Varahi Vedam V. A , Alphienes Stanley Xavier*
, Alphienes Stanley Xavier* and Darling Chellathai David
and Darling Chellathai David
Department of Pharmacology, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai - 600116, Tamil Nadu, India.
Corresponding Author E-mail: alphclinpharm@sriramachandra.edu.in
DOI : https://dx.doi.org/10.13005/bpj/1705
Abstract
Tagetes erecta, also known as African marigold has numerous medicinal values. With the rising need to explore better antifungal, anticancer agents in therapeutics, we have done this study to evaluate the antifungal and anticancer properties of Tagetes erecta petal extract. Antifungal activity against was evaluated against Candida albicans, Aspergillus niger, Aspergillus flavus, and Penicillium crysogenum fungal strains in disc diffusion method using Amphotericin-B, fluconazole as positive controls. Breast cancer line (MCF-7) was used to study the anticancer property of ethanolic petal extract using cytotoxicity assay, in which 5-fluorouracil was used as control. Compared to standard antifungal agents, T.erecta petal extract displayed good efficacy in increasing the diameter of zone of inhibition with disc diffusion method. In cytotoxicity assay, IC50 value was observed to be at concentration of 125µg/ml. This study demonstrated that the petal extract of Tagetes erecta could be a valuable lead, which has the potential to be explored for its use against fungal infection, and breast carcinoma in the upcoming years by the scientific fraternity.
Keywords
Anticancer Agent; African Marigold; Antifungal; Agar Disc Diffusion; MCF-7
Download this article as:| Copy the following to cite this article: Vedam V. V. A, Xavier A. S, David D. C. In-Vitro Evaluation of Antifungal and Anticancer Properties of Tagetes Erecta Petal Extract. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Vedam V. V. A, Xavier A. S, David D. C. In-Vitro Evaluation of Antifungal and Anticancer Properties of Tagetes Erecta Petal Extract. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/30VL6zG |
Introduction
Tagetes erecta, commonly known as Mexican marigold or Aztec marigold, belongs to the genus Tagetes, of Asteraceae family.1 Despite being native to America, it is often called as African marigold. Various medicinal uses of the different parts of this plant were explored in scientific literature, and have found place in alternative medicine. Alpha-tertheinyl is an active substance found in the plant, which causes a reduction in plant nematodes and inhibits hatching of eggs of nematodes, hence acting as a nematicidal agent.2 Flowers, roots, stems, and leaves of Tagetes erecta have thiophene and its derivatives which had an increased potential as a larvicidal agent against dengue vector.3 Tagetes erecta is used internally for joint pain, irregular menstruation, abdominal pain, and dysentery, as well as externally for ulcer, eczema, and wound healing. Other potential medicinal uses of Tagetes erecta are as central nervous system stimulant, antidepressant, antioxidant, antipyretic, antidiabetic, and hypolipidemic agent.4 Non-pharmacologically it is used as insecticidal, nematicidal, larvicidal, as well as in industries for dyeing textiles.4
The rising incidence of fungal infections is a real global concern, which is often overlooked. In India, the incidence of Candidemia is reported to be 6 per 1000 ICU admissions, and there are reports of drug resistant fungi, including azole resistant Aspergillus fumigatus, as well as newer unusual fungal infections have emerged.5 Many existing antifungal agents are derived from natural sources. Amphotericin-B and nystatin were separated from Streptomyces species, as well as griseofulvin was isolated from Penicillium griseofulvin. Several naturally available compounds such as terpene derivatives, alkaloids, peptides, flavans, saponins, and sterols have shown to have the potential to possess antifungal activity.6 The last three decades have seen many research initiatives published in literature which explored the efficacy of medicinal plants against fungal pathogens.7 The extracts from stem of Sandalwood and Arjuna bark which were used in traditional medicine have displayed potent antifungal activity against Candida albicans.8
Calendula species of plants which share the same family as T. erecta have been evaluated for their antifungal activity. The essential oil from the flowers of Calendula officinalis had displayed significant antifungal activity against various Candida strains including C.albicans in agar disc diffusion method.9 The methanolic extracts from aerial parts of Calendula species were observed to have good antifungal activity against dermatophytes. Caffeic acid and quercetin derivatives were the important constituents enriching these plants.10 Because of its vast availability and medicinal values, we decided to study the antifungal activity of Tagetes erecta petal extract against common pathogenic fungal strains such as Candida albicans, Aspergillus niger, Aspergillus flavus, and Penicillium chrysogenum.
