Worawat Songjang1 and Arunya Jiraviriyakul*1,2
and Arunya Jiraviriyakul*1,2
1Biomedical Science Graduated School, Faculty of Allied Health Sciences, Naresuan University, Phitsanulok 65000, Thailand.
2Department of Medical technology, Faculty of Allied Health Sciences, Naresuan University, Phitsanulok 65000, Thailand.
Corresponding Author E-mail: arunyaj@nu.ac.th
DOI : https://dx.doi.org/10.13005/bpj/1698
Abstract
Cholangiocarcinoma (CCA) is biliary tract malignancy. Because no specific biomarkers are available, CCA patients frequently present with disseminated tumour that is too late for curative treatment, leading to a high mortality rate. Honokiol and magnolol are the hydroxylated biphenyl compounds isolated from Magnolia officinalis. Many studies have reported that honokiol and magnolol have antitumour effects on various types of cancer, but the evidence of the effects of these compounds on CCA cells has not yet been reported. This study therefore aims to evaluate the antitumour activities of honokiol and magnolol on CCA cell lines. The CCA cell lines were incubated with honokiol and magnolol before determining their responses. The results indicate that low concentrations of honokiol and magnolol suppressed CCA proliferation by induction of cell cycle arrest at G0/G1 and down-regulation of cyclin D1 protein. Moreover, these compounds exhibited an antimetastasis ability mediated by inhibiting migration, adhesion, and the MMP activities of CCA cells. In addition, at high concentrations of honokiol and magnolol activated CCA cell death associated with the apoptosis signalling pathway, along either an intrinsic or extrinsic pathway. Our data provides evidence that honokiol and magnolol have potential anticancer properties and are promising compounds for alternative CCA treatment.
Keywords
Apoptosis; Cholangiocarcinoma; Honokiol; Magnolol; Metastasis; Proliferation
Download this article as:| Copy the following to cite this article: Songjang W, Jiraviriyakul A. Honokiol and Magnolol Inhibit Growth, Metastasis and Induce Apoptosis in Human Cholangiocarcinoma. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Songjang W, Jiraviriyakul A. Honokiol and Magnolol Inhibit Growth, Metastasis and Induce Apoptosis in Human Cholangiocarcinoma. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2X3OViW |
Introduction
Cholangiocarcinoma (CCA) is an epithelial malignancy which arises from cholangiocyte within either the intrahepatic or extrahepatic biliary tracts. CCA accounts for 3% of all gastrointestinal cancers and is the second most common primary hepatic tumour1. It is widely accepted that CCA development is strongly associated with liver fluke (Opisthorchis viverrini) infections, especially in the northeast of Thailand which has the highest incidence of CCA in the world2. CCA is asymptomatic in early stages of the disease, and the lack of specific biomarkers for clinical diagnosis are the major problems facing patients who frequently present metastasized tumours which are inappropriate for surgery. Furthermore, palliative treatment by chemotherapy is generally unsuccessful because of the extremely chemoresistant ability of this cancer, leading to poor prognoses and high mortality rates3. It is therefore imperative that new therapeutic agents against CCA are identified.
Honokiol (5,3′-Diallyl-2,4′-dihydroxybiphenyl) and magnolol (5,5′-Diallyl-2,2′-biphenyldiol) are bioactive natural compounds extracted from the bark, seed cones, and leaves of Magnolia officinalis. Magnolia is widely cultivated in Asian counties such as China, Japan and Korea, and has been used in traditional Chinese herbal medicine. Honokiol and magnolol have isomer structures, and the difference between them is only the position of one hydroxyl group. Previous studies report that these compounds showed multiple pharmacological proproteins including a neuroprotective effect4, an antithrombotic effect5, an antiviral effect6, a metabolic effect7, anti-inflammatory effects8, anti-oxidant effects9, as well as antitumour effects10. Previous reports also indicate that honokiol and magnolol show antitumour activities in various cancer cell lines such as in colon cancer11,12, lung cancer13,14, breast cancer15,16, prostate cancer17,18, gastric cancer19,20, ovarian cancer21, 22, lymphoma23,24, thyroid cancer25,26, glioblastoma27,28, melanoma29 and liver cancer30. The key mechanisms of low concentrations of these compounds on cancer cell are the antiproliferation and suppression metastasis ability of cancer cells, whereas high concentrations of honokiol and magnolol can induce cell death through cell apoptosis10. Therefore, honokiol and magnolol are potential antitumour agents. However, the mechanisms of these compounds in human CCA, especially in Thai liver fluke-related CCA, remain unknown.
For the first time, this study reports the antitumour activities of honokiol and magnolol on Opisthorchis viverrini– associated human CCA cell lines, KKU-100 and KKU-213L5. We demonstrate that these compounds inhibit cell proliferation as well as suppress the cancer cell metastasis ability. Moreover, the induction of cell apoptosis and their mechanisms related to signalling pathways were also investigated. Our data indicates that honokiol and magnolol are potential therapeutic agents for CCA patients.
Materials and Method
Honokiol and magnolol powders were purchased from Sigma-Aldrich (St. Louis, MO, USA) which is HPLC grade with ≥98% purity. The honokiol and magnolol powder was reconstituted with DMSO and ethanol, respectively.
