spread on PCA medium and PDA medium, respectively. Then, wells (7 mm diameter) were made using a sterile borer and loaded with 100 µl of Opuntia ficus-indica juice extracts at 100 mg/ml. A well with only 100 µl of DMSO (without extract) was used as a negative control. Gentamycin and Neomycin were used as positive references for bacteria. The Petri dishes were kept, first for 1 h at 4°C (Hajji et al., 2010), and then incubated for 24 h at 37 °C for bacteria and 72 h at 30 °C for fungal strains. The antimicrobial activity was evaluated by measuring the diameter of the inhibition zones in millimeters (including well diameter of 7 mm). The measurements of the inhibition zones were carried out for three sample replications and the values are the averages of three replicates.
Statistical Analysis
The values were expressed as mean and standard deviation of three parallel measurements. The data were evaluated by a one-way analysis of variance followed by Tukey HSD test, using SPSS V.20.0 software (SPSS Institute Inc., Cary, NC). Data considered statistically significant when p < 0.05. (Field, 2004).
Table 2: Some Physical Fruit Properties of four Opuntia ficus-indica cultivars
| Parameters | Ain Amara (Tunisia) |
Lengissima (Algeria) |
Ain Jemaa (Moraco) |
Sanguinea (Italy) |
| Length (cm) | 7.29 ± 0.56a | 6.62 ± 0.26b | 6.85 ± 0.26 b | 6.95 ± 0.67 b |
| Diameter (cm) | 4.77 ± 0.36 a | 4.17 ± 0.20 b | 4.51 ± 0.22 c | 4.13 ± 0.19 b |
| Fresh Fruit weight (g) | 99.67 ± 10.90 a | 68.84 ± 10.23b | 70.07 ± 14.11 b | 82.17 ± 13.37c |
| Fresh Pulp weight (g) | 71.27 ± 8.06 a | 27.16 ± 3.94 b | 37.28 ± 7.43 c | 42.68 ± 6.87 d |
| Fresh Peel weight (g) | 28.42 ± 2.94 a | 41.67 ± 6.31 b | 33.25 ± 6.62 c | 39.48 ± 6.59 b |
| Pulp (%) | 71.62 ± 0.23 a | 39.38 ± 0.62 b | 53.24 ± 0.54 c | 51.96 ± 0.94 d |
| Number of seeds | 237.0 ± 24.95 a | 235.0 ± 29.16 a | 141.0 ± 29.09 b | 250.0 ± 37.63 c |
| Dry weight of Seeds(g) | 2.00±0.21 a | 1.96±0.28 a | 1.27±0.23 a | 2.11±0.35 a |
| Seed/plup (%) | 2.87 ± 0.38 a | 7.22 ± 0.39 b | 3.94 ± 0.34 c | 4.90± 0.85 d |
| Volume of Juice (ml) | 28.6 ± 2.83a | 19.50 ± 3.96 b | 28.00 ± 4.87 a | 25.34 ± 3.90 c |
Values are means ± SD. Different letters in each row indicate significant difference (P < 0.05) among means of different cultivars
Results
Physical Fruit Properties of Cactus Pear cultivars
The physical characteristics of investigated cactus pear cultivars in this study are given in Table 2. The fruit properties differed significantly among locations. The samples from the Tunisia (Ain Amara) displayed higher fruit diameter and length together with high fresh fruit weight, fresh peel weight as well as fresh pulp weight and pulp% as compared to those collected from the other locations. Furthermore, Ain Amara samples possessed the highest extract juice volume (28.6±2.83 ml). the length of fruits from the other locations (Algeria, Moraco, Italy) were statistically of the same length. Regarding fruit diameter Algeria and Italy showed non significant different as compared with each other, while Moraco (Ain Jemaa) was differ significantly as compared with other cultivars. The mean values of fresh peel weights of the different locations ranged from 28.42 g to 41.67 g, with the Algeria (Lengissima) fruit samples having highest value. The same trend detected for percentage of seed/pulp. Regarding number of seeds; Italy (Sanguinea) showed the highest value and displayed significant different as compared with other cultivars.
Total Sugar Content
The levels of total sugar determined in juice and fruit of four Opuntia ficus-indica cultivars from different locations are presented in Table 3. There were significant differences in total sugar levels either in juice and fruits in relation to type of cultivars; varied from 76.8 to 215.0 (g/l) in juice and in fruit (g) from 5.77 to 15.44. Lengissima (Algeria) displayed the highest total sugar content either in juice and fruit and showed a significant different as compared with the other cultivars.
Table 3: Mean values of sugar content from four Opuntia ficus-indica cultivars
| Parameters | Ain Amara (Tunisia) |
Lengissima (Algeria) |
Ain Jemaa (Moraco) |
Sanguinea (Italy) |
| Juice (g/l) | 153.6 ± 1.63 a | 215.0± 2.00 b | 76.8± 2.00 c | 122.8± 6.11 d |
| Fruit (g/fruit) | 6.16 ± 0.02a | ± 0.02b 15.44 | 5.77± 0.02a | 7.29± 0.05a |
Values are means ± SD. Different letters in each row indicate significant difference (P < 0.05) among means of different cultivars.