The cancer burden is increasing tremendously in the recent years, according to the Indian cancer registry it was estimated to be 1.4 million cases in 2015, if untreated might even rise up to 1.84 million by 2020. Cancers of lung, stomach, and prostate, as well as among women cancers of breast, cervix, and uterus, are the common cancer conditions prevalent in South India.11 Plants have provided and will provide potential bioactive compounds for the development of new ‘leads’ to combat cancer diseases. In cancer therapy, novel plant extracts or bioactive compounds had contributed vastly for disease prevention and treatment, regardless of overshadowing by current drug discovery methods like combinatorial chemistry. Vincristine, camptothecin, vinblastine, and taxol were proven plant derivatives reported for their antitumor activity in the treatment of cancers. Many plants like Allium sativum, and Aloe-vera have anticancer phytochemicals as their constituents which can be used for breast carcinoma, and expressed anti-angiogenesis potential respectively.12 Curcuma longa has potent anticancer activity against various cancers such as colon, cervical, uterine, ovarian, head and neck, as well as skin cancer.13 Plants like Nardostachys jatamansi have been studied for treatment in breast carcinoma.14 The ethanol extract of Tagetes erecta contains syringic acid, quercetin, 6-hydroxykaempferol, protocatechuic acid, and quercetagetin. Among them compounds quercetin, protocatechuic acid, and quercetagetin are flavonoids. Compounds quercetin, and 6-hydroxykaempferol showed significant anticancer activity against A549 (Lung carcinoma) and HepG2 cells (Hepatocellular carcinoma). Compounds protocatechuic acid, and quercetagetin were effective against A549 cells.15 Based on these available information we conducted a study to assess the anticancer property of Tagetes erecta petal extract in breast carcinoma cell lines which can provide worthy information, as well as help in the novel anticancer drug development.
Materials and Method
Tagetes erecta plant was collected and authenticated. The petals of the plant were washed with tap water followed by distilled water, and dried for seven days prior to the study. The dried petals were crushed into powder with mortar and pestle. The powder was loaded into the Soxhlet apparatus, and processed with ethanol for 16 hours.16 After the procedure, the ethanolic extract was processed with rotavapor. The dried ethanolic extract obtained was stored in refrigeration until further use.
The pure powders of drugs fluconazole, and amphotericin-B were purchased from Bioderma solutions, Gujarat, and Bharat serum, Maharashtra respectively. The pathogens C.Albicans, A.Niger, A.Flavus, P. Crysogenum were obtained, and maintained in Sabouraud Dextrose Agar (SDA) medium. MCF-7 cell lines were obtained from National Centre for Cell Science (NCCS), Pune. 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide was purchased from Sigma-Aldrich, India.
Ethanolic Extract, Dilution, and Preparation of Impregnated Disc
Plant extract was diluted with Dimethyl Sulfoxide (DMSO) in serial two fold dilution across 96 well plate. The concentration was then further diluted to 16 fold in water correspondingly. Twenty microliter from each of the well was then used to impregnate a blank sterilized disc. The final concentration used for the test was 1mg/disc. The impregnated discs were dried at 37oC in incubator for 18 to 24 hours, and used immediately for sensitivity.
Agar Disc Diffusion Method
Antifungal activity of the extract was determined by disc diffusion method on SDA medium.SDA medium is poured into the Petri plate. After the medium was solidified, the inoculums were spread on the solid plates with sterile swab moistened with the fungal suspension. Four fungal strains Candida albicans, Aspergillus niger, Aspergillus flavus, and Penicillium chrysogenum were used. Amphotericin-B, and fluconazole were included as positive controls. Samples, and positive control of 20 µl each were added in sterile discs and placed in SDA plates. The plates were incubated at 37ºC for 24 hrs. Then antifungal activity was determined by measuring the diameter of zone of inhibition.
Cell Culture
The Michigan Cancer Foundation – 7 (MCF7) cells were maintained in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% Fetal Bovine Serum (10%FBS), Penicillin (100 U/ml), and Streptomycin (100µg/ml) in a humidified atmosphere of 50µg/ml CO2 at 37oC.