Cell Culture
Poorly and well differentiated human CCA cell lines, KKU-100 and KKU-213 were obtained from the Japanese Collection of Research Bioresources (JCBR) Cell Bank (Osaka, Japan) 31. The immortalized cholangiocyte, MMNK1 cell line was a gift from Prof. Kobayashi N. Cell lines were maintained according to international guidelines on good cell culture practice in Dulbecco’s modified Eagle’s medium (DMEM) at 37°C humidified atmosphere with 5% CO2, supplemented with 5% fetal bovine serum, 100 units/mL of penicillin, 100 µg/mL of streptomycin, and 0.25 µg/mL of amphotericin B.
Cell Cytotoxicity
The cell cytotoxicity was carried out by MTT assay. Briefly, CCA cell lines were seed at density 5×103 cells per well in 96-well plate. After cultivation for 12 h, honokiol and magnolol were added in different concentrations and incubated for 24 and 48 h. Subsequently, 0.5 mg/mL of MTT reagent was added and incubated for another 4 h. The formazan product was dissolved by DMSO and measured light absorbent by microplate spectrophotometer at 540 nm. The % cell viability was calculated following the formula ((honokiol or magnolol treated Abs540)/(control Abs540)) × 100 (%).
Colony Formation
CCA cell lines were treated with different concentrations of honokiol and magnolol for 24 h. After that, treated cells were counted and seeded into 6-will plate at 1×103 cells per well and continually cultured for 10 days. Cell culture medium was replaced with fresh medium every 3 days. The cell colonies were fixed with methanol and stained with 0.5% crystal violet for 30 minutes. Colony counting was performed under inverted microscope, only if they contain more than 50 cells per colony. The % colony formation was calculated following equation ((number colony of honokiol or magnolol treated cells/1000)/(number colony of control/1000) × 100 (%).
Cell Cycle Analysis
Cell cycle analysis was performed utilizing MuseTM Cell Analyzer from Millipore (MA, USA) following manufacturer’s instruction. Briefly, after incubated with different concentrations of honokiol and magnolol for indicated time, treated cells were washed with PBS before fixing with 70% ethanol for 3 h. Then, cells were stained with propidium iodide (PI) and processed for cell cycle analysis.
Apoptosis Analysis
Annexin V & Dead Cell and Caspase 3/7 expression assay were performed utilizing MuseTM Cell Analyzer from Millipore (MA, USA) following manufacturer’s instruction. Briefly, after exposed with different concentrations of honokiol and magnolol for indicated time, treated cell were incubated with Annexin V and Dead Cell Reagent (7-AAD), and MuseTM Caspase-3/7 reagent before analyzing for Annexin V/PI staining and Caspase 3/7 expression, respectively. The results were representing by event of live cell, apoptotic cell and dead cell.
Western Blot Analysis
After incubating CCA cell lines with indicated treatment, treated cell were washed with ice-cold PBS before lysing with RIPA lysis buffer plus protease inhibitor cocktail. Then, protein lysate was collected by centrifugation and qualified the concentration by using Bradford assay. The protein was then separate d by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto PVDF membranes. After that, membranes were blocked the non-specific binding by 5% skim milk buffer for 1 h before washing with TBST buffer. The membranes were then incubated with each primary antibody, anti-caspase 3, anti-caspase 8, anti-caspase 9 and anti-cyclin D1 (antibodies were purchased from Cell Signaling (MA, USA)) with gentle shaking at 4°C overnight. Then, membranes were washed with TBST and incubated with HRP-linked anti-rabbit antibody (Cell Signaling, MA, USA)) for 1 h at room temperature, and washed again before incubating with detection reagent. The image was developed by ChimidocTM XRS and analyzed by Image Lab (Bio-Rad, CA, USA).
Cell Adhesion Assay
CCA cell lines 2×104 cells were pretreated with honokiol or magnolol for 30 minutes before seeding onto 96-well plate precoated with Matrigel, incubated for 15-40 minutes (depending on cell type) at 37°C humidified atmosphere with 5% CO2. The unattracted cells were removed by washing with PBS for 3 times before staining with 0.5% crystal violet in 20% methanol for 30 minutes. Attracted cells were randomly photographed under microscopy at 10X objective. After that, crystal violet was eluted by 70% ethanol and measured light absorbent by microplate spectrophotometer at 570 nm. The % cell adhesion was calculated following the formula ((honokiol or magnolol treated Abs570)/(control Abs570)) × 100 (%).
Cell Scratch Assay
CCA cell lines were seeded onto 6-well plate and incubated at 37°C humidified atmosphere with 5% CO2 until 90-100% confluent. Then, cells were scratched by using 200 µL pipette tip before washing with PBS. Culture medium was replaced by complete medium containing honokiol or magnolol. After that, the scratching wound was monitoring photographed under microscope at 10X objective for desired time interval. Scratching wound area was measured using ImageJ program and calculated % migration following equation ((area at 0 h – area at x h)/(area at 0 h) × 100 (%).