Total Phenolic, Total Flavonoid and Total Flavonol Contents in extracts
Studies of the quantitative composition of cultivars extracts revealed the presence of high concentrations of phenolics in the ethyl acetate extract of Sanguinea (Italy) and methanolic extract of Ain Jemaa (Moraco). While the lowest concentration of phenolics displayed in Lengissima (Algeria) ethyl acetate extract and Sanguinea (Italy) methanolic extract. Concerning total flavonoids and flavonols of Ain Jemaa (Moraco) ethyl acetat extract displayed significant elevated concentrations as compared with other cultivars. While methanolic extract of Sanguinea (Italy) showed significant highest concentrations of Flavonoids and flavonols as compared with other cultivars.
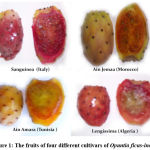 |
Figure 1: The fruits of four different cultivars of Opuntia ficus-indica |
 |
Figure 2: Chromatogram (HPLC-UV/SPD-10Avp) of the methanolic and ethyl acetate extract of four Opuntia ficus-indica cultivars (50 min) |
Chromatogram (HPLC-UV/SPD-10Avp) of the Methanol and Acetate Extract of Cactus Pear Fruit Juice
Separation of Compounds by High Performance Liquid Chromatography (HPLC)
In order to study the composition of the crude extracts obtained with the two organic solvents, were analyzed. Figure 2 shows, for the methanolic extracts of Tunisian and Algerian cultivars, similar profiles, while the Moroccan and Italian cultivars have different profiles. The chromatograms in diferent cultivars extracts showed a group of peaks that appear during the first 15 minutes, other less resolved peaks come out at the end, after 35 minutes. We notice a first peak (A) at 6 minutes, common to all profiles. A second peak (B) eluted at a retention time of 9 min is common to both spectra of Tunisian and Algerian cultivars only. Like a third peak (C) that comes out at 14 minutes.
With regard to the ethyl acetate extracts, the chromatograms have a different appearance. Tunisian shows a profile that differs from that of other cultivars, it has three peaks at the beginning of the spectrum (14 min). The other three cultivars have only one peak (D) out at 14 minutes.
Thus the methanolic extracts contain a wider variety of compounds than the ethyl acetate extracts. The two solvents did not extract the same molecules, the methanol selected the more polar compounds that come out on the chromatogram at the beginning of the spectrum. Instead, ethyl acetate dissolved more non-polar compounds eluted later and less well separated. At the beginning of the spectra, however, the peak common to the four cultivars (A), which appears at 14 minutes, was found interesting, to us to identify this product which would probably be responsible for the biological activities.
Analysis of the ethyl acetate extract of c. by Gas Chromatography Coupled to Mass Spectrometry (GC/MS)
We chose the ethyl acetate extract where there is the most abundant common peak (peak D) in figure 2, which is the case of the extract of Tunisian cultivar. We then sought to analyze this extract by coupling gas chromatography with mass spectrometry which allows the identification of the constituent compounds. It turns out that the major compound identified in the ethyl acetate extract of the fruit juice of Ain Amara cultivar is a phenolic compound: caffeic acid (peak 2, 73.70%) in figure 3. The second less abundant compound belonging to the same family is 4-hydroxyphenyl ethanol (peak 1; 0.381%). Other identified compounds correspond to saturated or unsaturated long-chain fatty acids such as hexadecanoic acid (peak 3), octadecanoic acid (peak 4), and oleic acid (peak 5). Finally, peak X originates from an experimental impurity from using plastic equipments.
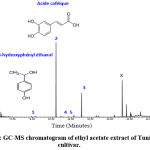 |
Figure 3: GC-MS chromatogram of ethyl acetate extract of Tunisian cultivar. |
Antioxidant Activity
The evaluation of the antioxidant activity by β-carotene method has affirmed that the result of the sample extracts is close to that of BHT. The obtained results have demonstrated that the activity methanol extract of Sanguinea and Ain Jemaa decreases after 30 min, while the ethyl acetate extract activity maintains the same level as that of BHT and even exceeds beyond 50 min.
Table 4: Mean values of total polyphenols, flavonoids, and flavonols from Ethyl acetate extracts of four Opuntia ficus-indica cultivars
| Parameters | Ain Amara (Tunisia) |
Lengissima (Algeria) |
Ain Jemaa (Moraco) |
Sanguinea (Italy) |
| TP (mg GAE /100g) | 1.50 ± 0.25 a | 0.25 b 0.50 ± | 1.00 ± 0.04 c | 2.20 ± 0.45 d |
| TF (mg CE /100g) | 57.00 ± 1.25 a | 42.00 ± 2.00 b | 65.00 ± 0.17 c | 56.00 ±1.52 d |
| TFL (mg RE /100g) | 7.80 ± 0.37 a | 9.10 ± 1.52 b | 9.70 ± 0.68 b | 2.50±0.68 c |
Values are means ± SD. Different letters in each row indicate significant difference (P < 0.05) among means of different cultivars. (TP) total polyphenols, (TF) total flavonoids, (TFL) total flavonols, (GAE) Galic acid equivalent, (CE) Catechin equivalent, (RE) Retin.