Cytotoxicity Assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay for determining the cytotoxicity of the two drugs were performed according to the method explained by Mossmann [17]. Briefly, cells (1 × 105/well) were plated in 24-well plates, and incubated at 370C with 5% CO2 atmosphere. Upon reaching confluence the cells were washed in PBS and the medium was changed. Marigold ethanolic extract and 5-Flurouracil were diluted serially. Increasing concentrations of both the drugs obtained through serial dilution were added to the different wells, and marked respectively. One of the wells was treated only with diluent which served as negative control. Both cell control and drug control were included in each assay. The culture plates were incubated for 24 hrs at 370C with 5% CO2 atmosphere. After incubation, the medium was removed from all the wells, and the cells were washed with phosphate-buffered saline (pH 7.4). 100µl/well (5mg/ml) of 0.5% 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide was added to each well and the cell plate was incubated for 4 hours. After incubation, 1 ml of DMSO was added in all the wells. This helped in dissolving the insoluble crystalline formazan product for effective absorbance measurement. The absorbance at 570 nm was measured with UV-visible Spectrophotometer using DMSO as the blank, and the results were tabulated.
Statistical Analysis
The observed results were interpreted based on comparing the zone of inhibition (in mm) values for petal extract and standard drugs for antifungal activity. The anticancer activity was assessed by graphically presenting the concentration of petal extract or standard drug at X-axis and percentage cell viability at Y-axis to calculate half-maximal inhibitory concentration (IC50) value. No specific statistical tests were used for analyzing the observed values.
Results
The Zone of Inhibition (ZI) of extract and standard drugs against four common pathogenic fungal species are shown in Table 1 and figure 1. The Zone of Inhibition (ZOI) was observed at three different concentrations of 1000µg/ml, 750µg/ml and 500µg/ml for the extract and at 1mg/ml for the two standard drugs, Amphotericin B and Fluconazole. The ZOI values which represent antifungal activity were higher for petal extract at all three concentrations compared to amphotericin B, fluconazole against Candida albicans, Aspergillus flavus, and Penicillium chrysogenum fungi strains. The ZOI value against Aspergillus niger was better than standard drugs (18mm Vs 14mm) only at the concentration of 1000µg/ml.
Table 1: Comparing antifungal activity of ethanolic extract with amphotericin-B and fluconazole as controls.
| Fungal strains | Tagetes erecta petal extract | Amphotericin-B | Fluconazole | ||
| 1000 µg/ml
ZOI (mm) |
750 µg/ml
ZOI (mm) |
500 µg/ml
ZOI (mm) |
1 mg/ml | 1 mg/ml | |
| Candida albicans | 15 | 12 | 11 | 9 | 7 |
| Aspergillus niger | 18 | 14 | 14 | 14 | 14 |
| Aspergillus flavus | 21 | 21 | 20 | 12 | 12 |
| Penicillium chrysogenum | 18 | 18 | 18 | 9 | 10 |
ZOI – Zone of Inhibition measured in millimeter, and graded concentrations of plant petal extract were compared with two standard antifungal drugs.
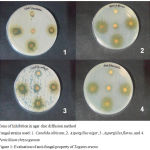 |
Figure 1: Evaluation of anti-fungal property of Tagetes erecta.
|
The Optical density (O.D) or absorbance was determined using ultra violet spectrophotometer for both test (Marigold ethanolic extract) [Table 2, Figure 2] and standard drug (5-fluorouracil) [Table 3, Figure 3] at serial dilutions of 1:1 to 1:64. Based on the optical density the percentage cell viability was calculated. Based on the observed values were plotted in graph and IC50 was determined. IC 50 for the T.erecta ethanolic extract was found to be 125µg/ml and for the standard drug it was around 15.6µg/ml.