Gelatin Zymography
CCA cell lines were seeded onto cell culture dish and incubated at 37°C humidified atmosphere with 5% CO2 until 80% confluent. Cell culture media was removed and washed with PBS for 3 times before replacing with serum-free medium containing honokiol or magnolol in different concentration. After continually cultured for 24 h, conditioned medium was collected, for KKU-213L5, conditioned medium was concentrated using Amicon® Ultra-2 Centrifugal Filter units (Millipore MA, USA) following manufacturer’s instruction for 10x final concentration. The conditioned medium was then mixed with sample loading buffer before subjecting to 10% SDS-PAGE containing 1% gelatin. Then, the gel was washed with washing buffer (pH 7.5, 2.5% Triton X-100) for 30 minutes, and incubated in developing buffer (pH 7.5, 1% Triton X-100, 50 mM Tri-HCl, 5 mM CaCl2 and 1 µM ZnCl2) overnight at 37°C. Gel was then stained with 0.5% Coomassie blue R-250 in 45% methanol, 10% acetic acid, and destained with 45% methanol, 10% acetic acid until clear bands. The gelatinolytic activity of MMPs was visualized by gel photographed using ChimidocTM XRS (Bio-rad®).
Statistical Analysis
All values were presented as mean ± SD. Significant differences between groups were assessed using one-way or two-way analysis of variances (ANOVA), followed by the Tukey-Kramer and Dunnett’s test when appropriate. The statistical testing was performed using the GraphPad Prism version 7. At ρ-value less than 0.05 were considered as statistically significant.
Results
Honokiol and Magnolol Inhibited Cancer Cell Proliferation in CCA Cells
To determine the inhibition effect on CCA cell line KKU-100 and KKU-213L5 were incubated with honokiol and magnolol before measuring the cell viability using MTT assay. The results indicated that these compounds significantly suppressed the proliferation ability of KKU-100 and KKU-213L5 in a dose- and time-dependent manner (Figure 1A). The IC50 of honokiol with KKU-100 at 24 and 48 h were 48.82 and 28.93 µM, and with KKU-213L5 were 49.99 and 26.31 µM (Table 1), respectively. Whereas, the IC50 of magnolol with KKU-100 at 24 and 48 h were 72.86 and 34.2 µM, and with KKU-213L5 were 69.51 and 50.64 µM (Table 1), respectively. Moreover, we also studied the cytotoxicity of these compounds on immortalized human cholangiocyte. Interestingly, the IC50 of honokiol and magnolol on treated MMNK-1 cell line was higher than the CCA cell lines (Table 1), which suggests that these compounds showed less cytotoxicity in human cholangiocyte. Furthermore, long term antiproliferation of honokiol and magnolol was confirmed by colony formation assay. Our data showed that concentrations less than IC50 significantly suppressed the colony formation rates of both CCA cell lines compared with the control group (Figure 1B-C).
Table 1: IC50 of honokiol and magnolol on cholangiocyte and CCA cell lines.
| Cell lines | Honokiol (µM) | Magnolol (µM) | ||
| 24 hours | 48 hours | 24 hours | 48 hours | |
| MMNK1 | 54.23 | 29.72 | 75.46 | 54.19 |
| KKU-100 | 48.82 | 28.93 | 72.86 | 34.2 |
| KKU-213L5 | 49.99 | 26.31 | 69.51 | 50.64 |
CCA cell lines were treated with honokiol and magnolol for 24 and 48 h, cell viability was carried out by MTT assay, and the IC50 was calculated using GraphPad Prism version 7.
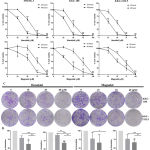 |
Figure 1: The antiproliferative effect of honokiol and magnolol on CCA cells.
|
(A) Chemical structure of honokiol and magnolol. (B) MMNK1, KKU-100 and KKU-213L5 were treated with honokiol and magnolol following indicated concentration, after 24 and 48 h, cell viability was determined using MTT assay. Cells were treated with DMSO and ethanol as negative control, and all groups were normalized with control group. The results represented as mean ± SD from three independent experiments. (C) Honokiol and magnolol suppressed colony formation ability of CCA cells. Treated KKU-100 and KKU-213L5 were cultured for 10 days to form colonies. The colonies were counted under microscope (×40), and quantified to % colony formation (D), all groups were normalized with control group. The results represented as mean ± SD from three independent experiments. A ρ-value < 0.05 was considered statistically significant; ρ -value < 0.05 (*), ρ -value < 0.01 (**) and ρ -value < 0.001 (***).
Honokiol and Magnolol Enhanced Cell Cycle Arrest in CCA Cell
To study the underlying antiproliferation mechanism of honokiol and magnolol, cell cycle analysis and the protein expression of treated CCA cell lines were investigated. Treatment of honokiol at 20 to 40 µM and magnolol at 40 to 60 µM significantly increased cell populations at G0/G1 phase, but depleted the cell populations at the S and G2/M phases (Figure 2A-D). The results indicate that both compounds arrested the cell cycle of KKU-100 and KKU-213L5 at the G0/G1 phase in a dose-dependent manner. Additionally, the same concentrations of honokiol and magnolol markedly decreased the expression of cyclin D1 in a dose-dependent manner (Figure 2E). These results suggest that these compounds suppressed CCA cell line proliferation mediated by cyclin D1 degradation and were the cause of cell cycle arrest.
