Kanishka Hrishi Das1 , V. Mangayarkarasi1 ⃰ and Maitrayee Sen2
, V. Mangayarkarasi1 ⃰ and Maitrayee Sen2
1Department of Microbiology, SRM Medical College Hospital and Research Centre, Kattankulathur- 603203, Tamil Nadu, India.
2Department of Gynaecology, SRM Medical College Hospital and Research Centre, Kattankulathur- 603203, Tamil Nadu, India.
Corresponding Author’s Email: mangaikumaran@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1765
Abstract
Vulvovaginal candidiasis (VVC) is caused by Candida species. It has been associated with impact on economic cost. Currently, Non-albicans Candida species are more resistant to azoles and get converted from harmless to pathogenic state due to several virulence factors. Monitoring of the antifungal susceptibility pattern is important to know the resistant pattern of Candida species. Thus the objective of this research was to the identification of Candida in species level and to evaluate the antifungal resistance pattern in Candida species isolated from the vaginal discharge of antenatal women with vulvovaginal candidiasis. This prospective study was done in SRM MCH & RC, Chennai, India, from March 2017 and December 2018. An aggregate of 342 vaginal swabs were gathered from antenatal women of symptomatic and asymptomatic VVC. Antifungal susceptibility test was done by the disk diffusion method as per the CLSI guidelines. A total of 112 Candida species were isolated from 342 high vaginal swabs. Out of 112 Candida isolates, 65 (58%) were Non-albicans Candida (NAC) and 47 (42%) were C. albicans. In this study, 103/112(91.6%) of Candida isolates had the highest sensitivity to voriconazole and 26/112(23.2%) of Candida isolates had the highest resistance to miconazole. NAC species are emerging as potential threats to cause infection and posing a therapeutic challenge. Early empirical antifungal therapy and further research to improve diagnostic, prevention and therapeutic strategies are necessary to reduce the considerable morbidity and mortality.
Keywords
Antifungal; Antenatal Women; Candida; Fluconazole; Non-Albicans Candida; Resistant; Vulvovaginal Candidiasis
Download this article as:| Copy the following to cite this article: Das K. H, Mangayarkarasi V, Sen M. Antifungal Resistant in Non-Albicans Candida Species are Emerging as a Threat to Antenatal Women with Vulvovaginal Candidiasis. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Das K. H, Mangayarkarasi V, Sen M. Antifungal Resistant in Non-Albicans Candida Species are Emerging as a Threat to Antenatal Women with Vulvovaginal Candidiasis. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/2mmGcvy |
Introduction
In antenatal women, Vulvo-Vaginal Candidiasis (VVC) is one of the commonest fungal infections caused by Candida species.1, 2 The prevalence rate of VVC is more than 40% worldwide and 5-10% antenatal women suffered recurrent VVC.3 The usual presentations are persistent curdy white vaginal discharge with itching, bad odour, irritation, pain in the lower abdomen and induration of vulva.4 During pregnancy, VVC has been related to adverse outcomes such as low birth weight, preterm birth, miscarriage and premature rupture of the membrane.5, 6 The etiological agents of VVC are Candida albicans and Non-albicans Candida (NAC). Among NAC, C. glabrata is the second most common Candida species isolates from VVC. Other species are C. tropicalis, C. krusei, C. parapsilosis, C. kefyr, C. rugosa, C. dubliniensis and C. guiliermondi.7, 8 There is increased scientific and epidemiological interest on NAC species as their prevalence is at increasing trend all around the world. In complicated VVC, NAC species are commonly found than C. albicans.9 Pathogenic mechanisms of NAC aren’t understood clearly as those of C. albicans where progressively broad research has been done. Prolonged antifungal treatment, diabetes mellitus, more established age and early antifungal uses are situations that lead to a rise in the prevalence rate of NAC species.2 C. albicans and NAC species possess several virulence factors like extracellular production of hydrolytic enzymes, hyphae formation, phenotype switching and cell adhesion.10 A significant characteristic of NAC in that they are intrinsically resistant to the first line azole drugs, resulting in treatment failure.11 In India, very limited data are available on NAC causing vaginal infection in antenatal women and its antifungal susceptibility. Incidence of antifungal resistance to Candida species has been on a growing trend over the past decade. Azoles are the drug of choice for VVC; yet, resistance has been reportable increased in NAC species.12, 13 Causes of azoles drugs resistance in Candida species is due to continued treatment and repeated use of antifungal for recurrent candidiasis.14 Several studies have described the mechanisms of azoles resistance, such as elevated expression of gene encoding lanosterol demethylase (ERG11), a point mutation in the ERG11, the gene coding the multidrug efflux pumps, CaMDR1, CDR1 & CDR2.8 It has become essential to have a close check of antifungal susceptibility and resistance mechanisms in an environment of rapidly changing resistant pattern. There are many studies, which have assessed the method of susceptibility patterns for Candida. “Clinical and Laboratory Standards Institute (CLSI)” has been described as the standard procedure for antifungal susceptibility test of fungus. Macro tube dilution method is time-consuming and more laborious in most of the clinical laboratories.15, 16 Micro broth dilution method has been found to be acceptable.16 Though micro broth dilution tests can commonly be read after 24 hours and 48 hours15, 17 the epsilometer test (E- test) is an exclusive method, within 24 hours of incubation can give trustworthy results with minimum labour.16 A simple disk diffusion test has some significant benefits for practical reasons in a clinical laboratory.18-21 Therefore, the aim of this study was to identify the Candida species level and investigate antifungal susceptibility patterns in Candida species from vaginal discharge in order to determine any emerging resistance and to develop standard treatment guidelines for VVC of antenatal women, particularly in settings where the diagnosis depends on clinical overview or restricted research facility testing.
