A. Ganesh Kumar1, Baby Joseph2, S. Nandagopal3, P. Sankarganesh*4 and S. K. Jagdish5
and S. K. Jagdish5
1Department of Microbiology, Hindustan College of Arts and Science, Padur, Chennai - 603 103, Tamil Nadu, India.
2-4Centre for Research and Consultancy, Hindustan Institute of Technology and Science, Padur, Chennai - 603103 Tamil Nadu, India.
3Department of Botany, Department of Botany, Government Arts College, Hosur - 635 001, Tamil Nadu, India.
5Department of Prosthodontics, Chettinad Dental College, Kelambakkam - 603 103, Tamil Nadu, India.
Corresponding Author E-mail: bilisankar@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1723
Abstract
The main aim of endodontic treatment is disinfection of root canal and to prevent chances of reinfection. The most commonly isolated species due to oral infections is Enterococcus faecalis. For nonsurgical endodontic procedures Sodium hypochlorite (NaOCl) has been the irrigant of choice. The mechanism by which endodontic irrigants induce cytotoxicity is still unclear. However, many studies clearly indicated that rapid expression of the reactive oxygen species (ROS) leads to free radicals formation which results in cytotoxicity and cell death. Hence this study was done to determine the viability of cells and oxidative stress mediated by NaOCl, an endodontic irrigant. The irrigants were tested for their effect against fibroblast isolated from human primary buccal mucosa and against 3T3 Cell line. Antibacterial activity was performed against Enterococcus faecalis. Cytotoxicity was determined by MTT. To determine the oxidative stress, total intracellular glutathione, superoxide radical scavenging activity, and catalase assays were performed. The MIC (Minimal Inhibitory Concentration) for the irrigants against Enterococcus faecalis was found to be 10 µl. 10 µl of NaOCl plain 5.2% produced the same effect as that of 10 μl of NaOCl plain 3%. The higher concentration of the irrigants decreased viability of the cells during dye exclusion assay. Enzyme based study showed there is a decrease in enzyme dehydrogenase when treat with irrigants. Glutathione, SOD level was increased gradually on 3T3 cells. But CAT level was increased when the irrigants concentration less. The results of this study indicated that endodontic irrigants were potentially controlling the Enterococcus faecalis and non-toxic/reduced viability of 3T3 cells by MTT which could be due to the oxidative stress and loss of cellular integrity probably due to the liberation of ROS evidenced by the alteration of antioxidant enzymes Glutathione, SOD and CAT.
Keywords
CAT; Enterococcus Faecalis; Glutathione; Irrigant; Sodium Hypochlorite; SOD
Download this article as:| Copy the following to cite this article: Kumar A. G, Joseph B, Nandagopal S, Sankarganesh P, Jagdish S. K. Experimental Human Root Canal Irrigant NaOCl Against Enterococcus Faecalis and 3T3, and Determination of Cytotoxicity Effect. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Kumar A. G, Joseph B, Nandagopal S, Sankarganesh P, Jagdish S. K. Experimental Human Root Canal Irrigant NaOCl Against Enterococcus Faecalis and 3T3, and Determination of Cytotoxicity Effect. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2KnZb2U |
Introduction
Infections in the root canals are of polymicrobial nature and microorganisms such as E. faecalis, Streptococcus mutans, and Candida albicans were generally detected in such cases (Li-Wan Lee et al. 2017, Anuradha et al. 2014) E. faecalis has the potential to get adapted in a variety of adverse environments for the survival. Shaping and cleaning is a vital step in the treatment of the root canal. This eliminates the microbial infection particularly from the space found in the root canal and from the tubules of dentin. This can be achieved by removing organic tissue, as well as the microorganisms. Irrigation also plays a role and its effectiveness on depends on many factors like the mechanism of action, and the ability to contact with the microorganisms and the tissue debris present (Rossi et al. 2006).
