Vijitra Luang-In1* , Manatchanok Yotchaisarn1
, Manatchanok Yotchaisarn1 , Worachot Saengha1
, Worachot Saengha1 , Piyachat Udomwong2
, Piyachat Udomwong2 , Sirirat Deeseenthum1
, Sirirat Deeseenthum1 and Kedsukon Maneewan1
and Kedsukon Maneewan1
1Natural Antioxidant Innovation Research Unit, Department of Biotechnology, Faculty of Technology, Mahasarakham University, Khamriang, Kantarawichai, Maha Sarakham, 44150, Thailand.
2International College of Digital Innovation, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding Author E-mail: vijitra.l@msu.ac.th
DOI : https://dx.doi.org/10.13005/bpj/1678
Abstract
The current work aimed to screen for and identify protease-producing bacteria from the untapped resource Nasinuan forest, Kantarawichai District, Mahasarakham Province, Thailand. Nineteen bacterial isolates with protease-producing capacity on 1% skimmed milk agar were identified using 16S rRNA sequencing. Seventeen bacteria were gram-positive, rod shaped and identified as Bacillus spp. and only two bacteria were identified as Enterobacter sp. and Staphylococcus cohnii. Their closest relatives were found in India, Oman, Italy, Indonesia, Malaysia, China and USA. The top six highest halo : colony ratios from pure isolates were ranked in the following order: 1.2PT1 (2.43) > 1.2PT2 (2.23) > 2.2PT3 (2.21) > 2.1PT3 (2.17) > 2.3PT3 (2.16) > 2.4PT1 (2.16). Bacillus thuringiensis 2.3PT3 was found to exhibit the highest protease enzyme activity of 3.72 ± 0.08 U/mg protein at the optimal conditions of 65°C and pH 8.0 after 30 min incubation with 1% casein in 0.05 M PBS buffer. This protease–producing bacterial strain can be of great potential for applications in food, agricultural and pharmaceutical industries in Thailand.
Keywords
Bacteria; Nasinuan Forest; Protease; Soil; Thailand
Download this article as:| Copy the following to cite this article: Luang-In V, Yotchaisarn M, Saengha W, Udomwong P, Deeseenthum S, Maneewan K. Protease-Producing Bacteria from Soil in Nasinuan Community Forest, Mahasarakham Province, Thailand. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Luang-In V, Yotchaisarn M, Saengha W, Udomwong P, Deeseenthum S, Maneewan K. Protease-Producing Bacteria from Soil in Nasinuan Community Forest, Mahasarakham Province, Thailand. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2xeqC7y |
Introduction
Proteases, one of the most import industrial enzymes, account for a major share of 60% of total global enzyme market1. Proteases are the hydrolytic enzymes which break down peptide bond between proteins with paramount applications in pharmaceutical and industrial sector. Proteases have a myriad of functions in food, textile industries and important biopharmaceutical applications such as infant formula preparation (American Academy of Pediatrics Committee on Nutrition, 1998), contact-lens enzyme cleaners and enzymatic deriders2. The proteolytic enzymes can also be used in clinical/medical field offering a gentle and selective debridement, promoting the natural healing step in the successful local management of skin ulcerations by removing the necrotic material efficiently3.
To supply sufficient industrial proteases to meet the global in
creasing demand, we have to investigate the cost effective way of producing industrially important enzymes. Proteases derived from microbial sources are preferred over the enzymes from plant or animal sources4 due to microbial wide-range biochemical diversity, their rapid proliferation, the limited space required for cell cultivation and the convenience with which the enzymes can be genetically manipulated to generate new enzymes for various application5 and they exhibit all most all the desirable characteristics for their biotechnological applications. A plethora of Bacillus derived alkaline proteases have been purified and characterized because of their significant proteolytic activity, broad substrate specificity, stability, short period of fermentation, simple downstream purification and low cost6,7. The protease producing bacterial strains are as Bacillus subtilis, Bacillus licheniformis, and Bacillus thuringiensi8.