Table 5: Mean values of total polyphenols, flavonoids, and flavonols from methanol extracts of four Opuntia ficus-indica cultivars
| Parameters | Ain Amara (Tunisia) |
Lengissima (Algeria) |
Ain Jemaa (Moraco) |
Sanguinea (Italy) |
| TP (mg GAE /100g) | 1.89 ± 0.02 a | 2.45 ± 0.20 b | 2.53 ± 0.06 b | 0.80 ± 0.35 c |
| TF (mg CE /100g) | 26.13 ± 0.02 a | 30.00 ± 2.64 b | 31.61 ± 1.41 b | 34.19 ± 2.08 b |
| TFL (mg RE /100g) | 1.20 ± 0.20 a | 0.20 a 1.40 ± | 0.90 ± 0.15 b | 1.50 ± 0.25 a |
Values are means ± SD. Different letters in each row indicate significant difference (P < 0.05) among means of different cultivars. (TP) total polyphenols, (TF) total flavonoids, (TFL) total flavonols, (GAE) Galic acid equivalent, (CE) Catechin equivalent, (RE) Retin.
DPPH is a free radical compound that has been widely used to determine the free radical-scavenging ability of various sample extracts. The DPPH radical scavenging effect for methanol and ethyl acetate extracts of four cultivars is shown in Figure (4 and 5). The results indicated as relative activities against the control (BHT). The relative activities of all extracts are high, between 66.0±2.0 % and 81.61±1.73 %, so they exhibit antioxidant activity at a similar level to that of BHT (84%). In addition, the relative activities of ethyl acetate were slightly higher than those of methanol for all cultivars. The maximum activity for methanolic extracts of cultivars juice were found in Algerian and Italine cultivars as compared with others and the minimum activity was observed in Moracain type. Radical scavenging activity of ethyl acetate extracts displayed non significant diffence among the four types of cultivars.
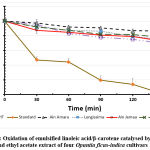 |
Figure 4: Oxidation of emulsified linoleic acid/β-carotene catalysed by BHT and ethyl acetate extract of four Opuntia ficus-indica cultivars |
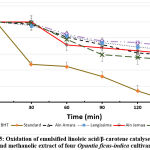 |
Figure 5: Oxidation of emulsified linoleic acid/β-carotene catalysed by BHT and methanolic extract of four Opuntia ficus-indica cultivars. |
Antimicrobial activity of methanolic and ethyl acetate extracts
The findings of this study show that methanolic crude extracts exhibited a considerably broader antimicrobial activity compared to ethyl acetate extracts (Table 6 and 7). The methanol extracts reveal positive tests against Staphylococcus aureus and the inhibition zone demonstrates larger diameters than those obtained with other bacteria (27.00±2.47, 24.00±1.16, 29.00±2.06 and 25.00±1.66) for Tunisia, Algeria, Moraco and Italy respectively. These values are the most highly recorded in comparison with other tested bacteria. The Moroccan cultivar has displayed the best antibacterial activity. The ethyl acetate extract also exhibits positive tests against Staphylococcus aureus, and the diameter of the inhibition zone were (14.00±1.77, 12.00±0.98, 11.00±1.37 and 13.00±1.47) for Tunisia, Algeria, Moraco and Italy respectively. Regarding both strains, Pseudomonas aeruginosa and Bacillus subtilis, the four methanolic extracts have shown moderately positive tests that. While, the inhibition diameter of neomycin against these two strains proves to be 25mm and 21mm, respectively. It can be noted that the ethyl acetate extract does not have any action against Pseudomonas aeruginosa and shows a positive action against Bacillus subtilis.
Table 6: Evaluation of the antibacterial and antifungal activities of methanol extracts of juices from Opuntia ficus-indica cultivars of using Agar diffusion method
| Parameters | Ain Amara (Tunisia) |
Lengissima (Algeria) |
Ain Jemaa (Moraco) |
Sanguinea (Italy) |
| Staphylococcus aureus | 27.00±2.47 a | 24.00±1.16 a | 29.00±2.06 a | 25.00±1.66 a |
| Escherchia coli | 0.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a |
| Salmonella enterica | 0.00±0.00 a | 0.00±0.00 a | 17.00±2.47 b | 16.00±2.47 b |
| Pseudomonas aeruginosa | 17.00±1.44 a | 17.00±1.25 a | 19.00±0.95 b | 20.00±1.92 b |
| Bacillus subtilis | 6.00±0.63 a | 6.00±0.68 a | 6.00±1.05 a | 7.00±1.21 a |
| Candida albicans | 15.00±0.91 a | 13.00±1.05 a | 0.00±0.00 b | 15.00±1.25 a |
| Aspergillus niger | 15.00±2.05 a | 17.00±2.05 b | 12.00±1.85 c | 14.00±1.35 a |
Values are means ± SD. Different letters in each row indicate significant difference (P < 0.05) among means of different cultivars.