There are many innovative approaches in the irrigation protocol to enhance the action of irrigants. The combined use of many irrigants sequential may help to eliminate the organisms effectively. However, caution should be exercised when using the combined irrigation protocol as the chemical interaction between the irrigation solutions that result in precipitation in the root canal space. The application of sodium hypochlorite (NaOCl), which acts as a deproteinizing agent, has been recommended for its efficient removal (Wright et al. 2017, Arias-Moliz et al. 2014, Svec & Harrison 1977). For effective action mostly such irrigants must be brought into contact with the walls of the canal. Many studies, with failed endodontic treatment particularly on the microbes of root canals in teeth, have used culture techniques for identification of bacteria. Cultivation, which is the traditional identification method has limitations when it comes to microbiological identification (Rossi et al. 2006).
E. faecalis is the best-known organism that has the potential to resist intra-canal medications and without the synergistic effect of other bacteria E. faecalis can survive in root canals (Qian-Qian Wang et al. 2012, Cohen & Burns 2006). For disinfecting the root canal system, various antimicrobial rinses have been introduced. Such disinfectants have many disadvantages such as antimicrobial activity, the risk of allergy, toxicity in patients and inability to penetrate into dentinal tubules (Gijo et al. 2015, Bufflier et al. 1997). So to date, there is no ideal intra-canal medication available.
Endodontic treatment failure results more often due to the colonization of the microorganisms in the root canal system and the success mainly depends on the elimination of microorganisms (Herzog et al. 2017, Sadia & Farhan 2016, Berutti & Marini 1997). For nonsurgical endodontic procedures, NaOCl dissolves vital and necrotic tissue, has antimicrobial activity, and acts as a lubricant in canal (Harrison 1984). NaOCl is also known to be very toxic to the periapical tissues if it goes beyond the apex of the tooth (Harrison et al. 1978) One of the broad-spectrum antimicrobial agents is Chlorhexidine gluconate (CHX) which has the advantage of substantivity (Kuruvilla & Kamath MP 1998). It is found to be very effective in inhibiting gram-positive organisms than gram-negative organisms (Zehender 2006). CHX in liquid form (0.2%–2%) and NaOCl (0.5%–5.25%) were found to be the most effective irrigants against Gram-positive and negative oral pathogens including yeasts (Luddin & Ahmed 2013).
The ability of endodontic irrigants to kill cells is still unclear. However, it is assumed that due to the excessive outcome of the ROS and the development of free radicals in the cells, may result in cell death. Hence, this study was carried out to assess the viability of cell and oxidative stress induced by endodontic irrigants NaOCl in fibroblast. This information will definitely give an idea on the possible mechanism of toxicity.
Materials and Method
Endodontic irrigants: NaOCl mint 3%, NaOCl mint 5%, NaOCl plain 3% and NaOCl plain 5.2% were the irrigants used in this study. All the irrigants were tested for their effect against fibroblast isolated from human primary buccal mucosa and against standard fibroblast cell line – 3T3 Cell line. Antibacterial activity was tested against Enterococcus faecalis.
Anti-Bacterial Activity
Agar disc diffusion method was used to determine the antibacterial sensitivity against Enterococcus faecalis. Agar plates were inoculated with an even lawn of cells. Sterile paper discs were prepared and autoclaved at a temperature of 121°C for 15 min. Using a sterile pipette, the paper disc was saturated with different concentration of the four irrigants (10µl, 20µl 40µl, and 80µl) and allowed to dry the agar surface for 3-5 min. To provide uniform contact, discs were placed and pressed on the surface of the medium. The bottom plates were marked to identify the irrigating solutions and then placed in an incubator at 37°C for 48 hrs. After 48hrs, zones of inhibition were measured across the diameter in millimeters (mm).