Thus far, a number of protease-producing bacteria has been reported, however, no study on protease-producing bacteria from soil in the Nasinuan Community Forest, Kantarawichai District, Mahasarakham Province, Thailand has been done. This forest seems to be rich in microbial biodiversity that can be applied for the production of industrial enzymes including protease. This is the first report to identify protease-producing bacteria isolated from Nasinuan Forest. These bacterial proteases have potential applications in food processing, animal feed, agriculture and pharmaceutical in Thailand.
Metrials and Methods
Soil Samples
Soil samples were randomly collected below the soil surface 15 cm and kept in polystyrene bags from Nasinuan Community Forest, Kantarawichai District, Mahasarakham Province, Thailand (area of 9.6 hectare; coordinate of 16.340941, 103.210799).
Isolation of Protease-Producing Bacteria
Soil sample (10 g) was suspended in 90 mL of sterile 0.85% NaCl solution. The suspensions (100 µL) of serial dilutions were spread on skimmed milk agar (g/L); 10.0 Tryptone, 5.0 Peptone, 3.0 (NH4)2SO4, 2.0 K2HPO4, 0.2 MgSO4, 1.0 Casein, 15.0 agar pH 7.0 and incubated at 37°C for 3 days. Any colonies with formation of clear zone around the colonies were subcultured in liquid broth and streaked at least five times to obtain pure isolates as confirmed by Gram staining and 1000X light microscopic observation. The pure isolates were point inoculated on skimmed milk agar and incubated at 37 °C for 7 days. The diameters of the clear zones over the diameters of the colonies were measured using a ruler as the halo : colony ratio.
16S rRNA Gene Sequencing and Phylogenetic Analysis
Pure bacterial isolates were identified using genomic DNAs obtained from the above method and universal primers: forward primer 27F 5′-GAGAGTTTGATYCTGGCTCAG-3′ and reverse primer 1492R 5’AAGGAGGTGATCCARCCGCA -3′. In 25 µL PCR mixture, it was composed of genomic DNA 0.5 ng, 2X Master Mix (One PCR) of 100 mM Tris-HCl (pH 9.1), 0.1% TritonTMX-100, 200 mM dNTP, 1.5 mM MgCl2, 0.005 U Taq DNA Polymerase and 0.2 µM forward and reverse primer with volume adjustment with nuclease-free water. PCR thermocycler (Thermo Scientific Hybaid Px2) was programmed as follows: (1) initial denaturation for 2 min at 94°C for 1 cycle; (2) denaturation at 94°C for 45 s; annealing at 54 °C for 45 s, and extension at 72°C for 1 min for 32 cycles; (3) final extension at 72°C for 7 min. Samples were held at 4°C till further analysis. The PCR products of 16S rRNAs (~ 1,500 bp) were detected on 0.8% agarose gel, purified using the PCR product purification kit (Vivantis, Malaysia), sent to First Base Co. Ltd. (Malaysia) for DNA sequencing. The 16S rRNA gene sequences were then compared with others available in GenBank using BLASTN program (Basic Local Alignment Search Tools)9. The Phylogenetic tree was constructed using Muscle method for sequence alignment and maximum likelihood method using MEGA X with 1,000 replicates of bootstrap values10. All 16S rRNA partial sequences of our protease-producing isolates were deposited on NCBI database.
Protease Enzyme Activity
The method followed the previous report11. Each isolate was subcultured in casein induction liquid broth (g/L): 10.0 Tryptone, 5.0 Peptone, 3.0 (NH4)2SO4, 2.0 K2HPO4, 0.2 MgSO4, 1.0 Casein and incubated at 37°C, 150 rpm for 3 days. The clear supernatant (crude extracellular protease enzyme) was obtained after centrifugation at 10,000g for 15 min at 4°C. The crude extract was concentrated using MWCO 10 kDa ultracentrifuge protein concentrator (Vivaspin, Sartorius, UK). Crude enzyme (0.5 mL) was mixed with 1 mL of 1% casein solution in 1 mL 0.05 M Potassium phosphate buffer pH 7.5. The samples were incubated at 37°C for 30 min. After incubation, 3 mL of 110 mM trichloroacetic acid (TCA) was added to each sample to stop the reaction and then centrifuged at 10,000 rpm for 15 min. The clear supernatant (1 mL) was mixed with 2 mL of 0.5 M sodium carbonate solution and 0.5 mL Folin’s reagent. The mixture was recorded for A280nm using a spectrophotometer. The reading was compared to a prepared blank solution (without crude enzyme). The A280nm values of samples at T30min were subtracted from those of samples at T0min since casein still remained in the T0min samples after enzyme induction process at 3 days. The process was carried out in triplicates. The concentration of tyrosine produced for each solution was obtained from the tyrosine standard curve. The activity of protease was calculated. One unit of protease activity is defined as the amount of protease required to catalyze the formation of 1 µmol tyrosine per min under assay conditions11 (Cupp-Enyard, 2008). The crude enzyme of the bacterial isolate having the highest activity was chosen for further work. In order to determine the specific enzyme activity of the selected isolates, the Folin-Lowry method for total protein estimation was used. The specific enzyme activity was measured using the following formula.