Material and Methods
This research was done in the Department of Microbiology and Obstetrics & Gynaecology (OG) at SRM MCH and RC, Kattankulathur, Tamil Nadu, India, from March 2017 to December 2018 after getting the approval from the “Institutional Ethical Committee (IEC NO-1090/IEC/2017)”. Informed consent of the patients was taken before including them into the study. Inclusion criteria for cases of VVC were all women attending the obstetric clinic with or without symptoms of curdy white discharge, itching, odour, pain, irritation, and swelling. Women with clinically diagnosed VVC on antifungal treatment were excluded from the study.
Collection of specimen and processing:
Totally 342 vaginal swabs were collected from antenatal women of symptomatic and asymptomatic VVC. Samples were processed for direct microscopy by 10 % potassium hydroxide mount (Figure 1) and Gram stain (Figure 2). The culture was performed on “Sabouraud’s Dextrose Agar (SDA)” (Figure 3) with gentamicin and incubated at 37oC as well as at 25oC for 24-48 hours.
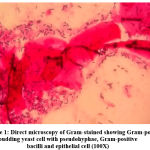 |
Figure 1: Direct microscopy of Gram-stained showing Gram-positive budding yeast cell with pseudohyphae, Gram-positive bacilli and epithelial cell (100X) |
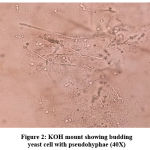 |
Figure 2: KOH mount showing budding yeast cell with pseudohyphae (40X) |
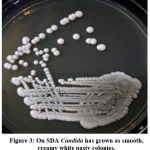 |
Figure 3: On SDA Candida has grown as smooth, creamy white pasty colonies. |
Candida Species Identification by the Conventional Method
Germ tube test (Figure 4) was done to differentiate Candida albicans from NAC.22 Subsequently, Candida species were identified by culture on Chrome agar (Himedia, India) according to manufactures instructions (Figure 5). Cornmeal agar with tween 80 (Dalmau plate technique) performed as described in the previous study for chlamydospores and blastopores formation23 (Figure 6). Sugar fermentation (Figure 7) and assimilation tests were done to identify Candida species as proposed by Giri et al.,24
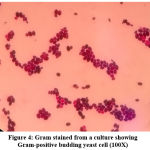 |
Figure 4: Gram stained from a culture showing Gram-positive budding yeast cell (100X) |
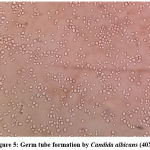 |
Figure 5: Germ tube formation by Candida albicans (40X) |
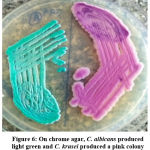 |
Figure 6: On chrome agar, C. albicans produced light green and C. krusei produced a pink colony |
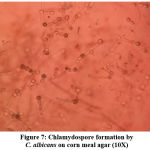 |
Figure 7: Chlamydospore formation by C. albicans on corn meal agar (10X) |
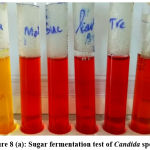 |
Figure 8 (a): Sugar fermentation test of Candida species |
Inoculum Preparation and Susceptibility Testing by Disk Diffusion Method
As per the “CLSI” (M44-A) procedures, the inoculum was set up by picking five particular colonies from a 24hr old culture of Candida species. Colonies were suspended in 5ml of sterile 0.9% normal saline.25 The suspension was vortexed to get uniform turbidity and adjusted visually to 0.5 McFarland standards. After 15 minutes, the suspension was inoculated onto “Mueller Hinton Glucose Agar (MHGA)” with 2% glucose and 0.5 µg/ml of methylene blue were used for susceptibility testing (Figure 8 & 9). Fowling antifungal disks were used: fluconazole (10 µg), ketoconazole (30 µg), clotrimazole (10 µg), nystatin (100 U), voriconazole (1 µg), miconazole (30 µg) and amphotericin-B (20 µg). Antifungal disks are placed evenly so that they are not closer than 24mm from the centre to centre. The plates were placed in an incubator at 37oC for 24-48hr. C. albicans ATCC90028, Candida tropicalis ATCC 750, Candida krusei ATCC6258 and C. Parapsilosis ATCC22019 were used as a control. All the antifungal disks, control strains and culture media were parched from Hi-Media, Mumbai, India. The zones of inhibitions were as resistant (R), susceptible dose-dependent (SDD) and susceptible (S) as per the CLSI guidelines for fluconazole and voriconazole, while for other antifungal agents, interpretive breakpoints were referred from published paper13, 25-27 (Table 1).
