Manuscript accepted on :11-April-2019
Published online on: 06-05-2019
Plagiarism Check: Yes
Reviewed by: Carla Guerreiro
Second Review by: Mina T. Kelleni
Jajang Sinardja1, Eryati Darwin2, Eva Decroli3 and Djong Hon Tjong4
1Program Biomedical Science, Faculty of Medicine, Andalas University, Padang, Indonesia.
2Department of Histology, Faculty of Medicine, Andalas University, Padang, Indonesia.
3Department of Internal Medicine, Faculty of Medicine, Andalas University, Padang, Indonesia.
4Department of Biology, Faculty of Mathematics and Natural Sciences, Andalas University, Padang, Indonesia.
Corresponding Author E-mail: sinardja@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1675
Abstract
Interleukin-6 (IL-6) has been reported to be related to coronary heart disease (CHD). It is proposed that the IL-6 trans-signaling pathway is responsible for the inflammatory effect in diseases, including CHD. In Asian countries, CHD tends to occur in younger age. However, no study has yet been done to assess the relationship between IL-6 trans-signaling pathway and young CHD patients in Indonesia. To assess whether there is a relationship between the levels of some components in the IL-6 trans-signaling pathway, including soluble interleukin-6 receptor (sIL-6R), soluble glycoprotein 130 (sgp130), and intercellular adhesion molecule 1 (ICAM-1) and CHD in young adults. A case-control study was conducted including 33 young CHD patients and 33 non-CHD patients as the control group (age and sex matched with CHD group) at Eka Hospital Pekanbaru, Indonesia, from July to November 2018. CHD was confirmed by coronary angiography, while non-CHD patients were subjects with normal ECG, without history of chest pain and family history of CHD. All participants were checked for sIL-6R, sgp130, and ICAM-1 serum levels using ELISA assays tests. The results were evaluated statistically using Student’s t test. The sIL-6R level tended to be higher in the CHD group compared to the control group (70.19+49.38 ng/ml vs 49.42+38.79 ng/ml) but did not reach statistical significance (p=0.062). The sgp130 level was 428.38+358.79 ng/ml and 474.08+389.43 ng/ml in CHD and control group, respectively (p=0.622). While the ICAM-1 level was 1829.53+1882.37 pg/ml and 2078.16+1595.25 pg/ml in CHD and control group, respectively (p=0.565). The IL-6 trans-signaling pathway, reflected by sIL-6R, sgp130, and ICAM-1 serum levels, was not significantly related with CHD in young adults.
Keywords
Coronary Heart Disease; Intercellular Adhesion Molecule-1; Interleukin-6 Trans-Signaling; Soluble Glycoprotein 130; Soluble Interleukin-6 Receptor; Young Adults
Download this article as:| Copy the following to cite this article: Sinardja J, Darwin E, Decroli E, Tjong D. H. Relationship of Interleukin-6 Trans-Signaling and Coronary Heart Disease in Young Adults: a Case-Control Study in Indonesia. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Sinardja J, Darwin E, Decroli E, Tjong D. H. Relationship of Interleukin-6 Trans-Signaling and Coronary Heart Disease in Young Adults: a Case-Control Study in Indonesia. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2WqFSch |
Introduction
Coronary heart disease (CHD) is still one of the most prominent diseases causing worldwide mortality.1 In some Asian countries, CHD tends to occur in younger age2 and inflammation is thought to play an important role in the pathogenesis of the disease in such patients, also is associated with worse prognosis.3,4 Interleukin-6 (IL-6) is one of the most studied inflammatory agents in relation to CHD.5-7
IL-6 has pro- and anti-inflammatory effects8-9, in which the unique IL-6 signaling pathways are thought to be responsible for the two opposite roles of this cytokine.10-12 There are two pathways of IL-6 signaling: classic and trans-signaling. In classic signaling, the IL-6 binds to membrane-bound IL-6 receptor (IL-6R) which was found in several cell types such as hepatocytes, monocytes, and lymphocytes, and then activates the signaling transduction cascade through the homodimerization of the membrane-bound glycoprotein 130 (gp130). In cells that do not have membrane-bound IL-6R, including endothelial cells, IL-6 activation occurs via trans-signaling pathway, in which the IL-6 binds to soluble IL-6 receptor (sIL-6R) in circulation, which then binds to gp130 in the cell, and finally initiates the intracellular signal cascade. Intercellular adhesion molecule 1 (ICAM-1) is one of the products of IL-6 activation in endothelial cells through the trans-signaling pathway. A soluble form of the gp130 (sgp130) is present in human plasma and acts as a natural inhibitor of the trans-signaling pathway by binding with the IL-6/sIL-6R complex and thus preventing the IL-6 complex to bind to gp130.13 It has been proposed that activation of IL-6 through classic signaling is responsible for the anti-inflammatory effect, while the trans-signaling pathway is responsible for the pro-inflammatory effect of IL-6. (Figure 1).