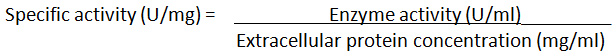
Optimal pH and Temperature For Protease Enzyme Activity
Casein (1%) in different pH solutions starting from 3 to 10 were tested. One mL of different 1% casein solution was added along with 1 mL of the respective buffers; 0.05 M citrate buffer (pH 3 to 5), 0.05 M sodium phosphate buffer (pH 6 and 7), 0.05 M Tris-HCl (pH 8 and 9) and 0.05 M glycine NaOH (pH 10). One mL of crude protease enzyme was added to these buffers as well. The samples were incubated at 37°C for 30 min. The specific protease activity was calculated. The pH at which the highest activity was observed was noted. Likewise, different substrate solutions were made by dissolving 1% casein in pH 7.0 solutions. One mL of 1% casein starch was added along with 1 mL of 0.05 M sodium phosphate buffer (pH 7). One mL of crude enzyme was added to the buffers as well. The samples were incubated at 4, 25, 35, 45, 55, 65, 75, 85, and 95°C for 30 min. The specific protease activity was calculated. The temperature at which the highest activity was observed was noted. Both optimal pH and temperature were used to determine the final specific protease enzyme activity.
Statistical Analysis
One-Way Analysis Of Variance (One-way ANOVA) was used with Duncan Multiple Range’s Test on SPSS Statistics Ver. 17.0. Results were expressed as means ± SD with statistical difference when p < 0.05.
Results and Discussions
Isolation of Protease-Producing Bacteria
In this study, 19 protease-positive isolates showed clear zones on skimmed milk agar with different halo : colony ratios. The colonies showing clear zones were taken as positive protein-degrading bacterial colonies. Seventeen bacterial isolates showed similar colony morphologies and appeared to be Gram-positive and rod-shaped (Table 1) while 3.5PT11 isolate was gram-negative and rod-shaped and 3.5PT7 isolate was gram-positive and coccus-shaped. The top six highest halo : colony ratios from pure isolates were ranked in the following order: 1.2PT1 (2.43) > 1.2PT2 (2.23) > 2.2PT3 (2.21) > 2.1PT3 (2.17) > 2.3PT3 (2.16) > 2.4PT1 (2.16) (Table 1). These six bacterial isolates were then used for secondary screening for protease activity in 3-day induction liquid broth containing 1% casein. It appeared that 2.3PT3 isolate had the highest protease activity (data not shown) among all isolates and thus used for further experiment.
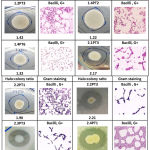 |
Table 1: Characteristics and halo : colony ratios of 19 protease-producing bacterial strains.
|
Strain Identification of Protease-Producing Bacteria
All 19 protease-positive bacterial strains were subjected to 16S rRNA gene sequencing for strain identification. The BLAST results displayed that all protease-positive isolates belong to the genus Bacillus, except for two isolate belonging to Enterobacter and Staphylococcus (Table 2). Their closest relatives were found in India, Oman, Italy, Indonesia, Malaysia, China and USA with a range of 95-99% sequence identity. Our results are similar to previous findings. Bacillus sp. APP-07 isolated from Laundromat soil of Solapur, Maharashtra, India produced alkaline protease with an optimum pH 10.5 and temperature 55°C12. Similarly, Bacillus licheniformis TKU004, an isolated bacterial strain from Taiwanese soil, was found to produce protease13. In addition, Enterobacter agglomerans and Enterobacter aerogenes have been previously discovered as protease producers with the highest proteolytic activities at pH 9.014. However, no report has identified Staphylococcus cohnii as a protease producer before.
