K. Kranthi*, V. V. M. Anand Priya, K. Punnagai and Darling Chellathai David
Department of pharmacology, Sri Ramachandra Medical College, Sri Ramachandra Institute of Higher Education, Porur, Chennai - 600116, Tamil Nadu, India.
Corresponding Author E-mail: anandpriyavvm@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1746
Abstract
To evaluated and compare the intrinsic antioxidant ability of amantadine and rasagiline drugs using in-vitro diphenyl-1-picrylhydrazyl assay method. Diphenyl-1-picrylhydrazyl assay method was used to compare the antioxidant activity of rasagiline and amantadine. At lower concentrations (200 - 400 µg/ml), there was a definite difference between amantadine and rasagiline with amantadine showing better antioxidant activity over rasagiline. But at higher doses (600 - 1000 µg/ml) both their antioxidant free radical scavenging activity were comparable. This study proved the intrinsic activity of rasagiline and amantadine which may be beneficial in attenuating the oxidative stress pathways, which were considered responsible for many degenerative diseases.
Keywords
Amantidine; 2,2-Diphenyl-1-Picrylhydrazyl Assay; Parkinson's Disease; Rasagiline; Reactive Oxygen Species
Download this article as:| Copy the following to cite this article: Kranthi K, Priya V. V. M. A, Punnagai K, David D. C. A Comparative free Radical Scavenging Evaluation of Amantadine and Rasagiline. Biomed Pharmacol J 2019;12(3). |
| Copy the following to cite this URL: Kranthi K, Priya V. V. M. A, Punnagai K, David D. C. A Comparative free Radical Scavenging Evaluation of Amantadine and Rasagiline. Biomed Pharmacol J 2019;12(3). Available from: https://bit.ly/2XNSgrT |
Introduction
Parkinson’s disease is the neurodegenerative disorder affecting more than 1% of population aged above 65 years.1 Most of the PD patients exhibit symptoms after 50-60% loss of dopaminergic neurons in substantia nigra pars compacta (SNpc).2,3 Free radicals or reactive oxygen species are certainly generated in living cells. They may also originate from external source. These free radicals were a known etiological factors for degenerative disorders.4 Antioxidants are the endogenous or exogenous compounds which fight free radical generation by intervening in pathways of oxidation.5 Health and vigour of the biological system was known to be decided by the balance between antioxidants and oxidants.6 The same was also postulated with “oxidative stress hypothesis”. Drechsel & Patel (2008), Liddell et al., (2013) have stated that oxidation of dopamine produces ROS species and causing chronic oxidative stress in SNpc dopaminergic neurons.7,8 Surendran, S., & Rajasankar, S. (2010); Ghanta, Mohankrishna, et al.,(2018) have shown oxidative stress in PD caused due to increased glutamate toxicity, lipid peroxidation, protein oxidation and DNA damage in SNpc.9,10 Treatment for PD included dopamine supplementation as gold standard with L-dopa and dopaminergic agonists since many years. Other drugs acting as enzyme inhibitors also aid in dopamimetic therapy.11-13 Selegiline and Rasageline decrease dopamine degradation by inhibiting monoamine oxidaze-B ( MAO-B ) enzyme. Amantadine, a tricyclic aminoadamantanes, synthetic drug acts as N-methyl D-aspartate receptor (NMDA) non competitive antagonist, antimuscarinic and proved beneficial in PD treatment.14-16 Apart from the above said mechanisms of amantadine and rasagiline drugs, this study have aimed to evaluate and compare the intrinsic antioxidant activity of these two drugs through in-vitro anti oxidant assay.
Materials and Method
Drugs and Reagents
Amantadine hydrochloride ( Amantrel Chennai, Cipla ), rasagiline mesylate ( Rasalect, Sun Pharma, Chennai ), butylated hydroxytoluene ( BHT ) (Sigma Aldrich USA), 2,2-diphenyl-1-picrylhydrazyl (DPPH•) (DPPH sigma Aldrich USA) were purchased. The drugs were mashed and dissolved in dimethyl sulfoxide ( DMSO ) (DMSO Sigma Aldrich USA). Other reagents used in this study were of high grade available commercially.
DPPH Assay
This assay was performed with standard method followed by many studies [ 17-21 ]. Absolute methanol ( 3.7 ml ) was allocated in all test tubes along with blank. 100µl of absolute methanol was added to blank tube. Then 100µl of BHT was added to tube marked as standard and 100µl of respective samples to all other tubes marked as tests. Finally 200µl of DPPH reagent was added to all the test tubes at room temperature condition for minimum of 30 minutes then, checked absorbance of all samples 517nm. BHT was used as the standard drug in the study. The percentage ( % ) inhibition and EC50 values are calculated by using the formulae
DPPH scavenged (% ) = [ (A initial DPPH – A final DPPH) / A initial DPPH ] × 100
The EC50 value, which represents the concentration of drug that gives rise to a 50% reduction in DPPH absorbance, was determined by linear regression analysis.