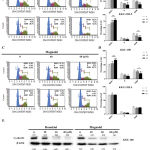 |
Figure 2: Honokiol and magnolol inhibited cell proliferation mediated by arresting cell cycle and cyclin D1 degradation.
|
(A) CCA cells were incubated with honokiol and magnolol for 24 h and cell cycle was analyzed using MuseTM Cell Analyzer (A, C), the percentage of G0/1, S and G2/M phase cell distribution was quantified (B, D). The results represented as mean ± SD from three independent experiments. A ρ-value < 0.05 was considered to significant; ρ -value < 0.05 (*), ρ -value < 0.01 (**) and ρ-value < 0.001 (***). (B) Honokiol and magnolol down-regulate the expression of cyclin D1. CCA cells were treated with honokiol and magnolol as indicated dose for 24 h, and the expression of cyclin D1 was evaluated by Western blot analysis. Indicating values showed protein expression normalized to β-actin.
Honokiol and Magnolol Induced CCA Cell Apoptosis
Previous studies indicate that honokiol and magnolol proficiently induced cancer cell apoptosis. To prove this hypothesis, CCA cells were incubated with honokiol and magnolol for 24 h before evaluating cell apoptosis using annexin V/PI staining. We found that 50 to 70 µM of honokiol significantly induced apoptosis in KKU-100 and KKU-213L5. Magnolol at 60 to 80 µM was found to significantly enhance apoptosis in both CCA cells (Figure 3A). These findings indicate that honokiol and magnolol exhibited apoptosis induction on CCA cells in a dose-dependent manner. Moreover, the induction of cell apoptosis was proved by caspase-3/-7 activation testing, which is the executioner enzyme for apoptosis induction. At the same concentration of the compounds, the results showed that caspase-3/-7 activity increased when incubated with honokiol and magnolol for 24 and 36 h in a dose-dependent manner (Figure 3B), suggesting that honokiol and magnolol potentially induced CCA cell apoptosis. Interestingly, these results indicate that KKU-100 and KKU-213L5 were more susceptible to apoptosis with honokiol than magnolol.
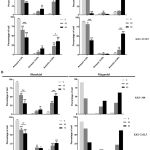 |
Figure 3: The induction of cell apoptosis of honokiol and magnolol on CCA cells.
|
(A) CCA cells were treated with honokiol and magnolol for 24 h before analyzing with Muse™ Cell Analyzer. Annexin V versus PI from the gated cell show the cell population as percentage of live, early apoptotic, late apoptotic/dead, and dead cell. (B) Treated CCA cells were stained with caspase-3/7 reagent kit and 7-AAD before analyzing with Muse™ Cell Analyzer. Caspase-3/-7 versus 7-AAD from the gated cell show the cell population as percentage of live, apoptotic, late apoptotic/dead, and dead cell. The results represented as mean ± SD from three independent experiments, except the activity of caspase-3/-7 experiment with magnolol treatment that represented data. A ρ-value < 0.05 was considered to significant; ρ -value < 0.05 (*), ρ -value < 0.01 (**) and ρ -value < 0.001 (***).
Honokiol and Magnolol Induced Apoptosis Mediated by Either Mitochondria-Dependent or Mitochondria-Independent Pathways
CCA cell lines confirmed the induction of cell apoptosis of honokiol and magnolol. Cells were treated with honokiol or magnolol at high concentrations (100 µM) for different incubation durations before determining the expression of caspase-3 by Western blot analysis. The biological compound treatment with KKU-100 and KKU-213L5 resulted in time-dependent increases of cleaved caspase-3 (17 and 19 kDa), beginning at 4 h for honokiol, and 8 h for magnolol (Figure 4A). Additionally, to elucidate the underlying mechanism of honokiol and magnolol-mediated apoptosis, the expression levels of caspase-8 and caspase-9 were also evaluated. Interestingly, honokiol and magnolol increased the cleaving of procaspase-8 (57 kDa) to p10 activated form (10 kDa), and also induced the proteolytic activity of procaspase-9 (47 kDa) to cleaved caspase-9 (35 and 37 kDa) in both CCA cells (Figure 4A). When the results were correlated by flow cytometer, the apoptosis induction ability of honokiol was found to be stronger than magnolol. Taken together, the results conclude that these herbal-derived compounds activated CCA cell apoptosis through the caspase-dependent pathway, which is either a mitochondria-dependent or a mitochondria-independent pathway.
 |
Figure 4: Honokiol and magnolol induced caspase-dependent apoptosis.
|
(A) KKU-100 and KKU-213L5 were incubated with honokiol and magnolol following indicated concentration and times, and apoptosis associated signaling proteins were determined using Western blot analysis. Inset values indicate protein expression normalizing with β-actin.
Honokiol and Magnolol Suppressed Adhesion and Migration of CCA Cells
Activation of cell invasion and metastasis is a hallmark of cancer. Therefore, we next determined whether honokiol and magnolol inhibited the metastatic behaviour of CCA cells. The adhesion assay was performed using a matrix gel-coated 96-well plate, with the results showing that 15 to 60 µM of honokiol significantly suppressed the adhesion of KKU-100 and KKU-213L5 in a dose-dependent manner (Figure 5A), while at 40 to 80 µM for magnolol (Figure 5B). Noticeably, at 15 µM of honokiol and 40 µM of magnolol inhibited KKU-213L5 adhesion, which is markedly lower than for KKU-100. To further examine the effects of honokiol and magnolol on cell migration, CCA cells were treated with compounds and the migration ability was monitored at 12 and 18 or 24 h. We found that 15 to 30 µM of honokiol and 40-60 µM of magnolol significantly inhibited the migration of KKU-100 and KKU-213L5 in both concentration- and time-dependent manners (Figure 6A-C). These results suggest that honokiol and magnolol exhibited anti-metastasis activities on CCA cells at sub IC50 concentrations.