Table 7: Evaluation of the antibacterial and antifungal activities of ethyl acetate extracts of juices from Opuntia ficus-indica cultivars of using Agar diffusion method
| Parameters | Ain Amara (Tunisia) |
Lengissima (Algeria) |
Ain Jemaa (Moraco) |
Sanguinea (Italy) |
| Staphylococcus aureus | 14.00±1.77a | 12.00±0.98b | 11.00±1.37 b | 13.00±1.47 a |
| Escherchia coli | 10.00±0.61 a | 10.00±0.56 a | 10.00±0.77 a | 11.00±0.40 a |
| Salmonella enterica | 0.00±0.00 a | 10.00±0.50b | 10.00±1.17 b | 10.00±0.81 b |
| Pseudomonas aeruginosa | 0.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a | 0.00±0.00 a |
| Bacillus subtilis | 10.00±0.86 a | 0.00±0.00 b | 10.00±0.56 a | 10.00±0.63 a |
| Candida albicans | 12.00±1.15 a | 0.00±0.00 b | 0.00±0.00 b | 0.00±0.00 b |
| Aspergillus niger | 16.00±1.07 a | 17.00±2.01 a | 16.50±1.54 a | 15.00±1.11 a |
Values are means ± SD. Different letters in each row indicate significant difference (P < 0.05) among means of different cultivars.
With respect to Salmonella enterica, the Tunisian and Algerian methanolic extracts appear to have no more activity, while the Moroccan and Italian extracts prove to have an antibacterial power. On applying ethyl acetate extract, the Tunisian samples are found to show no more activity, while the other samples turn out to have inhibitive power face to Salmonella enteric. Escherchia coli resists to all methaolic- extract samples. However, ethyl acetate extracts show a positive test against Escherchia coli.
The obtained results clearly highlight the persistence of a positive action of the sample extracts against the yeast and fungal species, except for the pertinent Ain jemaa extract. However, our extract seems not to show any resistance against Aspergillus Niger, contrarily to Candida albicans, in which the extract action has the ability to resist.
Discussion
The physical characteristics of the Cactus Pear fruits reported Tables 2. The analysis of the various morphological parameters studied shows that the Tunisian cultivar has the most favourable criteria: it has the fruit of larger size (length 7.29 cm and width 4.77 cm) and thus had the highest fresh weight. The percentage of the pulp compared to the whole fruit is 71.62%. On the other hand, the peel has the lowest percentage (28.38%), as well as that of the seeds in relation to the pulp (2.87%). Regarding fruit mass Arba (2004) reported same weight ranges for some Moroccan varieties as in our results. Moreover, Stintzing, Schieber, and Carle 2003) reported that the fruit mass of some South African varieties vary from 83 g to 194 g Also, Felker et al., (2005) reported that in Argentina, the cactus fruit mass is between 112 and 212 g. Indeed, many authors (Karababa, Coskuner, and Asay 2004) have suggested that the size of cactus pear fruit is influenced by the region, season and environment. Nevertheless, other authors have confirmed that cactus fruit size is controlled by genetic factors (Peter Felker et al., 2002).
Concerning, the fruit pulp percentage the present results displayed that Ain Amara was noticeably higher than Ain Jemaa and Sanguinea. Felker et al., (2005) reported that the pulp percentage of some varieties from Mexico and Argentina is between 40% and 60%. Indeed, the fruit pulp percentage may be due to the differences in cultivating practices, geographic location and climate conditions. It has been reported that during maturation, a higher rainfall can increase the pulp percentage, while a lower temperature can decrease the pulp percentage (Peter Felker et al., 2002; Lorenzo et al., 2008). The current results support the effect of cultivar on the fruit size and pulp percentage.
With regard to seeds, the current study revealed that Sanguinea presented the highest content of seeds with 4.9% of fruit; followed by Ain Amara and Lengissima. while Ain Jemaa, the seed content displayed the lowest value. This result in contrary with Pimienta-Barrios (1994) who reported a value between 10 and 15%. Indeed, these differences can explain the FAO collection of the cultivars.
The volume of juice is an important parameter of the quality of the fruit. The juice volumes recorded in the four cultivars (harvested in August) are close to that of prickly cactus harvested in (July-August) from the region of Kasserine (24.68 ml / fruit) studied by El-Guizani et al., (2012), but they are low compared to cultivars harvested (October-November) named Gialla and Rossa from the Soliman region in Tunise (44.77 ml and 55.43 ml / fruit, respectively). This highlights the effect of the environment, drought in August and precipitation in October-November.