Table 1: Interpretive break point of different antifungal agents
| Drugs with concentration (µg) | Susceptible (mm) | Susceptible dose-dependent (mm) | Resistant (mm) |
| Fluconazole (10 µg) | ≥19 | 15-18 | ≤14 |
| Ketoconazole (30 µg) | ≥28 | 21-27 | ≤20 |
| Clotrimazole (10 µg) | ≥20 | 12-19 | ≤11 |
| Miconazole (30 µg) | ≥20 | 12-19 | ≤11 |
| Voriconazole (1 µg) | ≥17 | 14-16 | ≤13 |
| Amphotericin-B (20 µg) | ≥15 | 10-14 | <10 |
| Nystatin (100 U) | ≥15 | 10-14 | No zone |
µg=micrograms, mm=millimetre.
 |
Figure 8 (b): Antifungal Susceptibility test of Candida isolates by disk diffusion methods |
 |
Figure 9: Antifungal Susceptibility test of Candida isolates by disk diffusion methods |
Statistical Analysis
Data were entered into an excel sheet and analysed by using Epi Tools epidemiological calculators online software. Z test has been used to find the difference in proportions and a 5% level of significance has been used.
Results
Totally 112 Candida species were isolated from 342 high vaginal swabs. Out of 112 Candida isolates, 65/112(58%) were Non-albicans Candida (NAC) and 47/112(42%) were C. albicans. Among NAC, 23/112(20%) were C. glabrata, followed by 21/112(19%) C. tropicalis, 11/112(10%) C. parapsilosis and 10/112(9%) were C. krusei as shown in figure 10.
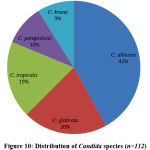 |
Figure 10: Distribution of Candida species (n=112) |
Totally 112 Candida isolates were tested for antifungal susceptibility testing by disk diffusion method. Among 112 Candida isolates, 65(58%) were Non-albicans Candida (NAC) and 47(42%) were C. albicans. In this study Candida species had shown highest sensitivity to voriconazole 101/112(90.1%) followed by amphotericin B 100/112(89.2%), nystatin 92/112(82.1%), fluconazole 83/102(81.3%), clotrimazole 85/112(75.8%), ketoconazole 79/112(70.5%) and miconazole 75/112(66.9%) as shown in table 2. In the present study Candida species had shown highest resistance to miconazole 26/112(23.2%) followed by ketoconazole 25/112(22.3%), clotrimazole 19/112(17%), fluconazole 16/102(15.6%), nystatin 16/112(14.2%), amphotericin B 12/112(10.8%) and voriconazole 9/112(8.1%). Candida albicans had shown the highest sensitivity to amphotericin B 43/47(91.4%) and voriconazole 40/47(85.1%) but the highest resistant to ketoconazole 10/47(22%). C.glabrata had shown the highest sensitivity to amphotericin B 21/23(93.3%) while 7/23(30%) resistance to Miconazole. C. tropicalis had shown a maximum of 20/21(95.2%) sensitivity against Voriconazole and 6/21(28.5%) resistance to Miconazole. C. parapsilosis had shown 11/11(100%) sensitivity against Voriconazole and 3/11(27%) resistance to Miconazole. C.krusei had shown 9/10(90%) sensitivity to Voriconazole and highest resistance 5/10(50%) against Clotrimazole (Table 2).