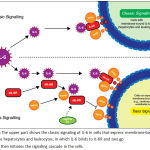 |
Figure 1: The upper part shows the classic signaling of IL-6 in cells that express membrane-bound IL-6R, like hepatocytes and leukocytes, in which IL-6 binds to IL-6R and two gp 130 and then initiates the signaling cascade in the cells.
|
The bottom part shows the trans-signaling of IL-6 in cells that do not express membrane-bound IL-6R, including cardiomyocytes and endothelial cells. In this pathway, IL-6 will bind to the circulating soluble IL-6R (sIL-6R), and then the IL-6/sIL-6R complex will bind with two gp130 on the membrane cells. There are also circulating gp130, known as soluble gp130 (sgp130) which can bind to IL-6/sIL-6R complex, and inhibit the complex to bind to gp130 on cells surface, hence acts as an inhibitor of the trans-signaling pathway of IL-6. A recombinant of sgp130, known as sgp130FC, can also act as the inhibitor of IL-6 trans-signaling pathway.
The trans-signaling pathway is responsible for most of the IL-6 adverse effects, especially in chronic inflammatory diseases.14-15 Genetic studies showed that IL-6 pathway seems to play a causative role in the development of CHD. These studies stated that SNP rs2228145, a single nucleotide polymorphism in IL-6R gene, was related to sIL-6R level and CHD.16-17 Some studies in CHD patients also showed that sIL-6R level was correlated with worse prognosis. The higher the sIL-6R level is, the higher the incidence of myocardial infarction events and mortality becomes.18-20 The sgp130 level, as the natural inhibitor of trans-signaling pathway, theoretically will be inversely related to the risk of CHD. This means a higher sgp130 level is associated with a lower risk of CHD. Animal studies had shown consistent results with this theory, whereas in human, the results of several studies were still controversial.18,19,21-23
To assess whether there is a relationship between IL-6 trans-signaling pathway and CHD in young adults, we examined the levels of sIL-6R, sgp130, and ICAM-1 in young CHD patients at Eka Hospital, Pekanbaru, Indonesia. As far as we know, this is the first study regarding the relationship of IL-6 trans-signaling pathway with young CHD patients in Indonesian population.
Methods
Patients
This study was a case-control study conducted at Eka Hospital Pekanbaru, Indonesia, in collaboration with Faculty of Medicine Andalas University, Padang, Indonesia, from July to November 2018. CHD patients, who were selected from Eka Hospital Pekanbaru catheterization laboratory, underwent coronary angiography examination between January 2017 until July 2018. Patients were included in this study if the coronary angiography showed >70% stenoses of at least one coronary artery and the age of patients for males were <45 years old and for females were <55 years old at the time of examination. The control group encompassed subjects who underwent a routine medical check-up at Eka Hospital Pekanbaru, from July to October 2018, with normal ECG recording, no symptom of chest pain, and no family history of CHD, matched by sex and age with the CHD patients group. The exclusion criteria were BMI >30 kg/m2, acute inflammatory states (including acute coronary syndromes), or history of autoimmune disease and malignancy.
