Quality by Design (QbD) Concept: A Potential Solution to Chinese Current Biomanufacturing Challenges
H. Fai Poon1, Fan Wu1, Liang Shen2 and Floris De Smet2
1Quacell Biotechology Co., Ltd., Zhongshan, Guangdong, China.
2Sartorius Stedim Biotech, Shanghai, China.
Corresponding Author E-mail: hungfaipoon@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1668
Download this article as:| Copy the following to cite this article: Poon H. F, Wu F, Shen L, Smet F. D. Quality by Design (QbD) Concept: A Potential Solution to Chinese Current Biomanufacturing Challenges. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Poon H. F, Wu F, Shen L, Smet F. D. Quality by Design (QbD) Concept: A Potential Solution to Chinese Current Biomanufacturing Challenges. Biomed Pharmacol J 2019;12(2). https://bit.ly/2KO5mgZ |
The China Biopharmaceutical Boom
Since China’s Reform and Opening Up policy in 1978, the country has propelled unprecedented business and industry growth. In the light of its rising economy, biotech has started to grow at an explosive rate in China within the past few years. The industry is anticipated to exceed 4% of GDP by 2020.1,2 National policy, attractive local market, and improved regulations are lowering the market entry barrier and accelerating this Chinese biotech boom.1–5 Part of the biotech boom is the rise of Chinese biopharmaceutical industry.5 China has the second-highest number of biosimilars in development after the United States.6 The biosimilar experience lays a good foundation for innovative biologics development in China. As the biosimilar begins to mature, the government announced the 13th five-year plan to encourage innovative biologics.4,7 Such announcements have generated a sudden push for biologics to the clinical trials.2,4,7 In the next five years, it is expected that a large amount of biologics will be authorized to market in China.2,6,7 However, commercial biomanufacturing are found challenging. These challenges can hinder the commercialization of these biologics and limit their globalization. Two of the most challenging issues are the industry’s lack of commercial biomanufacturing experience and immature platform technologies in China.
Lack of Biomanufacturing experience
Biomanufacturing is among the most sophisticated and elegant processes of biologics development 8. The huge, complex structures of biologics must be produced consistently to offer intended efficacy and safety to patients. Yet it is known that biomanufacturing is full of operational and technological challenges.7,9,10 Reproducing large molecules reliably at an industrial scale requires experienced experts. These experts need to have extensive hands-on experience in freezing cells for storage, thawing them out without damage, and growing them in a bioreactor. The molecules must then be separated from the cells and the media without destroying the complex, fragile structures of the biologics. This hands-on experience is typically absent in Chinese biopharmaceutical companies (Exhibit #1) due to the recently rapid rise of the industry. The lack of hands-on experience will typically compensated by using more workers on the same production line when compare to multinational companies (Exhibit #2). The current lack of experience in biomanufacturing operation will eventually solved as the industry matures, but the current inexperience workers can potentially lead to more frequent operation problems such as equipment failure, contaminations etc. These operation problems will manifested in failed batches which are extremely costly for current biopharmaceutical companies.8,11,12
Immature Platform Technology
Platform technologies of biomanufacturing are one of the most valuable tools to improving efficiency and quality in bioproduction and development. The platform technologies in combination with risk assessments are the most systematic methods to leverage prior knowledge for a given new molecule.7,9,13–15 Moreover, mature platform technologies require continuous improvement by adding data for every new molecule developed to increase the robustness of the platform.
Our recent survey showed that majority of the Chinese biopharmaceutical companies use CHO-S (Gibco) and CHO-K1 (ATCC) cell lines as their host cell for production (Exhibit 3). While these cell lines produced many commercial molecules successfully, they lacked the accompanied culture media and process as a platform technology for biomanufacturing. Moreover, the in-house platform technology typically does not have enough data from new molecule development to ensure biomanufacturing robustness (Exhibit 4). Therefore, the immaturity of platform technologies of the Chinese biopharmaceutical companies will create large amounts of uncertainty and reduce process robustness during the commercial biomanufacturing.