Table 2: Nineteen protease-positive bacterial strains identified by 16S rRNA analysis.
| Isolate | Accession no. a | Closest relative b | Accession no. c | % Identity d | Origin e |
| 1.1PT2 | MK648326.1 | Bacillus anthracis ANA1 | MG593550.1 | 99 | Marine sediments, India |
| 1.1PT3 | MK648327.1 | Bacillus cereus IARI-ME-36 | KJ752763.1 | 99 | Acidic soil, India |
| 1.1PT8 | MK648328.1 | Bacillus subtilis TP5 | KX822704.1 | 99 | Plant growth promoting endophytes, Oman |
| 1.1PT9 | MK648329.1 | Bacillus subtilis BCB-19 | MG832888.1 | 99 | Herbal vermicompost, India |
| 1.2PT1 | MK648330.1 | Bacillus thuringiensis BD17-E12 | HF584771.1 | 99 | Grapevine root, Italy |
| 1.2PT2 | MK648331.1 | Bacillus cereus F4a | MK088302.1 | 99 | Tea rhizosphere soil, India |
| 1.2PT3 | MK648332.1 | Bacillus subtilis subsp. stercoris HY5 | MK332369.1 | 99 | Fermented food, India |
| 1.4PT2 | MK648333.1 | Bacillus toyonensis DFT-2 | KY750686.1 | 97 | Seawater of industrial area, Indonesia |
| 1.4PT6 | MK648334.1 | Bacillus cereus DFT-4 | KY750688.1 | 99 | Seawater of industrial area, Indonesia |
| 2.1PT3 | MK648335.1 | Bacillus cereus F4a | MK088302.1 | 99 | Tea Rhizosphere soil, India |
| 2.2PT1 | MK648336.1 | Bacillus cereus RE01-BS05 | KJ742939.1 | 98 | Fermented shrimp paste, Malaysia |
| 2.2PT3 | MK648337.1 | Bacillus cereus DFT-6 | KY750690.1 | 99 | Seawater of industrial area, Indonesia |
| 2.3PT3 | MK648338.1 | Bacillus thuringiensis BD17-E12 | HF584771.1 | 98 | Grapevine root, Italy |
| 2.4PT1 | MK648339.1 | Bacillus cereus CP1 | JX544748.1 | 98 | Unknown source, China |
| 2.4PT2 | MK648340.1 | Bacillus cereus 2Y-2 | FJ493043.1 | 99 | Wheat, China |
| 3.1PT3 | MK648341.1 | Bacillus thuringiensis IARI-IIWP-38 | KF054891.1 | 99 | Wheat rhizospere, India |
| 3.5PT7 | MK648342.1 | Staphylococcus cohnii | AY395015.1 | 99 | Midgut of gypsy moth larva, USA |
| 3.5PT11 | MK648343.1 | Enterobacter sp. T311 | KM406403.1 | 96 | Irrigation water, Pakistan |
| 3.6PT7 | MK648344.1 | Bacillus cereus DFT-1 | KY750685.1 | 95 | Seawater of industrial area, Indonesia |
a GenBank accession no. of our strains deposited on NCBI website (http://www.ncbi.nlm.nih.gov/pubmed).
b Closest species with highest % identity and highest Max score on BLAST search.
c GenBank accession no. of closest relative strains on NCBI website.
d Based on BLAST search results, identity (%) of strains compared to the closest relatives.
e Based on BLAST search results, origin of the closest relatives.