Regarding total sugar content in the cactus fruit; Piga et al., (1996) reported that reducing sugar in cultivar from Italy is from 10 to 15 %. Indeed, the fruit of Opuntia sp. is considered as a very sweet fruit, which is due to the presence of reducing sugars in the pulp, especially glucose and fructose (Russel & Felker 1987; Stintzing, Schieber, and Carle 2003). From the above results it could be mentioned that the highest content of total sugar represented in Algeria cultivar followed by Tunisia. These variation may be explained as mentioned by Carle (2003).
A previous study showed that there are many types of polyphenols in plant, and various phenolic molecular structures exhibited different antioxidant activities (Agati et al., 2012). Currently, many researchers and dietary organizations are recommending an increase of the consumption of fruits and vegetables that contain natural antioxidant (Moure et al., 2001; Bazzano et al., 2002). Phenols are a major group of antioxidant phytochemicals with interesting properties for animal or human health. They display a remarkable group of biological and pharmacological activities (Ihme et al., 1996).
The solvent has an effect on the polyphenol content of the extract (Do et al., 2014). The phenolic compounds are polar compounds and the methanol is the most polar solvent and the most appropriate to extract the total phenol, this in agreement with our results (Roby et al., 2013). The above results revealed that; methanol extract of different cultivars displayed higher content of the total phenols as compared with ethyl acetate. Moreover, The majority phenolic compound found in the fruit pulp ethyl acetate extract of the cultivar of Opuntia ficus-indica is caffeic acid. The second least abundant of the same family compound was detected is 4-hydroxyphenyl ethanol. Other acids long-chain saturated and unsaturated were also identified as hexadecanoic acid, oleic acid, octadecanoic acid and hexanoic acid. Stintzing et al., (2001) reported that Cactaceae had low content of polyphenols. Moreover, Chavez-Santoscoy, et al., (2009) told that the total phenolic compounds are between 2.2 and 22.6 mg GAE /100g for some cactus species from Mexico.
Nonetheless, our results have shown that the content of flavonoids extracted with ethyl acetate higher than the one obtained with methanol. Moreover, the content of flavonols extracted using ethyl acetate is higher than that extracted with methanol in the tested cultivars. These results may be explained by the fact that cactus juice is rich in moderately polar flavonoids which are probably more soluble in ethyl acetate than methanol. This is in agreement with the results found by other authors (Stintzing et al., 2005) who reported that a considerable diversity of methods exists to assess these antioxidants in plant tissues.
Flavonoids can act as antioxidants, enzyme inhibitors, pigments that allow the absorption of light and are responsible for coloring in nature (Ninfali et al., 2007). The flavonoid contents of the ethyl acetate extracts are higher (41.61 to 65.16 EC mg/100g), and more variable in cultivar to the other. This result is unexpected since the flavonoids which make up half of the phenolic compounds (Hertog, 1995) are polar compounds and are usually extracted with methanol or water. This proves that the juice of prickly pear is rich in medium polar flavonoids and therefore more soluble in ethyl acetate.
Flavonols represent a group of flavonoids responsible for yellow, brown, and even white colors of flowers and fruits of plants. The contents of the methanolic extracts are less than those of the ethyl acetate extracts, they fluctuate from 1.10 to 1.56 mg Eq Rutin/100 g of extract. While with ethyl acetate, they reach 2.44 to 9.64 mg Eq Rutin/100 g Italian cultivars has the lowest and almost similar flavonol values, with both extraction solvents.
Thus, the polyphenols of the prickly pear juice of the four cultivars appear to be different in their content and their extractability with respect to the two solvents used, especially for Italian. Flavonoids, including flavonols, are better extracted with ethyl acetate. Some flavonoid activities are generally due to flavonols with several hydroxyl groups that have defense functions: antioxidant activity (Rice-Evans et al., 1996) and antimicrobial activity (Chattopadhay et al., 2001).
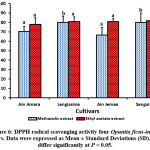 |
Figure 6: DPPH radical scavenging activity four Opuntia ficus-indica cultivars. Data were expressed as Mean ± Standard Deviations (SD). Values differ significantly at P < 0.05. |
(Figure 6, table 6) It was noted that the migration of deposits extracted with the fruit pulp ethyl acetate extract of Opuntia ficus-indica, F5 and F’5-5 (Bargougui et al., 2014) have the same revealed that beta-Sitosterol spots. So the extract, F5 and F’5-5 contain beta-Sitosterol has antiallergic activity by (Dok-Go, 2003). We can assume that the antileishmanial and antitrypanosomal activities of the extract, F5 and F’5 (Bargougui et al., 2014) are either the activity of caffeic acid or the activity of β-sitosterol.
Plants rich in secondary metabolites, including phenolics, flavonoids and carotenoids, have antioxidant activity due to their redox properties and chemical structures. The extract of cactus fruit juice cultivars had strong antioxidant activity. The DPPH radical is widely used in assessing free radical scavenging activity because of the ease of the reaction.