Data Collection
Appointments were made with each participant who met the inclusion and exclusion criteria. Health examinations and interviews were done by general practitioners and cardiologists of Eka Hospital Pekanbaru. The health examination included body weight, height, and blood pressure measurements. The questionnaire form included birth date, occupation, ethnicity, education level, smoking habits, family history of CHD, history of hypertension, diabetes, hypercholesterolemia, and medication history.
Hypertension was determined based on medical diagnosis by a physician or taking antihypertensive drugs, or when systolic blood pressure was >140 mm Hg, and/or diastolic blood pressure >90 mm Hg. Diabetes was determined based on medical diagnosis by a physician, or taking antidiabetic drugs, or when random blood glucose >200 mg/dl, or fasting blood glucose >126 mg/dl, or 2 hours postprandial blood glucose >200 mg/dl. Hypercholesterolemia was determined based on medical diagnosis by a physician, or when total serum cholesterol level >200 mg/dl. The family history of CHD was determined based on the history of CHD in their family; CHD of the father at the age of <45 years, and/or the mother at the age of <55 years. Patients were determined as smokers, if they had or were still smoking with the total amount of cigarettes >100 cigarettes in their entire lifetime; and as non-smokers, if they never smoke, or the number of cigarettes smoked were <100 cigarettes in their entire lifetime.
On the same day, after the interview, peripheral vein blood samples were drawn from all participants, placed into SST vacutainers, centrifuged with 3,600 rpm for 10 minutes, and stored in a -80°C freezer. After the blood samples were collected from all study participants, ELISA assay tests were performed at the Biomedical Laboratory, Faculty of Medicine, Andalas University, Padang, using ELISA kits from Bioassay Technology Laboratory (Shanghai, China). ELISA tests were done according to the manufacturer’s instruction. Data were obtained using ELISA Reader Bio-Rad and calculated using the Microplate Manager Software Bio-Rad. The low detection limit of the assay was 0.24 ng/mL, 2.55 ng/mL, and 9.98 pg/L for sIL-6R, sgp130 and ICAM-1, respectively. The mean inter-assay and intra-assay coefficients of variation were 10% and 8%, respectively, for all tests.
Statistical Analysis
Categorical data were analysed with Chi-square, while numerical data were analysed with Student’s t tests. Differences were considered statistically significant at the two-sided test with significance level p< 0.05.
Ethical Clearance
This study has been approved by the Ethical Review Board for Medicine and Health Research, Faculty of Medicine, University of Riau, Pekanbaru, Indonesia. All study participants gave their informed consent to be enrolled in this study.
Results
During the study period, we managed to obtain a total of 66 patients who met the study criteria, 33 for the CHD group and 33 for the control group. The baseline characteristics of the study participants can be seen in Table 1. Mean age of CHD patients was 45.82 years old, while for the control group was 45.37 years old, with 63.6% of patients were male. CHD patients were significantly more hypertensive (60.6% vs 6.1%, p value<0.001) compared to the control group. There was a tendency of higher diabetes (24.2% vs 6.1%, p value=0.086) in the CHD group compared to the control group, but the difference was not statistically significant. Smoking (57.6% vs 39.4%, p value=0.218) and hypercholesterolemia (81.8% vs 93.9%, p value=0.258) did not differ significantly for both groups.