Table 2: Antifungal susceptibility pattern of various Candida species
|
Antifungal agents |
C. albicans
n=47(%) |
Non-albicans Candida(NAC) | Total
n=112(%) |
||||
| C.glabrata
n=23(%) |
C. tropicalis
n=21(%) |
C. parapsilosis
n=11(%) |
C. krusei
n=10(%) |
||||
| FCZ | Susceptible | 39(83) | 19(82) | 16(76) | 9(81) | NA* | 83/102(81.3) |
| SDD | – | 1(4) | – | 2(19) | NA* | 3/102(2.9) | |
| Resistant | 8(17) | 3(14) | 5(24) | – | NA* | 16/102(15.6) | |
| VCZ | Susceptible | 40(85.1) | 21(91.3) | 20(95.2) | 11(100) | 9(90) | 101(90.1) |
| SDD | 2(4.2) | – | – | – | – | 2(1.7) | |
| Resistant | 5(10.6) | 2(8.7) | 1(4.7) | – | 1(10) | 9(8.1) | |
| KCZ | Susceptible | 35(74) | 15(65) | 15(71) | 8(72) | 6(60) | 79(70.5) |
| SDD | 2(4) | 2(8.6) | 2(10) | 1(9) | 1(10) | 8(7.1) | |
| Resistant | 10(22) | 6(26) | 4(19) | 2(19) | 3(30) | 25(22.3) | |
| CLOT | Susceptible | 40(85) | 16(69) | 17(80) | 9(81) | 3(30) | 85(75.8) |
| SDD | 1(2) | 3(13) | 1(4.7) | 1(9.5) | 2(20) | 8(7.1) | |
| Resistant | 6(13) | 4(17) | 3(14) | 1(9.5) | 5(50) | 19(17) | |
| MCZ | Susceptible | 36(76) | 14(61) | 12(57) | 7(63) | 6(60) | 75(66.9) |
| SDD | 4(4.5) | 2(8.6) | 3(14.2) | 1(9.5) | 1(10) | 11(9.8) | |
| Resistant | 7(14.8) | 7(30) | 6(28.5) | 3(27) | 3(30) | 26(23.2) | |
| AMP | Susceptible | 43(91.4) | 21(93.3) | 18(85.7) | 10(90.9) | 8(80) | 100(89.2) |
| SDD | – | – | – | – | – | – | |
| Resistant | 4(8.6) | 2(8.7) | 3(14.2) | 1(9.1) | 2(20) | 12(10.8) | |
| NS | Susceptible | 39(82.9) | 20(86.9) | 16(76.1) | 9(81.8) | 8(80) | 92(82.1) |
| SDD | 2(4.2) | – | 1(4.7) | – | 1(10) | 4(3.5) | |
| Resistant | 6(12.7) | 3(13) | 4(19) | 2(18.1) | 1(10) |
16(14.2) |
|
SDD= Susceptible Dose Dependent, NA*= C. krusei intrinsic resistance to fluconazole. FCZ=Fluconazole (10µg), KCZ=Ketoconazole (30µg), CLOT=Clotrimazole (10µg), NS=Nystatin (100U), VCZ=Voriconazole (1µg), MCZ=Miconazole (30µg) and AMP=Amphotericin-B (20µg).
Comparisons between C. albicans and NAC species with resistance antifungal agents as shown in table 3. There was a statistically significant difference (p<0.05) between C. albicans and NAC species in terms of resistance against amphotericin B and voriconazole.
Table 3: Comparison between C. albicans and NAC species with resistant antifungal agents.
| Antifungal agents | Number of resistance C. albicans, n=47(%) | Number of resistance NAC, n=65(%) | P-value |
| Amphotericin B | 4(8.6) | 8(12.3) | 0.0001* |
| Nystatin | 6(12.7) | 10(15.3) | 0.697 |
| Fluconazole | 8(17) | 8(12.8) | 0.482 |
| Voriconazole | 5(10.6) | 4(6.1) | 0.0001* |
| Clotrimazole | 6(13) | 13(20) | 0.33 |
| Ketoconazole | 10(22) | 15(23) | 0.9 |
| Miconazole | 7(14.8) | 19(29.2) | 0.0746 |
* Statistically significant (p<0.05)
Discussion
In antenatal women, VVC is one of the commonest fungal infections. The causative agents of VVC are C. albicans and NAC species such as C. glabrata and C. tropicalis appear to be increasing. C. glabrata is the 2nd commonest agent in vaginal infections.28-30 There are various effective antifungal drugs that are used for treating Candida vulvovaginitis, Understanding of the antifungal susceptibility patterns is key in guiding proper therapy and selection of antifungal drugs for vulvovaginal candidiasis.