Quality by Design: A Potential Solution
Quality by Design (QbD) is a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding, as well as process control, based on sound science and quality risk management.16 As a biopharmaceutical framework to robust drug quality, it implies a lifecycle perspective. QbD should start early in the drug’s development cycle, progressively building up a body of data and process models that in a structured, synergistic, risk- and science-based approach provide the desired process and product quality consistency. While comprehensive in nature, such lifecycle framework can be enabled through use of technical platforms and management tools. These have the potential to turn QbD into an accessible reality. Examples include, but are not limited to, Design of Experiments (DoE) strategy, High-Throughput Screening (HTS), applying high degrees of automation, Multivariate Data Analysis (MVDA), Process Analytical Technologies (PAT), Design Space and a risk assessing Process Control Strategy. If executed correctly, a QbD platform around process and product drastically enhances reliable product quality, minimizes impact of input variables and allows for continuous process improvement. Thus, a QbD approach will provide biopharmaceutical companies, regulators, and patients with confidence in assuring manufacturing process sturdiness and consistency of drug product quality. Not insignificantly, this process robustness also translates to the daily operational level, where impact of operator error, raw materials and process drift may not only be understood but also controlled and prevented. Aside from the Quality component, the economic impact follows suit: less failed batches, faster to approval, higher degree of consumer confidence, Cost of Goods (COGs) optimization and market share protection.
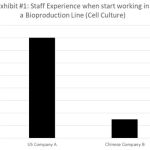 |
Figure 1
|
During the biopharmaceutical process development, preliminary risk assessments based on science and prior knowledge, will shorten the development timeline. Such systemic approach to Process Development, in turn, will identify the material attributes and process parameters that will have impact on drug product CQAs. DoE software support structured and scientific experimental design. Automated HTS technologies are powerful tools to perform the designed experiments within a shorter time. It ensures the performance consistency and reduces the contamination risks. Lastly, HTS generates multivariate dataset based on which further data analytics can be applied to link and verify material attributes and process parameters to their impact on CQAs in a design space. Multivariate data analysis (MVDA) is a powerful method to analyze complex datasets and provide accuracy and reliability during process development, process characterization and process control.
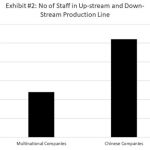 |
Figure 2
|
Scale-up is an important and potentially time-consuming step in the development of manufacturing processes. It involves much more than just doing the same at a larger volume. It requires the generation of solid process understanding at different scales to ensure consistent quality and titer throughout scale-up from early trials to final production scale 17. It is important to have a linear scale–up model to ensure that the scaling process will verify the previously defined process parameters and provide high productivity of drug samples available for clinical trials or assays. The importance of linearity in scale-up and scale-down can be derived from both the ICH Q8 as well as FDA Process Validation Guide.16,18
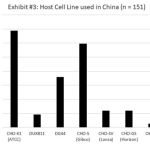 |
Figure 3
|
Previously, efforts were mainly focused on scaling up from bench to manufacturing scale, whereby a successful scale-up implied that the scale down model was adequate. Process characterization with a scale down mode that can represent the actual manufacturing scale performance is also crucial to the accuracy and reliability of the resulting design space.19 The representative equipment model, such as linearity, working mechanism, and performance consistency of drug contact material and equipment, plays an important role in both scaling up and scaling down to significantly shorten the process development timeline. A comprehensive DoE study can be used to better understand the process operating ranges and its impact on the product quality. Again, tools and techniques such as MVDA interpret the DoE study with an output of a design space. This will be part of the regulatory filing and ensure the process robustness.
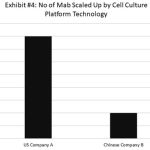 |
Figure 4
|
PAT has its origins in an initiative of the US Food and Drug Administration (FDA, first launched in mid-2002). Utilizing PAT can analyze and control manufacturing processes through timely measurements. The goal of PAT is to improve the understanding and control of the entire pharmaceutical and biopharmaceutical manufacturing process. One way of achieving this is through timely measurements of critical quality and performance attributes of raw and in-process materials and processes, combined with MVDA. This needs to be coupled with DoE to maximize the information content in the measured data. A central concept within the PAT paradigm is that quality should arise as a result of design-based understanding of the processes, rather than merely by aiming to generate products that meet minimum criteria within defined confidence limits, and rejecting those that fail to meet the criteria. During manufacturing, MVDA derived from all the batches data will visualize the data output and predict the manufacturing performance trend so that actions can be taken to reduce the risk of a failed batch. Thus, both PAT and MVDA can be viewed as enablers for continuous process verification.20
Conclusion
In summary, QbD provide a better control strategy during the stage of design space identification. These improved control strategies can compensate Chinese worker’s lack of biomanufacturing experience. Moreover, QbD demands risk assessment during the entire development stage. These risk assessment exercises result in development space to challenge their existing platform by performing additional experiment and data to expand the knowledge space for a more robust process. Therefore, we believe that QbD could be a potential solution for the industry’s lack of commercial biomanufacturing experience and immature platform technologies in China.