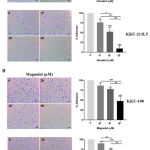 |
Figure 5: The inhibitory effects of honokiol and magnolol on cell adhesions of CCA cells.
|
(A) honokiol and (B) magnolol pretreated KKU-100 and KKU-213L5 were allowed to attach on Matrigel-coated plate for indicating times. Attached cell were photographed (×100) after crystal violet staining, cell number was quantified by measuring light absorbent at 570 nm before calculating to %adhesion by normalized with control group. The results represented as mean ± SD from three independent experiments. A ρ-value < 0.05 was considered to significant; ρ -value < 0.05 (*), ρ -value < 0.01 (**) and ρ -value < 0.001 (***).
 |
Figure 6: The inhibition effect of honokiol and magnolol on cell migration of CCA cells.
|
Confluent KKU-100 and KKU-213L5 were scratched and treated with (A) honokiol and (B) magnolol at indicating concentration and incubation times. The migrated cells were photographed (×100) and measured the migration area by using Image J, follow by calculation of percentage of migration (C). The results represented as mean ± SD from three independent experiments. A ρ-value < 0.05 was considered to significant; ρ -value < 0.05 (*), ρ -value < 0.01 (**) and ρ -value < 0.001 (***).
Honokiol and Magnolol Suppressed the Invasion of CCA Cells by Inhibiting MMP Activity
Metastasis is the accumulated result of multiple changes in cancer cells, not only in terms of cell adhesion and migration, but also cell invasion. Hence, we also studied the effect of honokiol and magnolol on MMP activity, which is the most crucial enzyme for tumour invasion owing to their ability to degrade ECM and basement membranes using gelatin zymography. We found that 10 to 40 µM of honokiol and 15 to 60 µM of magnolol markedly inhibited the MMP activities of KKU-100 in a dose-dependent manner, at 92 and 72 kDa for MMP-9 and MMP-2, respectively (Figure 7A). These results strongly support the previous data which suggested that honokiol and magnolol potentially inhibit the metastasis ability of CCA cells at noncytotoxic concentrations.
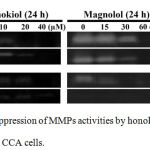 |
Figure 7: The suppression of MMPs activities by honokiol and magnolol on CCA cells.
|
(A) KKU-100 and KKU-213L5 were incubated with honokiol and magnolol for 24 h. The concentrated condition medium was using to assess the activities of MMPs by gelatin zymography before visualizing by Gel Dox RX+.
Discussion
Late diagnoses, insufficient treatment and the therapeutic resistance of CCA result in five-year survival rates for CCA patient is very low32. Therefore, development of an effective therapeutic agent for the treatment of CCA is imperative. Herbal medicine is one of the most commonly used alternative treatments for cancer patients. And effective antitumour effects that have reduced toxicity are the valuable sources of novel therapeutic agents. In the present study, for the first time we demonstrated the antitumour activities of honokiol and magnolol against CCA cells, these are well-known herbal-derived compounds that have multiple pharmacological effects.
Uncontrolled cell proliferation a hallmark of cancer which promotes unlimited cancer growth and the cause of widespread cancer metastasis. The results of this study showed that honokiol and magnolol significantly suppress the proliferation ability of CCA cells, while they exhibit less cytotoxicity in immortalized cholangiocyte. Moreover, the IC50 of honokiol on human monocyte-derived macrophage was further investigated and showed higher than CCA cells and MMNK-1, confirm that this compound shows less cytotoxicity on normal cell compared with cancer cell (manuscript in preparation). According to the size of compounds that makes them able to penetrate to tumour mass and potentially able to kill cancer cells. Previous study has reported that honokiol can pass through the blood-brain-barrier and can be a cause of neuroblastoma apoptosis33. This suggested that these compounds are interesting for the study and development of CCA treatment due to their anticancer proliferation while minimally toxic. In addition, the antiproliferation of honokiol and magnolol was further investigated. We demonstrated that these compounds exhibit cell cycle arrest at the G0/G1 phase for both KKU-100 and KKU-213L5. The induction of cell cycle arrest at G0/G1 with low concentrations of honokiol were found in various cancer cell lines, such as prostate cancer17 and pancreatic cancer34, whereas magnolol promoted cell arrest at G0/G1 and G2/M phases in breast cancer35 and colorectal cancer36. Previous studies indicated that the activation of STAT3 contributes to CCA carcinogenesis and progression which promotes cell proliferation mediated by the NF-kB signalling pathway, including cyclin D1 which is cell cycle regulation protein in the G1/S phase cell transition37,38. Therefore, the induction of cell cycle arrest at the G0/G1 to S phases by honokiol and magnolol were further demonstrated in the present study through the suppression of cyclin D1. These results are in accordance with previous reports that magnolol can inhibit the cell cycle of non-Opisthorchis viverrini-associated CCA and glioblastoma cell lines at G0/G1 by decreasing cyclin D1 expression through regulation of NF-κB pathway27,39, as well as KKU-213L5 treated berberine that causes cell cycle arrest at G0/G1 mediated by down-regulated cyclin D1 expression38. Taken together, the results demonstrated that honokiol and magnolol suppress CCA cells proliferation, mediated by cell cycle arrest and cyclin D1 degradation at non-cytotoxic concentrations, which are related to cell viability and colony formation assays.