Antifungal resistance in NAC is increasing day by day due to reiteration and long duration of antifungal therapy resulting in treatment failures and the emergence of azole resistance in Candida. Antifungal susceptibility testing may offer good treatment outcome by monitoring the drugs resistance and antifungal efficiency.31 Several studies have been described the mechanisms of azoles resistance such as elevated expression of gene encoding lanosterol demethylase (ERG11), alteration in the ERG11, the gene coding the multidrug efflux pumps, CaMDR1, CDR1 and 2.32
Candida identification at the species level is crucial, in the view of emerging different Candida species and the distinctive antifungal susceptibility profiles.33-35 In the present study, C. albicans were 42% but the prevalence of NAC species were 58%. An earlier study was done by El-Sayed et al.,36 has reported a higher prevalence rate of C. albicans in VVC as 86%, though the prevalence rates of 59%, 65% and 73% were reported by Al-Hedaithy et al.,37 Al-mamari et al.,38 Al-fouzan et al.,39 respectively. Worldwide the prevalence of C. albicans in VVC falls somewhere in the range of 47% and 89% in various investigations.40-46
In this study, there was an increasing rate of NAC species. In VVC C. albicans has been observed as the main disease-causing agent. Nevertheless, in complicated VVC, NAC species are commonly found than C. albicans.9 That might be because of the increased prevalence of drugs resistance, prolong antifungal treatment, diabetes mellitus, more established age, earlier antifungal uses and poor hygienic conditions that lead to increase in the prevalence of infection by NAC.2 However, there is increased scientific and epidemiological interest in NAC species as their prevalence is rising all around the world. In the present study, 20% of C. glabrata which was the second commonest species followed by 19% of C. tropicalis, 10% of C. parapsilosis and 9% of C. krusei. Results of the present study are concordant to the study done by the various researchers.37, 42, 43 Prior investigations have revealed rates of C. tropicalis in VVC extended from 5% to 26%36, 40, and 46 whereas rates of C. Krusei ranged from 5% to 15%37, 40, 43 and 46 and 0.6% for C. parapsilosis.37
In this study, overall resistance was 26(23.2%) of miconazole followed by 25(22.3%) ketoconazole, 19(17%) clotrimazole, 16(15.6%) fluconazole, 16(14.2%) nystatin, 12(10.8%) amphotericin B and 9(8.1%) voriconazole. Resistance to miconazole, ketoconazole and clotrimazole is of great concern as these are the first line azoles used for the treatment of vulvovaginal candidiasis. Fluconazole is a contraindication to antenatal women but the resistance of fluconazole have a great concern as it is the most common azole used for the treatment of systemic candidiasis such as candidemia. In the present study, there was an increase in resistance of azoles except for voriconazole but other drugs such as amphotericin B and nystatin showed good efficacy against Candida. The present finding revealed 15.6% were fluconazole resistant by Candida species, similarly, studies from Egypt37 and Taiwan47 has reported the same rates of fluconazole resistance. Higher resistance rates were accounted for from Brazil13 whereas no resistance from Kuwait.39 In the present study, 39(83%) were fluconazole sensitivity to C. albicans, which is higher than the study reported by Babin et al.,46 Highest fluconazole resistant seen in this study was C. tropicalis 5(24%) which is lower than the study reported by Sachin et al.,30 In addition to that, Dota et al reported increased resistance to fluconazole (32%) by Kirby Bauer method, while lower resistance was noticed by micro broth dilution method.13 In this study, clotrimazole had shown 40(85%) sensitivity to C. albicans followed by 9(81%) of C.parapsilosis which is quite high in the study reported by Ajitha et al.,28 Another study conducted by Sachin et al.,30 reported that 50% clotrimazole resistance to C. parapsilosis and 20% by C. albicans, whereas in our finding 5(50%) clotrimazole resistance was seen in C. krusei. The results of the present study revealed 35(74%) were ketoconazole sensitive to C. albicans and 3(30%) of ketoconazole was resistance against C. krusei which was highest, while a study was done by Sachin et al., reported that 25% ketoconazole was resistance by Candida species.30 Among the azole drugs, voriconazole showed good efficacy against Candida. In the present finding, all the C. parapsilosis was 11(100%) sensitive to voriconazole whereas 5(10.6%) resistance by C. albicans