Indeed, due to the different antioxidant potentials of the different compounds, the antioxidant activities of the extract strongly depend on the extraction solvent (Jang et al., 2007). antioxidant activity of phenolics is mainly due to their redox properties which make them act as reducing agents, hydrogen donors, singlet oxygen quenchers. These properties may also have a metallic chelating potential (Rice-Evans, et al., 1996).
The above results show that the activity of methanol extracts reveal positive tests against Staphylococcus aureus and the inhibition zone demonstrates larger diameters than those obtained with other bacteria. The Moroccan cultivar has displayed the best antibacterial activity. The ethyl acetate extract also exhibits positive tests against Staphylococcus aureus.
Regarding both strains, Pseudomonas aeruginosa and Bacillus subtilis, the four methanolic extracts have shown moderately positive activity. It can be noted that the ethyl acetate extract does not have any action against Pseudomonas aeruginosa and shows a positive action against Bacillus subtilis. This results in agreement with Ammar et al., (2012) who reported that different cultivars of Opuntia ficu -indica are more active than the flowers hexane extract against Pseudomonas aeruginosa, Esherchia coli and Staphylococcus aureus.
With respect to Salmonella enterica, the Tunisian and Algerian methanolic extracts appear to have no more activity, while the Moroccan and Italian extracts prove to have an antibacterial power. On applying ethyl acetate extract, the Tunisian samples are found to show no more activity, while the other samples turn out to have inhibitive power face to Salmonella enteric.
Concerning antimicrobial activity of cactus extracts, our findings confirm those reported by Bussmann et al., (2010) for Opuntia ficus indica collected in the North of Peru. Where Escherchia coli resists to all methaolic- extract samples. As well as the results obtained from exposure of Escherchia coli to ethyl acetate extract.
The obtained results clearly highlight the persistence of a positive action of the sample extracts against the bacterial and fungal species, except for the pertinent Ain jemaa extract. However, our extract seems not to show any resistance against Aspergillus Niger for more than two days, contrarily to Candida albicans, in which the extract action has the ability to resist for more than two days.
In the present work, the Opuntia ficus-indica extract appears to be more active than those reported by (Ammar et al., 2012) with regard to the flowers’ extract of the same species, especially against Aspergillus Niger and Candida albicans. It is noteworthy to mention that our results prove that the most effective extract against the studied microorganisms turns out to be the same extract which possesses the best antioxidant activity for Opuntia ficus-indica. Consequently, it is confirmed that the bioactive compounds important level, other than the phenolic compounds, is the one responsible for these biological properties. Indeed, many authors have substantiated that these compounds have an antibacterial effect (Ahmad and Beg 2001; Rodriguez et al., 2009; Taguri, et al., 2006).
Actually, the phenolic compounds have the ability to act at two different levels, namely, the cell membrane and cell wall of the microorganisms (Taguri, et al., 2006). In fact, they can interact with the membrane proteins, affect the membrane permeability and lead to cell destruction. Besides, they can penetrate into bacterial cells and coagulate their content. In addition, the phenolic compounds are widely known to be synthesized by plants in response to microbial infection (Doughari, El-Mahmood, and Tyoyina 2008; Sengul et al., 2009). Hence, it is logical that they can be found in vitro as effective antimicrobial substances against a wide range of micro-organisms (Cowan 1999).
Conclusions
The results of the present study have shown a large difference in fruit, pulp and juice characteristics of the four different cultivars of Opuntia ficus-indica. It is worthy to highlight that the present research contributes to demonstrate that the prickly pears potential plays an important role as a source of natural antioxidants, antibacterial and antifungal.
the antioxidant activity of cultivars may be due to presence of phenolic compounds which are the major contributor of the antioxidant capacities of these plants. Further research is needed in order to find the important bioactive compounds and if the in vitro results correlate with animal studies.
Acknowledgements
The authors would like to thank the director of the FAO station for the precious help in collecting the samples.
Conflict of Interest
The authors declare that they have no conflict of interest.
Reference
- Ahmad I and Arina Z. Antimicrobial and Phytochemical Studies on 45 Indian Medicinal Plants against Multi-Drug Resistant Human Pathogens. J Ethnopharmacol, 2001; 74 (2): 113-23.
- Agati G, Azzarellob E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci, 2012; 196: 67-
- Ammar I, Monia E, Bassem K, Thabèt Y, and Hamadi A. Variation in Chemical Composition and Biological Activities of Two Species of Opuntia Flowers at Four Stages of Flowering. Ind Crops Prod, 2012; 37 (1): 34-40.
- Arba M. ‘Dellahia’ a Cactus Pear Cultivar from the Mediterranean Coast of Northern Morocco. In V International Congress on Cactus Pear and Cochineal, 2004; 728, 37-42.
- Balentine C, Crandall G, O’bryan C, Duong D, and Pohlman F. The Pre-and Post-Grinding Application of Rosemary and Its Effects on Lipid Oxidation and Color during Storage of Ground Beef. Meat Sci, 2006; 73 (3): 413-21.