We demonstrate that high concentrations of honokiol and magnolol induced cell apoptosis. The same concentrations of honokiol and magnolol did not only cause cell membrane internalization that possibly bind with annexin V, but also activated caspase-3/-7 enzyme activity. Moreover, honokiol and magnolol remarkably elevated the expression level of cleaved caspase-3 on both KKU-100 and KKU-213L5 cells, in a time-dependent manner. Fascinatingly, 100 µM honokiol showed the proteolytic activity of caspase-3 at 4 h, whereas magnolol was 8 h after treatment, which is consistent with annexin V and caspase-3/-7 activity results that honokiol exhibit a stronger apoptotic inducer ability on CCA cells than magnolol. Moreover, we also demonstrated that at same concentrations of the two compounds upregulated the expression of cleaved caspase-8 and -9. Therefore, these results provide strong evidence that high concentrations of honokiol and magnolol intensively induce cell apoptosis on CCA cells, mediated by either intrinsic or extrinsic pathways. It has been reported that high concentrations of honokiol and magnolol can stimulate reactive oxygen species, DNA damage and also proapoptotic protein that consequently acts directly on the mitochondrial membrane, resulting in the releasing of cytochrome C and activating caspase-9 and caspase-340. On another hand, these compounds can cooperate with Fas and TRAI ligand to enhance apoptosis mediated caspase-8 and caspase-3, respectively40.
CCA is a highly aggressive malignancy that patients often present at an unresectable advanced metastasis stage, which is the cause of poor prognosis and high mortality rates41. In the present study, we showed that low concentration of honokiol and magnolol suppressed the migration and adhesion abilities of KKU-100 and KKU-213L5. These results are consistent with previous study which report that honokiol inhibits the adhesion activities of glioblastoma cell line through regulation of adhesion molecules such as ICAM-1 and VCAM-128. In addition to magnolol suppressing adhesion, the migration and invasion abilities of breast cancer and non-Opisthorchis viverrini-associated CCA cell lines are mediated by inhibiting of the NF-κB signalling pathway and MMP activities15,39. The role MMP activities on CCA has previously described that high expression of MMP-9 was associated with poor prognosis in CCA patients42. Consequently, we further examined the effect of honokiol and magnolol on cell invasion by gelatin zymography. The results showed that honokiol and magnolol suppress MMP activities, which are MMP-2 and MMP-9. These findings indicate that non-cytotoxic concentrations of honokiol and magnolol exhibited anti-metastasis activities on CCA cells by inhibiting cell adhesion, migration and MMP-9, -2 activities.
Previous studied have reported that the antitumour effect of honokiol is stronger than magnolol for various cancer types, such as squamous lung carcinoma13, 43 and ovarian cancer21,22. This phenomenon may be due to their own isomer structure, which are different by the position of the hydroxyl group that provides distinct properties, such as its solubility and binding pocket on target. Moreover, in this study we also investigated the effects of these compounds on two human CCA cell lines with different metastatic abilities. KKU-213L5, a high-invasive cell line, originated from adenosquamous CCA with well differentiation and KKU-100, a low-invasive cell line, was isolated from adenocarcinoma CCA with poor differentiation31. In this study, KKU-213L5 was seemly more sensitive to apoptosis from both compounds than KKU-100, which is similar to a previous study of cepharathine on CCA cell lines that found drug exhibited greater cell cytotoxicity on KKU-100 than KKU-213L544. Although the in vitro experiments in this study provided convincing results of the antitumour activities of honokiol and magnolol, it is still inadequate to ensure that phenomena. Further in vitro and in vivo experiments additional required to evaluate this effect on CCA.
In summary, we have demonstrated the antitumour activities of honokiol and magnolol on CCA cells. These herbal-derived compounds exhibited antiproliferation effects mediated by cell cycle arrest and cyclin D1 degradation. As well as inducing apoptosis through either mitochondria-dependent or independent pathways in two types of human CCA cell lines, honokiol and magnolol also inhibited the metastasis abilities by inhibiting cell adhesion, migration and MMP-9, -2 activities. We propose that honokiol and magnolol are promising therapeutic agents for CCA patient therapy.
Acknowledgements
We are grateful to Prof. Sopit Wongkham, Liver Fluke and Cholangiocarcinoma Research Center, Khon Kaen University, for kindly provided the CCA cell lines that use in this study, and Asst. Prof. Sarawut Kumphune for his excellent assistance. We would like to thank ProofRead4Sure service for English proof reading and editing.
Conflict of Interest
There is no conflict of interest.
Funding Source
This study was financially supported by Naresuan University Research endowment fund (Grant I.D. R2559B102).
References
- Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R and Wolfe C. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505-527.
- Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S and Thinkamrop B. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4(7):e201.
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ and Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016;13(5):261-280.
- Hoi CP, Ho YP, Baum L and Chow AHL. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother. Res.. 2010;24(10):1538-1542.
- Hu H, Zhang X-x, Wang Y-y and Chen S-z. Honokiol inhibits arterial thrombosis through endothelial cell protection and stimulation of prostacyclin. Acta. Pharmacol. Sin. 2005;26(9):1063-1068.
- Amblard F, Delinsky D, Arbiser JL and Schinazi RF. Facile Purification of Honokiol and its Antiviral and Cytotoxic Properties. J. Med. Chem. 2006;49(11):3426-3427.
- Atanasov AG, Wang JN, Gu SP, Bu J, Kramer MP, Baumgartner L, Fakhrudin N, Ladurner A, Malainer C, Vuorinen A, Noha SM, Schwaiger S, Rollinger JM, Schuster D, Stuppner H, Dirsch VM and Heiss EH. Honokiol: A non-adipogenic PPARγ agonist from nature(). Biochimica. et. Biophysica. Acta. 2013;1830(10):4813-4819.
- Kang JS, Lee KH, Han MH, Lee H, Ahn JM, Han SB, Han G, Lee K, Park SK and Kim HM. Antiinflammatory activity of methanol extract isolated from stem bark of Magnolia kobus. Phytother. Res. 2008;22(7):883-888.
- Kong CW, Tsai K, Chin JH, Chan WL and Hong CY. Magnolol attenuates peroxidative damage and improves survival of rats with sepsis. Shock. 2000;13(1):24-28.
- Lee YJ, Lee YM, Lee CK, Jung JK, Han SB and Hong JT. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011;130(2):157-176.
- Wang T, Chen F, Chen Z, Wu YF, Xu XL, Zheng S and Hu X. Honokiol induces apoptosis through p53-independent pathway in human colorectal cell line RKO. World J. Gastroenterol. 2004;10(15):2205-2208.
- Park JB, Lee MS, Cha EY, Lee JS, Sul JY, Song IS and Kim JY. Magnolol-induced apoptosis in HCT-116 colon cancer cells is associated with the AMP-activated protein kinase signaling pathway. Biol. Pharm. Bull. 2012;35(9):1614-1620.
- Yang S-E, Hsieh M-T, Tsai T-H and Hsu S-L. Down-modulation of Bcl-X L, release of cytochrome c and sequential activation of caspases during honokiol-induced apoptosis in human squamous lung cancer CH27 cells. Biochem. Pharmacol. 2002;63(9):1641-1651.
- Tsai JR, Chong IW, Chen YH, Hwang JJ, Yin WH, Chen HL, Chou SH, Chiu CC and Liu PL. Magnolol induces apoptosis via caspase-independent pathways in non-small cell lung cancer cells. Arch. Pharm. Res. 2014;37(4):548-557.
- Liu Y, Cao W, Zhang B, Liu YQ, Wang ZY, Wu YP, Yu XJ, Zhang XD, Ming PH, Zhou GB and Huang L. The natural compound magnolol inhibits invasion and exhibits potential in human breast cancer therapy. Sci. Rep. 2013;3:3098.
- Xu D, Lu Q and Hu X. Down-regulation of P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by honokiol. Cancer Lett. 2006;243(2):274-280.
- Hahm ER and Singh SV. Honokiol causes G0-G1 phase cell cycle arrest in human prostate cancer cells in association with suppression of retinoblastoma protein level/phosphorylation and inhibition of E2F1 transcriptional activity. Mol. Cancer. Ther. 2007;6(10):2686-2695.
- Lee DH, Szczepanski MJ and Lee YJ. Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt signaling pathway in human prostate cancer cells. J. Cell Biochem. 2009;106(6):1113-1122.
- Rasul A, Yu B, Khan M, Zhang K, Iqbal F, Ma T and Yang H. Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int. J. Oncol. 2012;40(4):1153-1161.
- Sheu ML, Liu SH and Lan KH. Honokiol induces calpain-mediated glucose-regulated protein-94 cleavage and apoptosis in human gastric cancer cells and reduces tumor growth. PLoS One. 2007;2(10):e1096.
- Chuang T-C, Hsu S-C, Cheng Y-T, Shao W-S, Wu K, Fang G-S, Ou C-C and Wang V. Magnolol down-regulates HER2 gene expression, leading to inhibition of HER2-mediated metastatic potential in ovarian cancer cells. Cancer Lett. 2011;311(1):11-19.
- Li Z, Liu Y, Zhao X, Pan X, Yin R, Huang C, Chen L and Wei Y. Honokiol, a natural therapeutic candidate, induces apoptosis and inhibits angiogenesis of ovarian tumor cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008;140(1):95-102.
- Tse AK, Wan CK, Shen XL, Yang M and Fong WF. Honokiol inhibits TNF-alpha-stimulated NF-kappaB activation and NF-kappaB-regulated gene expression through suppression of IKK activation. Biochem. Pharmacol. 2005;70(10):1443-1457.
- Tse AK, Wan CK, Zhu GY, Shen XL, Cheung HY, Yang M and Fong WF. Magnolol suppresses NF-kappaB activation and NF-kappaB regulated gene expression through inhibition of IkappaB kinase activation. Mol. Immunol. 2007;44(10):2647-2658.