Fibroblast Culture from Human Primary Buccal Mucosa
The tissue for fibroblast cell culture (submucosa) was harvested from oral buccal mucosa of a healthy donor after being informed andwritten consent. Before isolating the tissue, oral rinse with 0.2% chlorhexidine was done for 1 minute to prevent any chance of contamination. Tissue was excised by incisional biopsy under local anesthesia. The tissue fragments were immediately placed in Dulbecco modified Eagle’s medium (DMEM) for at least 1 hour. It was rinsed thrice with PBS and minced into small tissue pieces. By brief trypsin-EDTA application (0.02%), the fibroblast cells were collected and cultured in DMEM containing crude collagenase, 10% FBS and 100 µg/ml of streptomycin. Cultures were incubated at 37°C, in a humidified atmosphere of 5% CO2 for 48 hrs. At least two passages of the cells were cultured and maintained. The third level of passage cells was taken for further analysis.
Effect of Test Compounds in Fibroblast Cells
Fibroblast cells were poured at a density of 1 X 103 cells per well in 96-well tissue culture plates. Before serially diluting with culture medium, the test irrigants NaClO mint 3%, NaClO mint 5%, NaClO plain 3% and NaClO plain 5.2% were dissolved in dimethyl sulfoxide (DMSO). 0.5% DMSO was the maximum concentration used. In the culture media, the test compounds were immiscible hence they were dissolved in 0.5% DMSO. At this concentration, DMSO has shown to be non-cytotoxic. The cell viability and various enzyme activities were evaluated 24 hours after exposure to this treatment. All tests were done in triplicate. Trypan blue dye exclusion assay was used to assess the viability of cells.
Tryphan Blue Dye Exclusion Method
Tryphan blue is a stain that is used to selectively determine dead tissues or cells as blue. A determined concentration of the irrigant was taken and prepared with a known volume of PBS which was the stock concentration. From the stock different concentrations of the irrigants were prepared (10µl, 20µl 40µl, and 80µl) and used for the in-vitro analysis. The cultures were incubated after the addition of cell, media, and irrigants, with 5% CO2 and humidified condition at 37°C for 24 hours. After incubation 20 – 200µl was transferred in an appropriate tube and an equal ratio of 0.4% tryphan blue was gently mixed which was kept at room temperature for 5 minutes. The number of unstained (viable cells) and stained (dead) cells were enumerated by placing 10 µl of stained cells in a haemocytometer. In each quadrant, the average number of dead cells was counted and multiplied by 2 × 104 to find cells/ml. The cell viability is then determined.
Cell Culture – 3T3 Cell Line
3T3 cell line used in this study was obtained from the CIC Culture Collection, University of Granada (Spain). DMEM with 2mM of glutamine and 10% bovine calf serum was used for culturing. Flasks were kept until cells reached confluence, at 37°C, 5% CO2, and 95% humidity. Minimum of two passages the cells were cultured and maintained. The third level of passage cells was taken for the current study.
Effect of Test Compounds in 3T3 Cells
In 96-well tissue culture, plate cells were poured at a density of 1 X 106 cells per well. In DMSO the test irrigants were dissolved in culture medium after serial dilution. In this study, the compounds immiscible in the culture media were allowed to dissolve in 0.5% DMSO. The test concentration was determined based on previous studies using HPLC in artificial saliva (Chaves et al. 2010).
MTT Assay (Mosmann et al. 1983)
Various concentrations of the irrigants were prepared from the stock (10µl, 20µl 40µl, and 80µl) and analysed in-vitro. The cell cultures were incubated in an incubator (5% CO2 and humidified condition) at 37°C after the addition of cell, media and extract. The tetrazolium compound MTT was added to the wells and incubated. Mitochondrial dehydrogenase is expected to reduce MTT. The lysing solution was added to the wells to solubilize the formazan crystals. The absorbency at 570nm was recorded in ELISA reader. Cell proliferation rate is directly propositioned to the rate of tetrazolium reduction.