- Bargougui A, Champy P, Triki S, Bories C, Le Pape P, and Loiseau P. Antileishmanial Activity of Opuntia Ficus-Indica Fractions. Biomed Prev Nutr, 2014; 4 (2): 101-104.
- Bazzano, Lydia A, Jiang He, Lorraine G Ogden, Catherine M Loria, Suma Vupputuri, Leann Myers, and Paul K Whelton. Fruit and Vegetable Intake and Risk of Cardiovascular Disease in US Adults: The First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr, 2002; 76 (1): 93-99.
- Bouaziz M and Sayadi S. Isolation and Evaluation of Antioxidants from Leaves of a Tunisian Cultivar Olive Tree. Eur J Lipid Sci Technol, 2005; 107 (7-8): 497-504.
- Bussmann, RW, Malca-Garcia G, Glenn A, Sharon D, Chait G, Diaz D, Pourmand K, et al., Minimum Inhibitory Concentrations of Medicinal Plants Used in Northern Peru as Antibacterial Remedies. J Ethnopharmacol, 2010; 132 (1): 101-8.
- Chavez-Santoscoy RA, Gutierrez-Uribe JA, and Serna-Saldivar SO. Phenolic Composition, Antioxidant Capacity and in Vitro Cancer Cell Cytotoxicity of Nine Prickly Pear (Opuntia Spp.) Juices. Plant Food Hum Nutr, 2009; 64 (2): 146-52.
- Conway JG, Pink H, Bergquist ML, Han B, Depee S, Tadepalli S, and Selph, JL. Effects of the CFMS Kinase Inhibitor 5- (3-Methoxy-4- (4- in Normal and Arthritic Rats. Pharmacol, 2008; 326 (1): 41-50.
- Cowan MM. Plant Products as Antimicrobial Agents. Clin Microbiol Rev, 1999; 12 (4): 564-82.
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P and Vidal N. Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chem, 2006; 97 (4): 654-660.
- Doughari JH, El-Mahmood AM and Tyoyina I. Antimicrobial Activity of Leaf Extracts of Senna Obtusifolia (L). Afr J Pharm Pharmacol, 2008; 2 (1):7-13.
- Duru B, and Turker N. Changes in Physical Properties and Chemical Composition of Cactus Pear (Opuntia Ficus-Indica) during Maturation. J Prof Assoc Cactus Dev, 2005; 7: 22-33.
- Do Q, Angkawijaya A, Tran-Nguyen P, Huynh L, Soetaredjo F, Ismadji S, Ju Y. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal, 2014; 22(3), 296-302.
- Fazeli MR, Amin G, Attari MMA, Ashtiani H, Jamalifar H and Samadi N. Antimicrobial Activities of Iranian Sumac and Avishan-e Shirazi (Zataria Multiflora) against Some Food-Borne Bacteria. Food Control, 2007; 18 (6): 646-49.
- Felker, P., Rodriguez, SDC, Casoliba, RM, Filippini R, Medina D and Zapata R. Comparison of Opuntia ficus indica varieties of Mexican and Argentine origin for fruit yield and quality in Argentina. J Arid Environ, 2005; 60(3): 405-422.
- Felker P, Soulier C, Leguizamón, G and Ochoa, J. A comparison of the fruit parameters of 12 Opuntia clones grown in Argentina and the United States. Arid Environ, 2002; 52(3): 361-370.
- Field, A. Discovering statistics using SPSS: advanced techniques for the beginner (2nd Edition). London: Sage. 2004.
- Galati EM, Mondello MR, Giuffrida D, Dugo G, Miceli N, Pergolizzi S, and Taviano MF. Chemical characterization and biological effects of Sicilian Opuntia ficus indica (L.) Mill. fruit juice: antioxidant and antiulcerogenic activity. J Agric Food Chem, 2003; 51(17): 4903-4908.
- Gebremariam T, Melaku S, and Yami A. Effect of different levels of cactus (Opuntia ficus-indica) inclusion on feed intake, digestibility and body weight gain in tef (Eragrostis tef) straw-based feeding of sheep. Anim Feed Sci Technol, 2006; 131(1-2): 43-52.
- Hajji M, Masmoudi O, Souissi N, Triki Y, Kammoun S and Nasri M. Chemical composition, angiotensin I-converting enzyme (ACE) inhibitory, antioxidant and antimicrobial activities of the essential oil from Periploca laevigata root barks. Food Chem, 2010; 121(3): 724-731.
- Ihme N, Kiesewetter H, Jung FA, Hoffmann KH, Birk A, Müller A and Grützner KI. Leg oedema protection from a buckwheat herb tea in patients with chronic venous insufficiency: a single-centre, randomised, double-blind, placebo-controlled clinical trial. Eur J Clin Pharmacol, 1996; 50(6): 443-447.
- Jang HD, Chang KS, Huang YS, Hsu, CL, Lee SH and Su MS. Principal Phenolic Phytochemicals and Antioxidant Activities of Three Chinese Medicinal Plants. Food Chem, 2007; 103(3): 749-756.