Table 2: MTT assay and resultant cell viability with ethanolic extract of Tagetes erecta petals.
| S.no | Concentration (µg/ml) | Dilutions | Absorbance (O.D) | Cell viability (%) |
| 1 | 1000 | neat | 0.261 | 26.71 |
| 2 | 500 | 1:1 | 0.338 | 34.59 |
| 3 | 250 | 1:2 | 0.418 | 42.78 |
| 4 | 125 | 1:4 | 0.495 | 50.66 |
| 5 | 62.5 | 1:8 | 0.570 | 58.34 |
| 6 | 31.2 | 1:16 | 0.650 | 66.53 |
| 7 | 15.6 | 1:32 | 0.730 | 74.71 |
| 8 | 7.8 | 1:64 | 0.810 | 82.90 |
| 9 | Control | – | 0.977 | 100 |
O. D – Optical Density, %-percentage, Percentage cell viability of graded concentrations of plant petal extract.
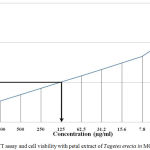 |
Figure 2: MTT assay and cell viability with petal extract of Tagetes erecta in MCF-7 cell line.
|
Table 3: MTT assay and resultant cell viability with positive control 5-Fluorouracil.
| S.no | Concentration (µg/ml) | Dilutions | Absorbance (O.D) | Cell viability (%) |
| 1 | 1000 | Neat | 0.089 | 959 |
| 2 | 500 | 1:1 | 0.155 | 16.70 |
| 3 | 250 | 1:2 | 0.219 | 23.59 |
| 4 | 125 | 1:4 | 0.285 | 30.71 |
| 5 | 62.5 | 1:8 | 0.352 | 37.93 |
| 6 | 31.2 | 1:16 | 0.414 | 44.61 |
| 7 | 15.6 | 1:32 | 0.481 | 51.83 |
| 8 | 7.8 | 1:64 | 0.544 | 58.62 |
| 9 | Cell control | – | 0.928 | 100 |
O.D – Optical Density, % – percentage, Percentage cell viability of graded concentrations of positive control 5-Fluorouracil.
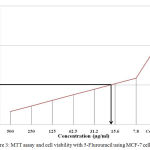 |
Figure 3: MTT assay and cell viability with 5-Flurouracil using MCF-7 cell line.
|
Discussion
The therapeutic effects of Tagetes erecta (petals) were extensively studied in literature. Indeed, it is reported to have broad pharmacological spectrum possessing anti-bacterial, anti-oxidant, hepato-protective, wound healing activity, and analgesic properties.18 Flowers of many plants were observed to have antifungal activity like, Cassia fistula L. flower against Candida, Aspergillus, and grass of Spinifex littoreus plant against Candida, Aspergillus, as well as Pencillium species.19,20 Since the incidence of adverse effects to antifungal drugs is found to be rising, it brings the need for exploring newer agents to target the fungal infections.21 In addition, there is a rising concern over resistance to multiple antifungal agents worldwide; it is of great importance to find effective treatment to overcome the same. Increased resistance is observed against Azole antifungals among Candida Albicans, and Non Candida Albicans species.22 Resistance in Aspergillus species against Azoles have been reported.23 The search for novel and effective substances from traditional medicine can benefit the future generations to effectively manage fungal infections. Mechanisms behind potential substances from traditional medicines can be explained by the presence of terpene / terpenoid compounds which have expressed principal effects on fungal cell wall, cell growth, fungal mitochondria, as well as inhibition on biofilm development.24 Hence we conducted this study to explore the potential of extract from petals of Tagetes erecta as an antifungal agent against the most common pathogenic fungi such as Candida albicans, Aspergillus niger, Aspergillus flavus, and Penicillium chrysogenum. Highest zone of inhibition which represents the antifungal activity was observed against Aspergillus flavus for Tagetes erecta extract followed by Penicillium chrysogenum and Aspergillus niger. Diamater of zonal inhibition was relatively less against Candida albicans. Compared to the two drug controls the ZOI diameter was better with the petal extract against all the four strains.