Phylogenetic Analysis
The phylogenetic tree of 19 protease-positive bacterial strains and 5 reference strains with putative protease enzymes showed that Enterobacter sp. 3.5PT11 was evolutionarily similar to Enterobacter sp. 638 (EU340965.1). These two bacteria evolved differently from the other bacteria. Likewise, B. subtilis 1.1PT8, 1.1PT9, and B. subtilis subsp. stercoris 1.2PT3 were evolutionarily similar to B. subtilis subsp. subtilis 168 (MH283878.1). The rest of Bacillus spp. evolved similarly. However, S. cohnii 3.5PT7 seemed to be evolutionarily different from all bacteria (Fig. 1).
 |
Figure 1: Phylogenetic tree of 19 protease-positive bacterial strains and 5 reference strains.
|
Optimal pH and Temperature of Protease Enzyme Activity
B. thuringiensis 2.3PT3 showed the highest specific activity at pH 8.0 when 37°C was fixed and at 65°C when pH 7.0 was fixed (Fig. 2). Thus, both optimal conditions (pH 8.0 and 65°C) were used to determine specific protease activity and 3.72 ± 0.08 U/mg (Table 3) was obtained.
It has been reported that Bacillus infantis SKS1 isolated from garden soil of north India showed specific protease activity at 7.93 U/mg15. Bacillus sp. SKS1 was active at pH 10 and wide range of temperatures (40°C to 70°C) suggesting its application in industry demanding moderate heat and alkaline conditions. Alkaline serine protease produced by Bacillus cereus strain S8 (MTCC NO 11901). The optimum activity of the protease was observed at pH 10.0 and 70°C16 (Lakshmi et al. 2019). In addition, the protease produced by Bacillus pumilis MK6-517 was active at 50°C-55°C (pH 11) whereas Bacillus licheniformis18 and Bacillus firmus 772819 produced proteases which are active at 37°C (pH 8.5) and 40°C (pH 9) respectively.
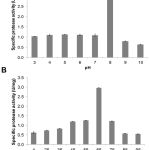 |
Figure 2: Optimal pH (A) and temperature (B) of protease enzyme activity from B. thuringiensis 2.3PT3.
|
Our results show that protease of B. thuringiensis 2.3PT3 showed its maximum activity at 65°C (pH 8) as it was thermostable under alkaline conditions. Similarly, the previous report of Bacillus subtilis RJAS 19 has shown the optimum conditions for protease at pH 9.5 and 65°C20. This suggests that our protease from B. thuringiensis 2.3PT3 has a potential to be used in industries with alkalinity and high temperature.
Table 3: Specific protease enzyme activity at optimal pH and temperature.
| Strain | Activity (U/mg) | Optimal Temp. (°C) | Optimal pH |
| B. thuringiensis 2.3PT3 | 3.72 ± 0.08 | 65 | 8.0 |
Further study is essential for the starting point of commercial protease production in Thailand to make it cost-effective. Thorough enzyme characterization, further study on the thermostability, pH stability, effect of different metal ions and different substrates is necessary. It is hoped that the production of commercial protease in Thailand enzyme industries may be more increased and in turn will be benefiting the country’s economy due to self-reliance on its own resources to produce protease enzyme.
Conclusion
This is the first report of identifying 19 protease-producing bacterial isolates from soil in Nasinuan Community Forest, Maha Sarakham. Most bacteria were identified as Bacillus spp. and two from Enterobacter and Staphylococcus genus. BY far, Bacillus-derived proteases are the most industrially exploited. The results in this work are in accordance with the previous reports, as several Bacillus species are known to be protease producers. These bacteria can be used for protease production and applied locally and nationally in food processing, agriculture, and pharmaceutical industries in Thailand. Thus, this will help lower the cost of industrial protease import from other countries, offer sustainability of local protease production and enhance the economy of the nation.
Acknowledgements
The authors would like to thank Mahasarakham University (Grant Year 2018) for financial support awarded to SD, Faculty of Technology, Mahasarakham University (Grant Year 2017) awarded to VL and National Research Council of Thailand through a research grant for postgraduate MSc students (Grant Year 2018) awarded to MY.
Conflict of Interest
Authors declare no conflict of interest.