Table 1: Baseline Characteristics.
| Variable | CHD patients (n=33) | Control Group (n=33) | p value |
| Age (year) | 45.82±5.05 | 45.37±4.70 | 0.76 |
| Male | 21 (63.6%) | 21 (63.6%) | 1.00 |
| Smoker | 19 (57.6%) | 13 (39.4%) | 0.218 |
| Hypertension | 20 (60.6%) | 2 (6.1%) | 0.001 |
| Diabetes Mellitus | 8 (24.2%) | 2 (6.1%) | 0.086 |
| Hypercholesterolemia | 27 (81.8%) | 31 (93.9%) | 0.258 |
Table 2 showed the sIL-6R, sgp130, and ICAM-1 serum levels. SIL-6R serum levels in CHD patients were higher (70.17+49.38 vs 49.42+38.79 ng/ml, p=0.062) compared to the control group, but failed to reach statistical significance. Sgp130 and ICAM-1 serum levels were (428.38+358.79 vs 474.08+389.43 ng/ml, p=0.622) and (1829.53+1882.37 vs 2078.16+1595.25 pg/ml, p=0.565) respectively, and also did not differ significantly for both groups.
Table 2: SIL-6R, sgp130, and ICAM-1 serum levels.
| Serum level | CHD patients (n=33) | Control Group (n=33) | p value |
| sIL-6R (ng/ml) | 70.19+49.38 | 49.42+38.79 | 0.062 |
| sgp130 (ng/ml) | 428.38+358.79 | 474.08+389.43 | 0.622 |
| ICAM-1 (pg/ml) | 1829.53+1882.37 | 2078.16+1595.25 | 0.565 |
Discussion
In this study, we recruited male patients <45 years old and female patients <55 years old in accordance to the definition of CHD in young adults used in Van Loon study.4 Hypertension was the only traditional cardiovascular risk factor related to young CHD patients in this study. Our study showed a much higher prevalence of hypertension in young CHD patients (60.6%) compared to Hussain et al. (20-25%) in Indonesian population24 and Aggarwal et al. (25%) in Indian young CHD patients.25 The hypercholesterolemia rate in our study was also very high, 81.8% in the CHD group and 93.9% in the control group. While Hussain et al. only reported 30% hypercholesterolemia in men and 39.6% in women.24 The high frequency of hypertension and hypercholesterolemia in our study might be due to the eating patterns of the people in our region (Riau province, Indonesia) which contains a lot of coconut milk and fat.
Studies on sIL-6R and sgp130, as novel members of the IL-6 trans-signaling cascade, in CHD patients are still rare. Previous studies, most were done in myocardial infarction patients, such as by Anderson et al. which compared the level of sIL-6R and sgp130 in AMI, CHD, and control groups18, Moreno et al. which studied the relationship between sIL-6R and sgp130 levels and the risk of myocardial infarction19, while Ritschel et al. studied the level of sIL-6R and sgp130 in STEMI patients to see future cardiovascular events20. In our study, patients with acute coronary events were excluded, so we focused on the role of IL-6 trans-signaling pathway in the chronic inflammatory process of CHD.
In this study, though failed to reach statistical significance, the sIL-6R level tended to be higher in the CHD group compared to the control group (70.19+49.38 ng/ml vs 49.42+38.79 ng/ml, p=0.062). Previous studies showed consistent results with a higher level of sIL-6R related to increased occurrence of MI, future cardiovascular events, and mortality in STEMI patients. However, the level of sIL-6R in our study was a bit higher compared to the previous related studies. For example, in Moreno’s study, which was done in Swedish population with more than 2,700 patients, the sIL6R level in the MI group was 43.3 ng/ml (32.0-65.2 ng/ml) and in the control group was 40.8 ng/ml (31.6-54.5 ng/ml, p<0.01).19 As we can see, the differences of sIL-6R levels between the two groups were much higher in our study compared to Moreno’s, but due to our limited number of study samples, we failed to reach statistical significance.19
Contrary to sIL-6R, the relationship between sgp130 level and CHD is still contradictory. Ritschel et al. reported that higher levels of sgp130 were related to more future cardiovascular and mortality events.20 On the other hand, Anderson and Moreno et al. did not find a relationship between sgp130 level, CHD, and MI incidences, which is also in line with our study results.18,19 In this study, the level of sgp130 was 428.38+358.79 ng/ml in the CHD group and 474.08+389.43 ng/ml in the control group. Again, the level of sgp130 in our study was higher as compared to previous studies. Moreno’s study reported 363.6 (306.7-431.7) ng/ml in the MI group and 365.6 (308.8-432.6) ng/ml in the control group.19