Results
The % inhibition of amantadine was found to be 16.1%, 49.4%, 62.9%, 71.9%, and 89.9 % inhibition while that of rasagiline was found to be 11.9%, 25.6%, 61,6%, 68.7% and 85.6% inhibition compared with that of standard BHT which had 58.6%, 88.9%, 96.1%, 97.9% and 99.4% inhibition at concentrations of 200, 400, 600, 800, 1000 µg/ml respectively. This was depicted in Figure 1.
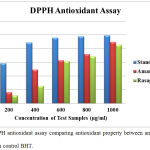 |
Figure 1: DPPH antioxidant assay comparing antioxidant property between amantadine and rasagiline with control BHT.
|
Discussion
In order to evaluate the intrinsic antioxidant property of amantadine and rasagiline, DPPH assay was performed which is a standard technique, developed by Blois ( 1958 ),22 for antioxidant in-vitro assay. The principal of this assay is based on reduction of DPPH, a constant free radical. The colour of DPPH solution is purple due to the presence of free/an odd electron. When DPPH and antioxidants react the stable free radical get reduced to DPPH-H in the company of a hydrogen donor .This results in yellow decolourization as the absorbance reduced from the DPPH radical to the DPPH-H form. This reduction is proportional to the quantity of electrons paired.22 Both the drug compounds showed dose dependent increase in free radical scavenging activity. The activity was evident in lower doses ( 200 ug/ml ) and showed a steady increase up to 1000ug/ml which was the highest dose tested. At lower concentrations, there was a definite difference between amantadine and rasagiline with amantadine showing better antioxidant activity over rasagiline. But at higher doses, the antioxidant free radical scavenging activity were comparable between the two drugs. This intrinsic antioxidant activity of these drugs could be characterized to the chemical structures of the drug compounds.23 As oxidative stress caused due to free radicals was evidenced both clinically and preclinically in PD, this assay could reveal the drugs capable of augmenting or enhancing the treatment of PD apart from their known mechanism of actions.24 Rasagiline a known drug for treatment of PD, inhibits MAO-B enzyme and increases dopamine availability in SNpc and striatum by reducing the dopamine degradation. It was also shown to reduce oxidative stress in rodent models.25-28 Structural activity studies revealed that propargyl moiety of rasagiline was related to the MAO-B enzyme inhibition activity while some other studies have also revealed the MAO-B independent neuroprotective activity of rasagiline.29-32 This present study have proved the intrinsic antioxidant activity of rasagiline. Amantadine is a known antiparkinsonian drug with NMDA receptor antagonist property.23-35 Lupp et al. (1998) have reported the antioxidant property of amantadine in their in-vitro study.36 In this present study also, amantadine have shown in-vitro antioxidant activity and proved to have free radical scavenging activity.
Conclusion
Both Amantadine and Rasagiline have shown in-vitro antioxidant activity in DPPH assays, which is one of the standard assays for antioxidant activity. Even though some works have previously stated into this path, there was a lack in the comparative research works among antiparkinsonian drugs along with an absence of healthy discussion on the theories that could clarify the antioxidant mechanisms of these drugs. The antioxidant action of amantadine and rasagiline may propose neuroprotection apart from their known mechanism of actions.
Acknowledgements
The author(s) received no specific funding for this work.
References
- Perfeito R, Cunha-Oliveira T, Rego AC. Revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease—resemblance to the effect of amphetamine drugs of abuse. Free Radical Biology and Medicine. 2012 Nov 1;53(9):1791-806.
- Datla KP, Zbarsky V, Rai D, Parkar S, Osakabe N, Aruoma OI, Dexter DT. Short-term supplementation with plant extracts rich in flavonoids protect nigrostriatal dopaminergic neurons in a rat model of Parkinson’s disease. Journal of the American College of Nutrition. 2007 Aug 1;26(4):341-9.
- Mercado G, Valdés P, Hetz C. An ERcentric view of Parkinson’s disease. Trends in molecular medicine. 2013 Mar 1;19(3):165-75.
- Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson’s disease. Progress in neurobiology. 2007 Jan 1;81(1):29-44.
- Cui K, Luo X, Xu K, Murthy MV. Role of oxidative stress in neurodegeneration: recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004 Aug 1;28(5):771-99.
- Halliwell B. Commentary oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free radical research. 1996 Jan 1;25(1):57-74.
- Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radical Biology and Medicine. 2008 Jun 1;44(11):1873-86.
- Liddell JR, Obando D, Liu J, Ganio G, Volitakis I, San Mok S, Crouch PJ, White AR, Codd R. Lipophilic adamantyl-or deferasirox-based conjugates of desferrioxamine B have enhanced neuroprotective capacity: implications for Parkinson disease. Free Radical Biology and Medicine. 2013 Jul 1;60:147-56.
- Surendran S, Rajasankar S. Parkinson’s disease: oxidative stress and therapeutic approaches. Neurological Sciences. 2010 Oct 1;31(5):531-40.