- Karababa E, Coskuner Y and Asay S. Some physical fruit properties of cactus pear (Opuntia spp.) that grow wild in the Eastern Mediterranean region of Turkey. J Profes Assoc Cactus Develop, 2004; 6: 1-8.
- Li H, Cheng K, Wong C, Fan K, Chen F, Jiang Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem, 2007; 102: 771-776.
- Lorenzo N, Domínguez MC, Hernández A, Barberá A, Merino N, Vazquéz A and Padrón, G.., Characterization of two experimental rodent models for evaluating novel drugs for Rheumatoid Arthritis. Biotecnol Apl, 2008; 25(3): 236-241.
- Medina ED, Rodríguez, ER and Romero C. Chemical characterization of Opuntia dillenii and Opuntia ficus indica fruits. Food chem, 2007; 103(1):38-45.
- Meot-Duros L and Magne C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol Biochem, 2009; 47(1): 37-41.
- Miliauskas G, Venskutonis PR and Van Beek, TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food chem, 2004; 85(2): 231-237.
- Miller G, Use of dinitrosalicylic acid reagent for détermination of reducing sugar. Analyt Chem, 1959; 31: 426-428.
- Moure C, Gutierrez D, Tartaj J, Capel F and Duran P. Chemical and electrical features of the interphase: electrode–electrolyte in the system (Y,Ca) MnO3–CeO2–Y2O3. Solid state ionics, 2001; 141: 381-386.
- Natarajan D, Britto SJ, Srinivasan K, Nagamurugan N, Mohanasundari C and Perumal G. Anti-bacterial activity of Euphorbia fusiformis—A rare medicinal herb. J ethnopharmacol, 2005; 102(1): 123-126.
- Piga, A. Cactus pear: a fruit of nutraceutical and functional importance. J Profes Assoc Cactus Develop, 2004; 6: 9-22.
- Pimienta-Barrios E. Prickly pear (Opuntia spp.): a valuable fruit crop for the semi-arid lands of Mexico. J Arid Environ, 1994; 28(1): 1-11.
- Rice-Evans CA, Miller NJ and Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med, 1996; 20(7): 933-956.
- Roby M, Sarhan M, Selim K, Khalel K. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind Crops Prod, 2013; 43: 827-831.
- Rodríguez H, Curiel JA, Landete JM, de las Rivas B, de Felipe FL, Gómez-Cordovés C, and Muñoz, R. Food phenolics and lactic acid bacteria. Int J Food Microbiol, 2009; 132(2-3): 79-90.
- Sáenz C, Arriagada S, Fiszman SM, and Calvo C. Influence of PH and Content of Carrageenan during the Storage of Cactus Pear Gels. In III International Congress on Cactus Pear and Cochineal, 1996; 438: 131-134.
- Sáenz C, Estévez AM, Sepúlveda E, and Mecklenburg P. Cactus Pear Fruit: A New Source for a Natural Sweetener. Plant Food Hum Nutr, 1998; 52 (2): 141-149.
- Saenz C. Processing technologies: an alternative for cactus pear (Opuntia spp.) fruits and cladodes. J Arid Environ, 2000; 46(3): 209-225.
- Sengul M, Yildiz H, Gungor N, Cetin, Eser Z and Ercisli S. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak J Pharm Sci, 2009; 22: 1.
- Shetty AA, Rana MK and Preetham SP. Cactus: a medicinal food. J Food Sci Technol, 2012; 49(5):530-536.
- Stintzing FC, Herbach KM, Mosshammer MR, Carle R, Yi W, Sellappan S. and Felker P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J Agric Food Chem, 2005; 53(2): 442-451.
- Stintzing FC, Schieber A and Carle R. Phytochemical and nutritional significance of cactus pear. Eur Food Res Technol, 2001; 212(4): 396-407.
- Stintzing FC, Schieber A, Carle R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur Food Res Technol, 2003; 216(4): 303-311
- Taguri T, Tanaka T and Kouno I. Antibacterial Spectrum of Plant Polyphenols and Extracts Depending upon Hydroxyphenyl Structure. Biol Pharm Bull, 2006; 29 (11): 2226-2235.
- Vieira EL, Batista ÂM, Mustafa AF, Araújo RFS, Soares PC, Ortolane EL and Mori CK. Effects of feeding high levels of cactus (Opuntia fícus-indica Mill) cladodes on urinary output and electrolyte excretion in goats. Livest Sci, 2008; 114(2-3), 354-357.
- Vogel AI. A Text-Book Of Quantitative Inorganic Analysis-Theory And Practice. Longmans, Green And Co.; London; New York; Toronto, 2013.
- de Wit M, Nel P, Osthoff G and Labuschagne, MT. The effect of variety and location on cactus pear (Opuntia ficus-indica) fruit quality. Plant Foods Hum Nutr, 2010; 65(2):136-145.