ICAM-1 has been known to be associated with subclinical atherosclerosis in young adults and the risk of developing atherosclerotic disease in the future.26,27 IL-6 trans-signaling exerts its pro-atherogenic effects by stimulating chemokine production in endothelial cells and enhances adhesion molecule expression.28 Endothelial cells, in response to IL-6 activation, causes upregulation of ICAM-1. The vascular smooth muscle cells also secrete IL-6 and thus increase ICAM-1 expression, in addition to stimulating cells proliferation.29 In this study, the level of ICAM-1 in both groups were not significantly different, 1829.53+1882.3 pg/ml in CHD group and 2078.16+1595.25 pg/ml in the control group with p=0.565, but tended to be higher in the control group. The level of cholesterol was associated with ICAM-1 expression in the endothelium.30 This study showed high rates of hypercholesterolemia in both groups, 81.8% in the CHD group versus 93.9% in the control group. The rate of hypercholesterolemia was higher in the control group, although the p value was not significant (p=0.258). This result might explain the higher level of ICAM-1 in the control group.
This study has some limitations. First is the small sample size at only 33 subjects per group. Secondly, the non-CHD group (control group) was recruited only by history taking and ECG recording, not by angiography, so there might be some bias.
Conclusion
In conclusion, although we failed to show a relationship between some components of the IL-6 trans-signaling pathway (sIL-6R, sgp130, and ICAM-1) with CHD in young adults, our study showed that sIL-6R level tended to be higher in the CHD group. This finding might require further research with larger sample size.
Acknowledgements
We thank the nurses and staffs of Eka Hospital Pekanbaru for excellent assistance in recruiting participants and completing all the needed data.
Conflict of Interest
There is no conflicts of interest.
Funding Source
There is no funding source.
References
- World Health Organization. Global status report on noncommunicable diseases 2014. Attaining the nine global noncommunicable diseases targets, a shared responsibility, 2014.
- Joshi P., Islam S., Pais P., Reddy S., Dorairaj P., et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA., 2007; 297(3): 286-294.
- Puranik R., Fox O. J., Sullivan D. S., Duflou J., and Bao S. Inflammatory characteristics of premature coronary artery disease. J. Cardiol., 2010; 145(2): 288-290.
- van Loon J. E., de Maat M. P. M., Deckers J. W., van Domburg R. T., and Leebeek F. W. Prognostic markers in young patients with premature coronary heart disease. Atherosclerosis., 2012; 224: 213-217.
- Schuett H., Luchtefeld M., Grothusen C., Grote K., and Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb. Haemost., 2009; 102: 215–
- Su D., Li Z., Li X., Chen Y., Zhang Y., et al. Association between serum interleukin-6 concentration and mortality in patients with coronary artery disease. Mediators Inflamm., 2013; 2013: 726178, DOI: 10.1155/2013/726178.
- Tomas M. J. S., Baluyot P. M., Santos A. L. D., Faustino J., Licudine E., et al. Interleukin 6 as a biomarker of ischemic heart disease. International Journal of Scientific and Research Publications., 2015; 5(7): 1-4.
- Fuster J. J., and Walsh K. The good, the bad, and the ugly of interleukin-6 signaling. J., 2014; 33(13): 1425–1427.
- Scheller J., Chalaris A., Schmidt-Arras D., and Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biophys. Acta., 2011; 1813(5): 878–888.
- Jones S.A., Richards P.J., Scheller J., and Rose-John S. IL-6 transsignaling: the in vivo consequences. J. Interferon. Cytokine. Res., 2005; 25(5): 241–
- Morieri M. L., Passaro A., and Zuliani G. Interleukin-6 trans-signaling and ischemic vascular disease: the important role of soluble gp130. Mediators Inflamm., 2017; 2017: 1396398, DOI: 1155/2017/1396398.