- Ghanta M, Panchanathan E, Lakkakula BV, Narayanaswamy A, Murkunde Y, Tamrakar S. 1H-[1, 2, 4] oxadiazolo [4, 3-a] quinoxalin-1-one Attenuates Oxidative Trauma and Recuperate Beam Walk and Adhesive Removal Behavior in MPTP Parkinsonian Mice Model. Biomedical and Pharmacology Journal. 2018 Dec 25;11(4):2011-7.
- Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radical Biology and Medicine. 2013 Sep 1;62:132-44.
- Nolan YM, Sullivan AM, Toulouse A. Parkinson’s disease in the nuclear age of neuroinflammation. Trends in molecular medicine. 2013 Mar 1;19(3):187-96.
- Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH, Halliday G. Missing pieces in the Parkinson’s disease puzzle. Nature medicine. 2010 Jun;16(6):653.
- Boll MC, Alcaraz-Zubeldia M, Rios C. Medical management of Parkinson’s disease: focus on neuroprotection. Current neuropharmacology. 2011 Jun 1;9(2):350-9.
- Thomas A, Bonanni L, Gambi F, Di Iorio A, Onofrj M. Pathological gambling in Parkinson disease is reduced by amantadine. Annals of neurology. 2010 Sep;68(3):400-4.
- Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of amantadine benefit on dyskinesia of severe Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 2004 Jan 1;75(1):141-3.
- Olugbami JO, Gbadegesin MA, Odunola OA. In vitro free radical scavenging and antioxidant properties of ethanol extract of Terminalia glaucescens. Pharmacognosy research. 2015 Jan;7(1):49.
- Mishra K, Ojha H, Chaudhury NK. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food chemistry. 2012 Feb 15;130(4):1036-43.
- Müller L, Fröhlich K, Böhm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chemistry. 2011 Nov 1;129(1):139-48.
- Shalaby EA, Shanab SM. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis.
- Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food chemistry. 2009 Apr 15;113(4):1202-5.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958 Apr;181(4617):1199.
- Maruyama W, Takahashi T, Youdim M, Naoi M. The anti-Parkinson drug, rasagiline, prevents apoptotic DNA damage induced by peroxynitrite in human dopaminergic neuroblastoma SH-SY5Y cells. Journal of neural transmission. 2002 Apr 1;109(4):467-81.
- Danielson SR, Andersen JK. Oxidative and nitrative protein modifications in Parkinson’s disease. Free Radical Biology and Medicine. 2008 May 15;44(10):1787-94.
- Weinreb O, Bar-Am O, Prosolovich K, et al. Does 1-(R)-aminoindan possess neuroprotective properties against experimental Parkinson’s disease? Antioxid Redox Signal 2011 Mar; 14 (5): 767-75.
- Speiser Z, Mayk A, Litinetsky L, Fine T, Nyska A, Blaugrund E, Cohen S. Rasagiline is neuroprotective in an experimental model of brain ischemia in the rat. Journal of neural transmission. 2007 May 1;114(5):595-605.
- Eliash S, Shteter N, Eilam R. Neuroprotective effect of rasagiline, a monoamine oxidase-B inhibitor, on spontaneous cell degeneration in a rat model. Journal of neural transmission. 2005 Aug 1;112(8):991-1003.
- Blandini F, Armentero MT, Fancellu R, Blaugrund E, Nappi G. Neuroprotective effect of rasagiline in a rodent model of Parkinson’s disease. Experimental neurology. 2004 Jun 1;187(2):455-9.
- Weinreb O, Bar-Am O, Amit T, et al. Neuroprotection via pro-survival protein kinase C isoforms associated with Bcl-2 family members. FASEB J 2004 Sep; 18 (12): 1471-3.
- Naoi M, Maruyama W. Functional mechanism of neuroprotection by inhibitors of type B monoamine oxidase in Parkinson’s disease. Expert Rev Neurother 2009 Aug; 9(8): 1233-50.
- Jenner P, Langston JW. Explaining ADAGIO: a critical review of the biological basis for the clinical effects of rasagiline. Mov Disord 2011 Nov; 26 (13): 2316-23.
- Oldfield V, Keating GM, Perry CM. Rasagiline: a review of its use in the management of Parkinson’s disease. Drugs 2007; 67 (12): 1725-47.
- Transm JN. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J Neural Transm. 1994;43:91-104.
- Crosby NJ, Deane K, Clarke CE. Amantadine in Parkinson’s disease. Cochrane Database of Systematic Reviews. 2003(1).
- Danysz W, Parsons CG, Kornhuber J, Schmidt WJ, Quack G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents—preclinical studies. Neuroscience & Biobehavioral Reviews. 1997 Jan 1;21(4):455-68.
- Lupp A, Kerst S, Karge E, Quack G, Klinger W. Investigation on possible antioxidative properties of the NMDA-receptor antagonists ketamine, memantine, and amantadine in comparison to nicanartine in vitro. Experimental and Toxicologic Pathology. 1998 Sep 1;50(4-6):501-6.