Antifungal activity of the extract was assessed by comparing the diameter of ZOI in agar diffusion method. Amphotericin B, a fungicidal agent and fluconazole, an azole antifungal which is primarily fungistatic were used as controls. The diffusion method was selected as initial screening tool because it is simple and economical. The possible mechanism of antifungal activity has to be explored further, as well as measuring minimum inhibitory concentration (MIC), minimum fungicidal concentration (MFC) of the extract by serial dilution method would take this compound further as a lead compound.25
Plants from asteraceae family were found to possess fungicidal activity against pathogenic fungi of humans as well as plants. The possible mechanism of action of Tagetes erecta extract as botanical fungicide against Fusarium was explained to be due to inhibition of fungal cell wall synthesis.26 Tagetes patula, a plant closely related to T. erecta was found to be active against pathogenic fungi including candida, aspergillus, and trichophyton. Significant antifungal activity was appreciated by using disc diffusion method with patula flower extract, and petroleum ether extract from aerial parts as well as roots.27
Medicinal plants play a vital role in anticancer drug development. Many plants like Allium sativum, Ginkgo biloba, Withania somnifera, Zingiber officinale with their active ingredients have potent anticancer activity against lymphomas, breast, ovarian, lung, liver, stomach, prostate and testicular cancers.28 Flowers of plants in specific are explored to have cytotoxic effect against cancers including hepatic carcinoma. Extract from Bauhinia tomentosa flowers were observed to express anticancer effect against HePG2 Cell lines using MTT assay.29 Leaves of the species Ageratum conyzoides L. which belongs to the same family as Tagetes erecta (Asteraceae) were found to have anticancer activity against human breast carcinoma cell line (MDA-MB-231), human prostate carcinoma cell line (DU-145), and human hepatic carcinoma cell line (BEL-7402).30 MTT assay using MCF-7 cell line for breast carcinoma suggested cytotoxic activity Marrubium persicum extract.31 Hence, natural products can serve as successful leads in novel anticancer drug discovery. Active ingredients like polyphenolic compounds including flavonoids, tannins, curcumin, are responsible for the potent anticancer activity in plants which alter the regulation of signal transducer, transcription proteins, and also inhibit NF-KB, needed for cancer cells survival and angiogenesis.32 Rising concerns over occurrence of drug resistant cancers and safety concerns with the available anticancer medications are emphasizing the need to explore safer and more effective novel anticancer drugs.33 In the present study MCF-7 cell line for breast carcinoma was used, which represents one of the common cancers in India, and all over the world. The present study revealed good anticancer activity of T.erecta petal extract using MTT assay in MCF-7 cell line for breast carcinoma which was compared with standard drug 5-Fluorouracil.
MTT assay is a widely used colorimetric assay in screening of compounds with cytotoxic potential, since it is easy, rapid, economical, and reliable as early screening tool. Ability of the NADPH dependent oxido-reductase enzyme present in the viable cells to convert tetrazolium to colored formazan product forms the basis of this assay. This reducing enzyme is majorly present in mitochondria and also seen in cytosol, lysosome, and plasma membrane.34 But the assay has its own limitations, such as interference with other compounds, and it measures only the metabolic activity of the cell not the actual number of viable cells.35
The standard drug, 5-Fluorouracil is an antimetabolite anticancer drug commonly used in the treatment of solid cancers. The drug acts by inhibiting thymidylate synthase causing DNA and RNA damage, hence resulting in cell death.36 The possible mechanism of 5-FU in causing mitochondrial apoptosis and dysfunction has also been discussed in scientific literature.37,38 5-FU is widely used as standard drug in in-vitro studies using MCF7 cell line, and it is preferred over capecitabine because of its more potent action.39
The bioactive constituents of T. erecta flowers were found to be falavonoids such as quercetin, quercetagetin, and 6-hydroxykaempferol. These compounds were demonstrated to have growth inhibitory and cytotoxicity action against human liver cancer cell lines (HepG2) and lung cancer cell lines (A549).40 The related flower T. patula (French marigold) has displayed significant cytotoxic, growth inhibitory and free radical scavenging properties with major contribution from the components such as patuletin, patulitrin. The anticancer properties were demonstrated using HeLa cell lines.41
Conclusion
Our study has indicated the potential of Tagetes erecta petal extract to be a lead compound to be further evaluated in the management of fungal infections and breast cancer. We have assessed simple screening methods to get an initial idea about the flower extract’s activity. Further in-vitro assays are required to confirm this observation, and take it forward to do in-vivo animal studies to verify its’ possible practical use. Active chemical constituents of the plant possessing antifungal and anticancer properties have to be further explored. Undertaking cell cycle analysis can be helpful to identify the distinct anticancer mechanism of action of Tagetes erecta.
Acknowledgements
The author(s) received no specific funding to acknowledge for this research work.
Conflicts of Interest
There is no conflict of interest.
References
- Gilman EF, Howe T. Tagetes erecta. Fact Sheet FPS-569. Gainesville: Institute of Food and Agricultural Science, University of Florida. 1999 Oct.
