Ahmed Anwar Albir1 and Aedah Z. Al-Kaisy2
1Department of Basic Sciences, College of Dentistry, University of Baghdad, Iraq.
2Department of Physiology, College of Medicine, University of Baghdad, Iraq.
Corresponding Author E-mail: dr.alkarkhi@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1726
Abstract
Our experimental study was conducted on twenty seven healthy male albino mice, whose ages ranged from 5 to 6 weeks and weight range was 23-26gm with standard deviation (Std) equals to 1.83. The mice were randomly divided into three groups (9 mice each). The first group of mice was served as a control and not irradiated with laser, while both second and third groups of mice were anaesthetized and irradiated by using diode laser (λ = 890nm) which was focused on the upper right quadrant of the abdomen where the liver is located. It was concluded from our study that although laser exposed to both second and third groups of mice for 5 and10 minutes once daily respectively during the entire period of experimentation (6 days), the laser did not affected their hepatic tissue structure.
Keywords
Histological Alterations; Laser Treatment; Liver; Mice
Download this article as:| Copy the following to cite this article: Albir A. A, Al-Kaisy A. Z. Histological Investigation of the Hepatic Tissue in Mice Induced by Soft Laser with Different Doses. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Albir A. A, Al-Kaisy A. Z. Histological Investigation of the Hepatic Tissue in Mice Induced by Soft Laser with Different Doses. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2FNSunB |
Introduction
Liver is the largest internal organ in the body.1,2 It is located just below the diaphragm, in the right upper quadrant of the abdominal cavity.1 Liver consists mainly of important cells called hepatocytes, which constitute 70-80% of the liver’s cytoplasmic mass.3 Bile is formed and secreted from the hepatocytes.3 The vascular channels which are known as sinusoids separate the hepatocytes which are arranged in plates. The hepatocytes which have an average life span of 5 months, have the capability to regenerate.3 The aged erythrocytes are phagocytosed by a type of cells known as Kuppfer cells, which are located in the hepatic sinusoids.4
Many types of liver diseases or (disorders) are present including viral hepatitis, autoimmune hepatitis as well as toxic and alcoholic hepatitis which represent a global human dangerous problem.5-7 Furthermore, histological examination including conventional histology is very useful method for diagnosing most liver diseases.8
Liver fibrosis can be caused by abnormal hepatic extracellular matrix accumulation. The advanced conditions of liver fibrosis may lead to liver cirrhosis, portal hypertension and liver failure and usually need liver transplantations.9, 10
Terms like “cold laser”, “soft laser”, are used to describe low-level laser (or light) therapy (LLLT),11 which is considered as a treatment for pain control or tissue repair.12 In both soft tissue and connective tissue injuries, LLLT can increase the final tensile strength of the healed tissue. By increasing the amount of collagen production/synthesis and by increasing the intra and inter-molecular hydrogen bonding in the collagen molecules, laser therapy contributes to improved tensile strength.13 In dentistry, LLLT is effectively used to accelerate recovery in cases of recurrent aphthous stomatitis, oral mucositis, traumatic ulcers, herpetic lesions,14,15 and treatment of temporomandibular disorders.16
The main goal of this study was to evaluate the role of soft laser in activating the structure of mice liver by using different doses of laser irradiation during the experimentation period.
Materials and Method
This experimental study was carried out on twenty seven mature apparently healthy male albino mice (age, five to six weeks, weight range was 23-26gm with standard deviation (Std) equals to 1.83. All measures were taken to protect the animals from pain or discomfort. The mice were housed at room temperature (20 -24ºC) on a 12h light and 12 h dark cycle, with unlimited access to food and tap water. The mice were randomly divided into three groups (9 mice each). The first group of mice which was not irradiated with laser served as a control group. Both second and third groups of mice were anaesthetized and irradiated by using diode laser emitting wavelength (λ=890 nm) with power density of 50 mW/cm2. The laser was focused on the upper right quadrant of the abdomen where the liver is located, and provided at a distance of 1cm from the target abdomen of the mice. The second group of mice was irradiated with laser for 5 minutes once daily during the entire period of experimentation (6 days), whereas the third group of mice was irradiated with laser for 10minutes once daily during the entire period of experimentation (6 days). For the light microscopy purposes, all the mice were sacrificed by exposing them to inhaled anaesthesia continuously until they died. Their livers were excised and fixed in 10% formalin until ready to be used. After fixation, mice livers were routinely processed for histological investigation. The prepared sections were stained with hematoxylin and eosin stain, and examined under bright field light microscope.
Results
The present study proved clearly that soft laser did not adversely affected the hepatic tissue structure of the mice as per the results of histological examinations (Figures 2 and 3) in comparison with the sections of mice livers of the control group (Figure 1) as shown in table (1):
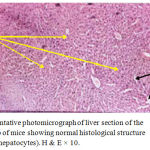 |
Figure 1: Representative photomicrograph of liver section of the first control group of mice showing normal histological structure of hepatic tissue (hepatocytes). H & E × 10.
|
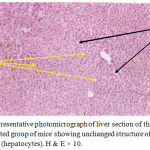 |
Figure 2: Representative photomicrograph of liver section of the secondirradiated group of mice showing unchanged structure of hepatic tissue (hepatocytes). H & E × 10.
|
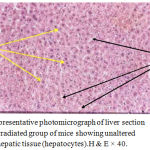 |
Figure 3: Representative photomicrograph of liver section of the third irradiated group of mice showing unaltered structure of hepatic tissue (hepatocytes).H & E × 40.
|
Table 1: Histological Investigation of the Hepatic Tissue Structure of the Mice Exposed to Laser Irradiation.