- Rose-John S. IL-6 Trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. J. Biol. Sci., 2012; 8(9): 1237-1247.
- Heinrich P.C., Behrmann I., Haan S., Hermanns H. M., Müller-Newen G., et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. J., 2003; 374(Pt 1): 1–20.
- Barkhausen T., Tschernig T., Rosenstiel P., van Griensven M., Vonberg R. P., et al. Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Care. Med., 2011; 39(6): 1407–1413.
- Garbers C., Aparicio-Siegmund S., and Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Opin. Immunol. 2015; 34: 75–82.
- IL6R Genetics Consortium Emerging Risk Factors Collaboration, Sarwar N., Butterworth A.S., Freitag D. F., Gregson J., et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet., 2012; 379(9822): 1205-1213.
- The Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium, Swerdlow D. I., Holmes M. V., Kuchenbaecker K. B., Engmann J. E., et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet., 2012; 379(9822): 1214-1224.
- Anderson D. R., Poterucha J. T., Mikuls T.R., Duryee M. J., Garvin R. P., et al. IL-6 and its receptors in coronary artery disease and acute myocardial infarction. Cytokine., 2013; 62: 395–
- Moreno Velásquez I., Golabkesh Z., Kallberg H., Leander K., de Faire U., et al. Circulating levels of interleukin 6 soluble receptor and its natural antagonist, sgp130, and the risk of myocardial infarction. Atherosclerosis., 2015; 240(2): 477-481.
- Ritschel V. N., Seljeflot I., Arnesen H., Halvorsen S., Eritsland J., et al. Circulating Levels of IL-6 Receptor and gp130 and Long-Term Clinical Outcomes in ST-Elevation Myocardial Infarction. J Am Heart Assoc., 2016; 5(6): e003014, DOI:10.1161/JAHA.115.003014.
- Schuett H., Oestreich R., Waetzig G. H., Annema W., Luchtefeld M., et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol., 2012; 32: 281-290.
- Morieri M. L., Passaro A., and Zuliani G. Interleukin-6 Trans-Signaling and Ischemic Vascular Disease: The Important Role of Soluble gp130. Mediators. Inflamm., 2017; 2017:1396398, DOI:10.1155/2017/1396398.
- Askevold E.T., Nymo S., Ueland T., Gravning J., Wergeland R., et al. Soluble glycoprotein 130 predicts fatal outcomes in chronic heart failure: analysis from the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Circ. Hear.t Fail., 2013; 6: 91-98.
- Hussain M. A., Al Mamun A., Peters S. A. E., Woodward M., and Huxley R. R. The Burden of Cardiovascular Disease Attributable to Major Modifiable Risk Factors in Indonesia. J. Epidemiol., 2016; 26(10): 515-521.
- Aggarwal A., Srivastava S., and Velmurugan M. Newer perspectives of coronary artery disease in young. World. J. Cardiol., 2016; 8(12): 728-734.
- Gross M. D., Bielinski S. J., Suarez-Lopez J. R., Reiner A. P., Bailey K., et al. Circulating soluble ICAM-1 and subclinical atherosclerosis: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin. 2012; 58(2): 411–420.
- Ridker P. M., Hennekens C. H., Roitman-Johnson B., Stampfer M. J., and Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet., 1998; 351(9096): 88-92.
- Romano, Sironi M., Toniatti C., Polentarutti N., Fruscella P., et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity., 1997; 6(3): 315-325.
- Boekholdt S. M., and Stores E. S. , The interleukin-6 pathway and atherosclerosis. Lancet. 2012; 379(9822): 1176-1178.
- Fotis L., Agrogiannis G., Vlachos I. S., Pantopoulou A., Margoni A., et al. Intercellular Adhesion Molecule (ICAM)-1 and Vascular Cell Adhesion Molecule (VCAM)-1 at the Early Stages of Atherosclerosis in a Rat Model. In Vivo., 2012; 26(2): 243-250.