- Huang SH, Chen Y, Tung PY, Wu JC, Chen KH, Wu JM and Wang SM. Mechanisms for the magnolol‐induced cell death of CGTH W‐2 thyroid carcinoma cells. J. Cell. Biochem.. 2007;101(4):1011-1022.
- Lu C-H, Chen S-H, Chang Y-S, Liu Y-W, Wu J-Y, Lim Y-P, Yu H-I and Lee Y-R. Honokiol, a potential therapeutic agent, induces cell cycle arrest and program cell death in vitro and in vivo in human thyroid cancer cells. Pharmacol. Res. 2017;115:288-298.
- Chen LC, Liu YC, Liang YC, Ho YS and Lee WS. Magnolol inhibits human glioblastoma cell proliferation through upregulation of p21/Cip1. J. Agric. Food Chem. 2009;57(16):7331-7337.
- Jeong JJ, Lee JH, Chang KC and Kim HJ. Honokiol exerts an anticancer effect in T98G human glioblastoma cells through the induction of apoptosis and the regulation of adhesion molecules. Int. J. Oncol. 2012;41(4):1358-1364.
- You Q, Li M and Jiao G. Magnolol induces apoptosis via activation of both mitochondrial and death receptor pathways in A375-S2 cells. Arch. Pharm. Res. 2009;32(12):1789-1794.
- Rajendran P, Li F, Shanmugam MK, Vali S, Abbasi T, Kapoor S, Ahn KS, Kumar AP and Sethi G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell. Physiol. 2012;227(5):2184-2195.
- Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C and Miwa M. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J. Gastroenterol. 2005;11(22):3392-3397.
- Razumilava N and Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168-2179.
- Lin JW, Chen JT, Hong CY, Lin YL, Wang KT, Yao CJ, Lai GM and Chen RM. Honokiol traverses the blood-brain barrier and induces apoptosis of neuroblastoma cells via an intrinsic bax-mitochondrion-cytochrome c-caspase protease pathway. Neuro. Oncol. 2012;14(3):302-314.
- Arora S, Bhardwaj A, Srivastava SK, Singh S, McClellan S, Wang B and Singh AP. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS One. 2011;6(6):e21573.
- Zhou Y, Bi Y, Yang C, Yang J, Jiang Y, Meng F, Yu B, Khan M, Ma T and Yang H. Magnolol induces apoptosis in MCF-7 human breast cancer cells through G2/M phase arrest and caspase-independent pathway. Pharmazie. 2013;68(9):755-762.
- Lin SY, Liu JD, Chang HC, Yeh SD, Lin CH and Lee WS. Magnolol suppresses proliferation of cultured human colon and liver cancer cells by inhibiting DNA synthesis and activating apoptosis. J. Cell. Biochem. 2002;84(3):532-544.
- Dokduang H, Techasen A, Namwat N, Khuntikeo N, Pairojkul C, Murakami Y, Loilome W and Yongvanit P. STATs profiling reveals predominantly-activated STAT3 in cholangiocarcinoma genesis and progression. J. Hepatobiliary Pancreat. Sci. 2014;21(10):767-776.
- Puthdee N, Seubwai W, Vaeteewoottacharn K, Boonmars T, Cha’on U, Phoomak C and Wongkham S. Berberine Induces Cell Cycle Arrest in Cholangiocarcinoma Cell Lines via Inhibition of NF-kappaB and STAT3 Pathways. Biol. Pharm. Bull. 2017;40(6):751-757.
- Zhang FH, Ren HY, Shen JX, Zhang XY, Ye HM and Shen DY. Magnolol suppresses the proliferation and invasion of cholangiocarcinoma cells via inhibiting the NF-kappaB signaling pathway. Biomed. Pharmacother. 2017;94:474-480.
- Xu HL, Tang W, Du GH and Kokudo N. Targeting apoptosis pathways in cancer with magnolol and honokiol, bioactive constituents of the bark of Magnolia officinalis. Drug. Discov. Ther. 2011;5(5):202-210.
- Vabi BW, Carter J, Rong R, Wang M, Corasanti JG and Gibbs JF. Metastatic colon cancer from extrahepatic cholangiocarcinoma presenting as painless jaundice: case report and literature review. Int. J. Gastrointest. Cancer. 2016;7(2):E25-E30.
- Sun Q, Zhao C, Xia L, He Z, Lu Z, Liu C, Jia M, Wang J and Niu J. High expression of matrix metalloproteinase-9 indicates poor prognosis in human hilar cholangiocarcinoma. Int. J. Clin. Exp. Pathol. 2014;7(9):6157-6164.
- Yang S-E, Hsieh M-T, Tsai T-H and Hsu S-L. Effector mechanism of magnolol-induced apoptosis in human lung squamous carcinoma CH27 cells. Br. J. Pharmacol. 2003;138(1):193-201.
- Seubwai W, Vaeteewoottacharn K, Hiyoshi M, Suzu S, Puapairoj A, Wongkham C, Okada S and Wongkham S. Cepharanthine exerts antitumor activity on cholangiocarcinoma by inhibiting NF-kappaB. Cancer Sci. 2010;101(7):1590-1595.