but lower resistant rates was reported by Baghdadi et al.,48 Nystatin was 20(86.9%) sensitive to C. glabrata and 6(12.7%) resistance against C. albicans; the finding of this study is concordant with the study done by Sherin et al.,19 Amphotericin B was 21(93.3%) sensitive to C. glabrata and 2(20%) resistance against C. krusei in this study which is higher than the rate reported by Ajitha et al.,28 In the present study, overall fluconazole was 83(81.3%) sensitive, while the study done by Kelen et at.,8 showed 35.5% and Dharmik et al.,4 showed 97.2%. In this study, the overall Clotrimazole was 85(75.8%) sensitive and ketoconazole was 79(70.5%) which were almost near to the study done by Dharmik et al.,4 as 80%. However, in this present study, as compared to C. albicans the majority of NAC had shown a high level of resistance towards antifungal drugs (Table 3). A huge alteration in the epidemiologic patterns of Candida and furthermore the development of resistance among already susceptible species because of increased uses of over-the-counter antifungal agents. The prior study reported that there is a higher MIC value by most NAC species; consequently, it is very hard to treat.9 An enormous report discovered that among NAC of vaginal isolates, C. glabrata is emergence as more resistant to azoles as compared to isolates from bloodstream infection.11, 21 In the present study, there was a statistically significant difference (p<0.05) between C. albicans and NAC species in terms of resistance against amphotericin B and voriconazole. Whereas, no statistically significant difference (p>0.05) between C. albicans and NAC species resistance against other antifungal agents as shown in table 3.
Conclusion
The study offers information about Candida species distribution and antifungal susceptibility activity of Candida isolated from antenatal women of VVC. In this study, there was a clear shift in the prevalence of infection by Candida albicans to those by NAC. NAC species are emerging as a potential threat for causing cause infection and posing a therapeutic challenge. Early empirical antifungal therapy and further research to improve diagnostic, prevention and therapeutic strategies are necessary to reduce the considerable mortality and morbidity. Appropriate selection of drugs for the treatment of Candida infections and antifungal susceptibility testing must be performed regularly. The study was directed on a low number of isolates and in a tertiary care hospital which is the limitation of the study.
Acknowledgement
We are grateful to SRM Medical College Hospital and Research Centre to avail us with reagents, equipment’s and supporting our research work.
Author’s Contribution Statement
Dr V. Mangayarkarasi designed the study, guided in conducting this research study, drafted the manuscript and also reviewed the manuscript. Mr Kanishka Hrishi Das carried out the research study, evaluated the results and also drafted the manuscript. Dr Maitrayee Sen helped for sample collection from antenatal women and also reviewed the manuscript for this research study.
Conflict of Interest
Conflict of interest declared none.
Funding
Authors did not receive any funding to conduct this research.
Ethics
The study was conducted after getting approval from the Institutional Ethical Committee (IEC, 1090/ IEC/2017).
References
- Foxman B, Marsh J.V, Gillespie B, and Sobel J.D. Frequency and Response to Vaginal Symptoms among White and African American Women: Results of a Random Digit Dialing Survey. Journal of Women’s Health., 1998; 7(9):1167-1174.
- Sobel J.D, Faro S, Force R.W, Foxman B, Nyirsejy W.P and Reed B.D et al. Vulvovaginal Candidiasis: Epidemiologic, Diagnostic, and Therapeutic Considerations. American Journal of Obstetrics and Gynaecology., 1998; 178(2):203- 211.
- Sobel J.D. Vaginitis. New England Journal of Medicine, 1997; 337(26):1896-1903.
- Dharmik P.G, Gomashe A.V and Upadhyay V.G. Susceptibility Pattern of Various Azoles Against Candida Species Causing Vulvovaginal Candidiasis. The Journal of Obstetrics and Gynecology of India., 2013; 63(2):135–13
- Viudes A, Peman J, Canton E, Ubeda P, Lopez-Ribot J.L and Gobernado M. et al. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. J. Clin. Microbiol. Infect. Dis., 2002; 21(11):767–774.
- Pfaller M.A. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. J. Med., 2012; 125(1):3–13.
- Adhikary R and Joshi S. Species distribution and antifungal susceptibility of Candidaemia at a multi super-speciality centre in southern India. Indian Journal of Medical Microbiology., 2011; 29(3):309-11.