|
Number of Mice Per Group = 9 |
|||
| Group | Entire Period of Experimentation | Time of Irradiation | Histological Investigation of then Hepatic Tissue Structure |
| First (control) | ________ | ________ | Normal structure of hepatic tissue |
| Second (irradiated) | 6 days | 5 minutes once daily | No structural alterations were observed in the hepatic tissue in spite of laser exposure |
| Third (irradiated) | 6 days | 10 minutes once daily | No structural alterations were observed in the hepatic tissue in spite of increasing time of laser exposure |
Discussion
Results obtained from this experimental study demonstrated that soft laser did not affected the hepatic tissue structure including second and third groups of mice according to the histological findings of the examinations of the mice liver sections. Sections of liver revealed normal histological pictures of hepatic tissues (Figures2 and 3) in comparison with the sections of mice livers of the control group which were not irradiated with laser (Figure 1). The spaces or gaps between hepatocytes are normal and present in the three figures of liver (1, 2, and 3) and called hepatic sinusoids as mentioned in the introduction and laser did not affected or reduced them. The structures of hepatic tissues which belong to the second and third groups of mice (Figures 2 and 3) irradiated with laser for 5 and 10 minutes per day respectively during the experimentation period (6 days) remained unchanged depending on a fact that there were no responses of hepatic tissues to the action of laser. We reckoned that our findings were due to the selection of both time of laser irradiation per day (5 and 10 minutes consecutively) and the experimentation period (6 days) which might be insufficient or not appropriated to make activations to produce alterations in the hepatic tissues. That was exactly occurred in second and third groups of mice (Figures 2 and 3) when compared with the hepatic tissue structure in the first control group of mice not irradiated with laser (Figure 1). However, the laser action principles in body component such as cells and tissues are still not well known.17
Finally, the action of laser and its effects on cells and tissues will be understood through time and carful investigation.
Conclusion
It was expected that during our experimental work that changes in the hepatic tissue including hepatocytes will be occurred, but actually, there were no changes at all. We recommend that other investigations are needed using different times of laser irradiation and different periods of experimentation to find out and identify more about the action of laser and its effects on the histological systems of animal.
References
- Mescher, A.L. (2013), “Junqueira’s Basic Histology Text & Atlas”, Organs Associated with the Digestive Tract, 13th edn, Lange, New York, 323-342.
- Ramadori, G., Moriconi, F., Malik, I. & Dudas, J. (2008), “Physiology and Pathophysiology of Liver Inflammation, Damage and Repair”, Journal of Physiology and Pharmacology 59, Suppl 1,107-117.
- Grisham, J.W., Nopanitaya, W., Compagno, J. & Nägel, A.E. (1975), “Scanning electron microscopy of normal rat liver: the surface structure of its cells and tissue components”, Am J Anat 144, 295-321.
- Haubrich, W. S. (2004), “Kupffer of Kupffer cells”, Gastroenterology 127, 16.
- Tu, C-T., Yao, Q-Y., Xu, B-L & Zhang, S-C. (2013), “Curcumin protects against concanavalin A-induced hepatitis in mice through inhibiting the cytoplasmic translocation and expression of high mobility group box1”, Inflammation 36 (1), 206-215.
- Verma, S.& Thuluvath, P.J.(2007), “Complementary and alternative medicine in hepatology: review of the evidence of efficacy”, Clinical Gastroenterology and Hepatology 5 (4), 408-416.
- Czaja, A. J. (2014), “Hepatic inflammation and progressive liver fibrosis in chronic liver disease”, World Journal of Gastroenterology 20 (10), 2515-2532.
- Wang, H., Wang, H., Chen, T., Liang, X., Song, Y. & Wang, J. (2014), “Evaluation of the POSSUM, P-POSSUM and E-PASS scores in the surgical treatment of hilar cholangiocarcinoma”, World J Surg Oncol 12 (1), 191.
- Ramadori, G. & Saile, B. (2004), “Portal tract fibrogenesis in the liver”, Lab Invest 84, 153-159.
- Saile, B. & Ramadori, G. (2007), “Inflammation, damage repairand liver fibrosis-role of cytokines and different cell types”,Z Gastroenterol 45, 77-86.
- Roelandts, R. (2002), “The history of phototherapy: something new under the sun?”, J Am Acad Dermatol 46, 926- 30.
- Enwemeka, C.S., Parker, J.C., Dowdy, D.S., Harkness, E.E., Sanford, L.E & Woodruff, L.D. (2004), “The Efficacy of Low- Power Lasers in Tissue Repair and Pain Control: A Meta-Analysis Study”, Photomedicine and Laser Surgery 22 (4), 323-329.
- Reddy, G.K., Stehno-Bittel, L., & Enwemeka, C.S. (2001), Laser photo stimulation accelerateswound healing in diabetic rats. Wound Repair Regen 9, 248-255.
- Lima, A. G., Antequera, R., Peres, M. P. S. M., Snitcosky, I. M. L., Federico, M. H. H,& Villar, R. C. (2010), Efficacy of low-level laser therapy and aluminum hydroxide in patientswith chemotherapy and radiotherapy-induced oral mucositis. Braz Dent J 21, 186-192.
- Colvard ,M., & Kuo, P. (1991), Managing apthous ulcers: laser treatment applied. J Am DentAssoc 122, 51-53.
- Mazzetto, M. O., Hotta, T. H., & Pizzo, R. C. A. (2010), Measurements of jaw movements and T.M.J pain intensity in patients treated with Ga. Al. As. Laser. Braz Dent J 21, 356-360.
- Rocha Jr, A.M., Vieira, B.J., de Andrade, L.C.F. & Aarestrup, F.M. (2007), “Effects of low-level laser therapy on the progress of wound healing in humans: the contribution of in vitro and in vivo experimental studies”, J Vasc Bras 6(3), 258-266.