References
- Rayda S., Fakher F., Samiha M., Moncef N., and Alya S.K. Optimization of acid protease production by Aspergillus niger I1 on shrimp peptone using statistical experimental
The Sci World J. 2012:01-11. (2012). - Anwar A. and Saleemuddin M. Alkaline protease from Spilosomaoblique: potential applications in bio-formulations. Biotech App Biochem. 31:85-89 (2000).
- Sjodahl J., Emmer A., Vincent J., and Roeraade J. Characterization of proteinases from Antarctic krill (Euphausia superba). Protein Expr Purif. 26:153-161 (2000).
- Mukesh Kumar D.J., Premavathi V., Govindarajan N., Balakumaran M.D., and Kalaichelvan P.T. Production and purification of alkaline protease from Bacillus MPTK 712 isolated from dairy sludge. Global Veterinaria 8(5):433-439 (2012).
- Singhal P., Nigam V.K., and Vidyarthi AS. Studies on production, characterization and applications of microbial alkaline proteases. Int J Advanc Biotechnol Res. 3(3):653-669 (2012).
- Maurer K.H. Detergent proteases. Curr Opin Biotechnol. 15:330-334 (2004).
- Haddar A., Agrebi R., Bougatef A., Hmidet N., Sellami-Kamoun A., and Nasri M. Two detergent stable alkaline serine-proteases from Bacillus mojavensis A21: purification, characterization and potential application as a laundry detergent additive. Biores
Technol. 100: 3366-3373 (2009). - Sharma A.K., Sharma V., Saxena J., Yadav B., Alam A., and Prakash A. Optimization of protease production from bacteria from soil. Appl Res J. 1:388-394 (2015).
- Altschul S.F., Gish W., Miller W., Myers E.W., and Lipman D.J. Basic local alignment search tool. J Mol Biol. 215:403–410 (1990).
- Tamura K., and Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 10:512-526 (1993).
- Suganthi C., Mageswari A., Karthikeyan S., Anbalagan M., Sivakumar A., and Gothandam K.M. Screening and optimization of protease production from a halotolerant Bacillus licheniformis isolated from saltern sediments. J Genet Eng Biotechnol. 11:47–52 (2013).
- Shaikh I.K., Dixit P.P., and Shaikh T.M. Purification and characterization of alkaline soda-bleach stable protease from Bacillus APP-07 isolated from Laundromat soil. J Genetic Eng Biotechnol.16(2):273–279 (2018).
- Doan C.T., Tran T.N., Nguyen M.T., Nguyen V.B., Nguyen A.D., and Wang S.L. Anti-α-Glucosidase activity by a protease from Bacillus licheniformis. Molecules (Basel, Switzerland), 24(4):691 (2019).
- Rodarte P., Dias D.R., Vilela D.M., and Schwan R.F. Proteolytic activities of bacteria, yeasts and filamentous fungi isolated from coffee fruit (Coffea arabica L.). Acta Sci Agron. 33(3):457-464 (2011).
- Saggu S.K. and Mishra P.C. Characterization of thermostable alkaline proteases
from Bacillus infantis SKS1 isolated from garden soil. PLoS ONE. 12(11):e0188724 (2017). - Lakshmi B., Muni Kumar D., and Hemalatha K. Purification and characterization of alkaline protease with novel properties from Bacillus cereusstrain S8. J Genetic Eng 16(2):295–304 (2018).
- Kumar C.G. Purification and characterization of a thermostable alkaline protease from alkalophilic Bacillus pumilus. Lett Appl Microbiol. 34: 13–17 (2002).
- Paul D., Rahman A., Ilias M., Hoq M.M. Production and characterization of keratinolytic protease of Bacillus licheniformis MZK-03 grown on feather mill. Bangladesh J Microbiol. 24: 57–61 (2007).
- Rao K., Narasu M.L. Alkaline protease from Bacillus firmus Afr J Biotechnol. 6: 2493–2496 (2007).
- Kumar D.J., Rakshitha R., Vidhya M.A., Jennifer P.S., Prasad S., Kumar M.R. et al. Production, optimization and characterization of fibrinolytic enzyme by Bacillus subtilis RJAS19. Pak J Biol Sci. 17:529–534 (2014).