- Kelen F.D, Alessandra R, Marcia E.L and Terezinha I.E. A Challenge for Clinical Laboratories: Detection of Antifungal Resistance in Candida Species Causing Vulvovaginal Candidiasis. Journal of Lab medicine., 2011; 42(2):87-93
- Fidel P.L, Vazquez J.A and Sobel J.D. Candida glabrata: Review of epidemiology, pathogenesis, and clinical disease with comparison to albicans. Clin. Microbiol. Rev., 1999; 12(1):80–96.
- Odds F.C. Candida and candidiasis. 2nd ed. London: Balliere Tindall 1988; 30(5):382-383
- Richter S.S, Galask R.P, Messer S.A, Hollis R.J, Diekema D.J and Pfaller M.A. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. Clin. Microbiol., 2005; 43(5):2155–2162.
- Trick W.E, Fridkin S.K, Edwards J.R, Hajjeh R.A and Gaynes R.P. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin Infect Dis., 2002; 35(5):627–30.
- Dota K, Freitas A, Consolaro M and Svidzinski T. Challenge for clinical laboratories: detection of antifungal resistance in Candida species causing vulvovaginal candidiasis. Science., 2011; 42(2):87–93.
- Chander J. Candidiasis. In: A textbook of Medical Mycology, 3rd ed. Mehta Publishers, New Delhi, 2009; 266-90.
- Arthur L.B and Steven D.B. Fluconazole Disk Diffusion Procedure for Determining Susceptibility of Candida Journal of clinical microbiology., 1996; 34(9):2154–2157.
- Debora M, Marcos E.A and Luciana S.R. Species Distribution and Antifungal Susceptibility of Yeasts Isolated from Vaginal Mucosa. Rev Patol Trop., 2014; 43(1):48-56.
- Vandenbossche I, Vaneechoutte M, Vandevenne M, Baere T.D and Verschraegen G. Susceptibility testing of fluconazole by the NCCLS broth microdilution method, E- test, and disk diffusion for application in the routine laboratory. J Clin Microbiol 2002; 40(3):918-921.
- Arul M.S, Viswanathan T, Malarvizhi A, Lavanya V and Moorthy K. Isolation, characterisation and antifungal susceptibility Pattern of Candida albicans and non-albicans Candida from Integrated counselling and testing centre (ICTC) patients. African Journal of Microbiology Research., 2012; 6(31):6039-6048.
- Sherin ME, Abeer AE, Ahmed WA. Exoenzymes Production and Antifungal Susceptibility of Candida Species Isolated from Pregnant Women with Vulvovaginitis. Journal of American Science 2012;8(12):1392-1399.
- Keyvan P, Leila B and Zahra R. In vitro activity of six antifungal drugs against clinically important dermatophytes. Jundishapur Journal of Microbiology., 2009; 2(4):158-163.
- Pfaller M.A, Diekema D.J, Gibbs D.L, Newell V.A, Ellis D and Tullio V. et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-Year Analysis of Susceptibilities of Candida Species to Fluconazole and Voriconazole as Determined by CLSI Standardized Disk Diffusion. J Clin Microbiol., 2010; 48(5):1366–1377.
- Howell S.A and Hazen K.C. Candida, Cryptococcus and other yeasts of medical importance. Manual of clinical microbiology. 10th ed. Washington, DC: ASM Press; 2012;1793–821.
- Pasligh J, Radecke C, Fleischhacker M and Ruhnke M. Comparison of phenotypic methods for the identification of Candida dubliniensis. J Microbiol Immunol Infect., 2010; 43(2):147–54.
- Giri S and Jyoti A.K. Phenotypic Tests for Identification of Candida National Journal of Laboratory Medicine., 2015; 4(4):13-18.
- Clinical and Laboratory Standard Institute (CLSI). Reference method for disk diffusion antifungal susceptibility testing of yeasts. Approved standard M44-A, Wayne, PA: Clinical Laboratory Standard Institute; 2008.
- Vandeputte P, Larcher G, Berge S.T, Renier G, Chabasse D and Bouchara J.P. Mechanisms of azole resistance in a clinical isolate of Candida tropicalis. Antimicrob Agents Chemother., 2005; 49(11):4608–15.
- Pam V, Akpan J, Oduyebo O, Nwaokorie F.O, Fowora M.A and Oladele R.O. et al. Fluconazole susceptibility and ERG11 gene expression in vaginal Candida species isolated from Lagos Nigeria. Int J Mol Epidemiol Genet., 2012; 3(1):84–90.
- Ajitha R, and Maimoona M. Phenotypic identification of Candida species and their susceptibility profile in patients with genitourinary candidiasis. International Journal of Advanced Research., 2014; 2(12):76-84.