Reference
- Ellis, S. Biotech booms in China. Nature (2018). doi:10.1038/d41586-018-00542-3
CrossRef - Greenwood, J. C. Biotech in China. EBR – Eur. Biopharm. Rev. (2013).
- Lundh, E. Assessing the impact of China’s Thousand Talents Program on life sciences innovation. Nat. Biotechnol. (2011). doi:10.1038/nbt.1894
CrossRef - Yang, B. Innovation The Big Winner As China Joins ICH :: Pink Sheet. Informa Pharma Intelligence (2017). Available at: https://pink.pharmaintelligence.informa.com/PS120840/Innovation-The-Big-Winner-As-China-Joins-ICH. (Accessed: 12th March 2019)
- Hu, X., Ma, Q. & Zhang, S. Biopharmaceuticals in China. Biotechnology Journal (2006). doi:10.1002/biot.200600083
CrossRef - Chen, C. & Yin, S. China: the Next Frontier for Biologics. (2016).
- Yuan, J. et al., Scientific Strategy in China: Starting with Biosimilar Platforms. in Advances in Biopharmaceutical Technology in China, 2nd ed. (eds. Xia, V. (Qing) & Cai, L. (yang)) 51–64 (BioPlan Associates, Inc, 2018).
- Le, H., Vishwanathan, N., Jacob, N. M., Gadgil, M. & Hu, W. S. Cell line development for biomanufacturing processes: recent advances and an outlook. Biotechnology Letters 37, 1553–1564 (2015).
CrossRef - Yuan, J., Xu, W. W., Yu, H. X., Jiang, L. Y. & Poon, H. F. Cell Culture Technologies in Successful Biosimilar Development. Bioequivalence Bioavailab. Int. J. 2, 1–7 (2018).
CrossRef - Barnes, L. M., Bentley, C. M. & Dickson, A. J. Stability of protein production from recombinant mammalian cells. Biotechnology and Bioengineering 81, 631–639 (2003).
CrossRef - Kirchhoff, C. F. et al. Biosimilars: Key regulatory considerations and similarity assessment tools. Biotechnology and Bioengineering 114, 2696–2705 (2017).
CrossRef - Shire, S. J. Monoclonal Antibodies: Meeting the Challenges in Manufacturing, Formulation, Delivery and Stability of Final Drug Product. Mi colección Mi historial Libros en Google Play Monoclonal Antibodies: Meeting the Challenges in Manufacturing, Formulation, Delivery and Stability of Final Drug Product (2015). doi:10.1016/B978-0-12-639380-4.50001-4
CrossRef - Challener, C. A. Platform technologies improve protein expression. BioPharm International (2017).
- Sinclair, A. & Monge, M. Implementing disposables technology, delivering innovation, and transforming an organization. BioPharm Int. (2010).
- Langer. On the Horizon: New Expression Systems to Become Common Industry Platforms. BioPharm Int. (2009).
- ICH-8-R2-quality. 0042-Pharmaceutical Development Q8. ICH Harmon. Tripart. Guidel. (2009).
- De Wilde, D. et al. Superior scalability of single-use bioreactors. Bioprocess Int. (2014).
- FDA. Guidance for Industry Process Validation: General Principles and Practices. Guid. Ind. (2011).
- Tsang, V. L., Wang, A. X., Yusuf-Makagiansar, H. & Ryll, T. Development of a scale down cell culture model using multivariate analysis as a qualification tool. Biotechnol. Prog. (2014). doi:10.1002/btpr.1819
CrossRef - Eriksson L, Byrne T, Johansson E, Trygg J, V. C. Multi- and Megavariate Data Analysis Basic Principles and Applications. Technometrics (2013). doi:10.1198/tech.2003.s162
CrossRef