- Krueger R, Dover KE, McSorley R, Wang KH. Marigolds (Tagetes spp.) for nematode management. ENY-056 (NG045), Entomology & Nematology Department, Florida Cooperative Extension Service, University of Florida, Gainesville, FL. 2010.
- Marques MM, Morais SM, Vieira ÍG, Vieira MG, Silva AR, De Almeida RR, Guedes MI. Larvicidal activity of Tagetes erecta against Aedes aegypti. Journal of the American Mosquito Control Association. 2011 Jun;27(2):156-9.
- Shetty LJ, Sakr FM, Al-Obaidy K, Patel MJ, Shareef H. A brief review on medicinal plant Tagetes erecta Linn. Journal of Applied Pharmaceutical Science. 2015;5(3):091-5.
- Vallabhaneni S, Mody RK, Walker T, Chiller T. The global burden of fungal diseases. Infectious Disease Clinics. 2016 Mar 1;30(1):1-1.
- Di Santo R. Natural products as antifungal agents against clinically relevant pathogens. Natural product reports. 2010;27(7):1084-98.
- Murtaza G, Mukhtar M, Sarfraz A. A review: Antifungal potentials of medicinal plants. Journal of Bioresource Management. 2015;2(2):4.
- Rathod M, Das NA, Dhale D. Antifungal activity of two medicinal plants against fungus Candida albicans. Int J Pharma Bio Science. 2015;6:701-6.
- Gazim ZC, Rezende CM, Fraga SR, Svidzinski TI, Cortez DA. Antifungal activity of the essential oil from Calendula officinalis L.(Asteraceae) growing in Brazil. Brazilian Journal of Microbiology. 2008 Mar;39(1):61-3.
- Faustino MV, Pinto DC, Gonçalves MJ, Salgueiro L, Silveira P, Silva AM. Calendula L. species polyphenolic profile and in vitro antifungal activity. Journal of Functional Foods. 2018 Jun 30;45:254-67.
- Mathew A, George PS, KM JK, Vasudevan D, James FV. Transition of cancer in populations in India. Cancer epidemiology. 2019 Feb 1;58:111-20.
- Shukla S, Mehta A. Anticancer potential of medicinal plants and their phytochemicals: a review. Brazilian Journal of Botany. 2015 Jun 1;38(2):199-210.
- Imran M, Ullah A, Saeed F, Nadeem M, Arshad MU, Suleria HA. Cucurmin, anticancer, & antitumor perspectives: A comprehensive review. Critical reviews in food science and nutrition. 2018 May 24;58(8):1271-93.
- Chaudhary S, Chandrashekar KS, Pai KS, Setty MM, Devkar RA, Reddy ND, Shoja MH. Evaluation of antioxidant and anticancer activity of extract and fractions of Nardostachys jatamansi DC in breast carcinoma. BMC complementary and alternative medicine. 2015 Dec;15(1):50.
- Lu H, Yang S, Ma H, Han Z, Zhang Y. Bioassay-guided separation and identification of anticancer compounds in Tagetes erecta L. flowers. Analytical Methods. 2016;8(15):3255-62.
- Redfern J, Kinninmonth M, Burdass D, Verran J. Using soxhlet ethanol extraction to produce and test plant material (essential oils) for their antimicrobial properties. Journal of microbiology & biology education. 2014 May;15(1):45.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 1983 Dec 16;65(1-2):55-63.
- Gopi G, Elumalai A, Jayasri P. A concise review on Tagetes erecta. International Journal of Phytopharmacy Research. 2012;3(1):16-9.
- Panda SK, Brahma S, Dutta SK. Selective antifungal action of crude extracts of Cassia fistula L.: A preliminary study on Candida and Aspergillus species. Malaysian J Microbiol. 2010 Jan 1;6(1):62-8.
- Thirunavukkarasu P, Ramanathan T, Ramkumar L, Balasubramanian T. Anti microbial effect of a coastal sand dune plant of Spinifex littoreus (Burm. f.) Merr. Current Research Journal of Biological Sciences. 2010 Jul 20;2(4):283-5.
- Chen SC, Sorrell TC. Antifungal agents. Medical Journal of Australia. 2007 Oct 1;187(7):404.
- Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Frontiers in microbiology. 2017 Jan 12;7:2173.