- Zahra S, Seifi Z, Zarei A and Mahmoudabadi A. Sensitivity of Vaginal Isolates of Candida to Eight Antifungal Drugs Isolated From Ahvaz, Iran. Jundishapur J Microbiology., 2012; 5(4):574-577.
- Sachin C.D and Santosh S. Vulvovaginal Candidiasis due to non albicans Candida: its species distribution and antifungal susceptibility profile. J. Curr. Microbiol App. Sci., 2013; 2(12):323-328.
- Espinel-Ingroff A. Clinical relevance of antifungal resistance. Infect Dis Clin North Am., 1997; 11(4):929–944.
- Sanglard D, Ischer F, Koymans L and Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother., 1998; 42(2):241–253.
- Ayatollahi S.A, Khalesi E, Shahidi G.H, Aghighi S, Sharifi F and Aram F. Rapid molecular diagnosis for Candida species using PCR-RFLP. ,2007; 6(4):583–7.
- Wei Y.P, Feng J and Luo Z.C. Isolation and genotyping of vaginal non-albicans Candida in women from two different ethnic groups in Lanzhou China. Int J Gynecol Obstetrics., 2010; 110(3):227–30.
- Fallahi A.A, Korbacheh P, Zaini F, Mirhendi H, Zeraati H and Noorbakhsh F et al. Candida species in cutaneous candidiasis patients in the Guilan province in Iran; identified by PCR-RFLP method. Acta Med Iran., 2013; 51(11):799–804.
- El-sayed H and Hamouda A. Candida albicans causing vulvovaginitis and their clinical response to antifungal therapy. Egypt J Med Microbiol., 2007; 16(1):53–62.
- Al-Hedaithy S. Spectrum and proteinase production of yeasts causing vaginitis in Saudi Arabian women. Med Sci Monit., 2002; 8(7):498–501.
- Al-mamari A, Al-buryhi M, Al-heggami M.A and Al-hag S. Identify and sensitivity to antifungal drugs of Candida species causing vaginitis isolated from vulvovaginal infected patients in Sana’a city. Der Pharma Chemica., 2014; 6(1):336–42.
- Alfouzan W, Dhar R, Ashkanani H, Gupta M, Rachel C and Khan Z.U. Species spectrum and antifungal susceptibility profile of vaginal isolates of Candida in Kuwait. J Mycol Med. 2015; 25(1):23–8.
- Darce B.M, Gonzalez A, Barnabe´ C and Larrouy G. First characterization of Candida albicans by Random amplified polymorphic DNA method in Nicaragua and comparison of the diagnosis methods for vaginal candidiasis in Nicaraguan women. Mem Inst Oswaldo Cruz., 2002; 97(7):985–9.
- Holland J, Young M, Lee O and Lee S. Vulvovaginal carriage of yeasts other than Candida albicans Sex Transm Infect., 2003; 79(3):249–50.
- Pirotta M and Garland S. Genital Candida species detected in samples from women in Melbourne, Australia, before and after treatment with antibiotics. J Clin Microbiol., 2006; 44(9):3213–7.
- Gultekin B, Yazici V and Aydin N. Distribution of Candida species in vaginal specimens and evaluation of CHROMagar Candida Microbiol Bul., 2005; 39(3):319–24.
- Pakshir K, Yazdani M and Kimiaghalam R. Etiologic of vaginal candidiasis in Shiraz, Southern Iran. Res J Microbiol., 2007; 2(9):696–700.
- Xu Y, Chen L and Li C. Susceptibility of clinical isolates of Candida species to fluconazole and detection of albicans ERG11 mutations. J Antimicrob Chemother., 2008; 61(4):798–804.
- Babin D, Kotigadde S, Rao P and Rao TV. Clinico-mycological profile of vaginal candidiasis in a tertiary care hospital in Kerala. Int J Res Biol Sci., 2013; 3(1):55–9.
- Tseng Y, Tsung W and Tsungcheng L. In vitro susceptibility of fluconazole and amphotericin B against Candida isolate from women with vaginal candidiasis, Taiwan. J Food Drug Anal., 2005; 13(1):12–6.
- Baghdadi E, Sadegh K, Sassan R, Sara A, Neda K and Zahra S. et al. Antifungal Susceptibility Patterns of Candida Species Recovered from Endotracheal Tube in an Intensive Care Unit. Advances in Medicine., 2016; Article ID 9242031:1-6.