- Shishodia SK, Tiwari S, Shankar J. Resistance mechanism and proteins in Aspergillus species against antifungal agents. Mycology. 2019 Feb 8:1-5.
- Nazzaro F, Fratianni F, Coppola R, Feo VD. Essential oils and antifungal activity. Pharmaceuticals. 2017 Dec;10(4):86.
- Liliana S, Tatiane B, Fusco AAM, Siqueira SDH, Silva BV, Mendes GMJS. The use of standard methodology for determination of antifungal activity of natural products against medical yeasts Candida sp and Cryptococcus sp. Braz. J. Microbiol. 2007: 38(3), 391-7. https://dx.doi.org/10.1590/S1517-83822007000300001.
- Du R, Liu J, Sun P, Li H, Wang J. Inhibitory effect and mechanism of Tagetes erecta L. fungicide on Fusarium oxysporum f. sp. niveum. Sci Rep. 2017 Oct 31;7(1):14442.
- Faizi S, Siddiqi H, Bano S, Naz A, Lubna, Mazhar K, et al. Antibacterial and antifungal activities of different parts of Tagetes patula.: Preparation of patuletin derivatives, Pharm. Biol. 2008: 46:5: 309-20
- Shaikh AM, Shrivastava B, Apte KG, Navale SD. Medicinal plants as potential source of anticancer agents: a review. Journal of Pharmacognosy and Phytochemistry. 2016 Mar 1;5(2):291.
- Solomon S, Muruganantham N, Senthamilselvi MM. Anti-cancer activity of Bauhinia tomentosa (Flowers) against human liver cancer. Journal of Pharmacognosy and Phytochemistry. 2016 Jan 1;5(1):287.
- Adebayo, A. H., Tan, N. H., Akindahunsi, A. A., Zeng, G. Z., & Zhang, Y. M. (2010). Anticancer and antiradical scavenging activity of Ageratum conyzoides L. (Asteraceae). Pharmacognosy magazine, 6(21), 62-6.
- Hamedeyazdan S, Fathiazad F, Sharifi S, Nazemiyeh H. Antiproliferative activity of Marrubium persicum extract in the MCF-7 human breast cancer cell line. Asian Pacific Journal of Cancer Prevention. 2012;13(11):5843-8.
- Greenwell M, Rahman PK. Medicinal plants: their use in anticancer treatment. International journal of pharmaceutical sciences and research. 2015 Oct 1;6(10):4103.
- Wahlang JB, Laishram PD, Brahma DK, Sarkar C, Lahon J, Nongkynrih BS. Adverse drug reactions due to cancer chemotherapy in a tertiary care teaching hospital. Therapeutic advances in drug safety. 2017 Feb;8(2):61-6.
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol. Annu. Rev. 2005;11:127-52.
- van Tonder A, Joubert AM, Cromarty AD. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes. 2015 Feb 20;8:47.
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003 May;3(5):330-8.
- Pagliara V, Saide A, Mitidieri E, d’Emmanuele di Villa Bianca R, Sorrentino R, Russo G, Russo A. 5-FU targets rpL3 to induce mitochondrial apoptosis via cystathionine-β-synthase in colon cancer cells lacking p53. Oncotarget. 2016 Aug 2;7(31):50333-48.
- Sommer J, Mahli A, Freese K, Schiergens TS, Kuecuekoktay FS, Teufel A, Thasler WE, Müller M, Bosserhoff AK, Hellerbrand C. Analysis of molecular mechanisms of 5-fluorouracil-induced steatosis and inflammation in vitro and in mice. Oncotarget. 2017 Feb 21;8(8):13059-72.
- Akbari R, Javar HA. Efficacy of Capecitabine and 5-Fluorouracil (5-FU) on the human breast cancer cell line (MCF7)–effect of concentration. Am. J. Res. Commun. 2013;1(16):75-91.
- Lu H, Yang S, Ma H, Han Z, Zhang Y. Bioassay-guided separation and identification of anticancer compounds in Tagetes erecta L. flowers. Anal. Methods. 2016;8(15):3255–62.
- Kashif M, Bano S, Naqvi S, Faizi S, Lubna, Ahmed Mesaik M, et al. Cytotoxic and antioxidant properties of phenolic compounds from Tagetes patula flower. Pharm Biol. 2015 May 4;53(5):672–81.