Total Intracellular Amount of Glutathione (GSH) Determination
The 3T3 cells were treated with all the 4 endodontic irrigants for 24 hours. After attaining 80% confluence, cells were detached by trypsinization, remixed in fresh medium, and poured in six-well dishes (106 cells/well). The untreated cells served as control. The cells were added into 1mL PBS after washing. The cells were then subjected into sonicator to extract cellular GSH. Protein was determined using an aliquot of sonicating (Lowry et al. 1951). To control spontaneous oxidation of GSH 5%, sulfosalicylic acid was used in the remainder sonicate. Then sonicate was centrifuged at 10000 rpm for 10 min at 4°C to takeout the denaturated proteins. DTNB-GSSG reductase recycling assay (Tietze 1969) with minor modifications was used to determine GSH levels. Samples were calibrated to pH 7.0 with 0.2N NaOH to measure GSH. The increases in absorbance at 412nm were converted to GSH. DTNB-GSSG reductase and NADPH reduced GSSG into GSH.
Superoxide Radical Scavenging Activity
Superoxide anion scavenging activity was measured according to the method described by Fridovich (Fridovich 1989). The assay mixture was prepared by adding 0.05 ml of Nitro blue tetrazolium (1.5 mM NBT) solution and 0.05 ml L- methionine (200mM), into 25µl cell supernatant (treated with all four irrigants). The reaction mixture was allowed for 30 min to record the absorbency at 560 nm. All the tests were performed in triplicate.
Catalase Assay
3T3 cells were mixed with different concentrations of the irrigants and plated in 48 well plates with 500 µl of DMEM. CFA-NPs was also added 100 ug/well and incubated for 60 min. Quercetin –PLA particles with various concentrations (5-50 µg/ml) were mixed with fresh DMEM media whereas the spent media was discarded. The treated cells were incubated for 23 hour and catalase levels were calculated after the exposure. CFA-NPs were a positive control and wells with cell alone served as negative control.
Statistical Analysis
Statistical analysis was done using EXCEL STATPRO to determine SD Mean, Standard error and ANOVA. The p-value < 0.05 indicates statistical significance between groups. p > 0.05 indicates there is no statistical significance between groups.
Results
The antibacterial activity of the Endodontic irrigants was assessed using disc diffusion method. The results indicate that the Endodontic irrigants Sodium hypochlorite mint 5%, Sodium hypochlorite plain 3% and Sodium hypochlorite plain 5.2% possessed strong antibacterial activity except for Sodium hypochlorite mint 3% against Enterococcus faecalis. The highest antibacterial activity was found in both NaOCl 5.2% and NaOCl plain 3%. In a dose-dependent manner, the irrigants were successful in killing the bacteria. The MIC (Minimal Inhibitory Concentration) for the irrigants was found to be 10 µl. 10 µl of NaOCl plain 5.2% produced the same effect as that of 10 μl of NaOCl plain 3% (Table 1), (Fig. 1a – 1d).
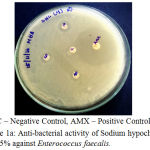 |
Figure 1a: Anti-bacterial activity of Sodium hypochlorite mint 5% against Enterococcus faecalis.
|
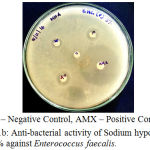 |
Figure 1b: Anti-bacterial activity of Sodium hypochlorite plain 3% against Enterococcus faecalis.
|
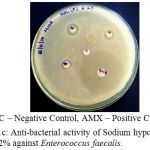 |
Figure 1c: Anti-bacterial activity of Sodium hypochlorite plain 5.2% against Enterococcus faecalis.
|
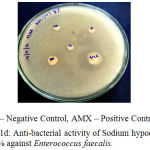 |
Figure 1d: Anti-bacterial activity of Sodium hypochlorite mint 3% against Enterococcus faecalis.
|
Table 1: Anti bacterial activity of the endodontic irrigants against Enterococcus faecalis.
| Endodontic Irrigants | Zone of inhibition in (mm) (Mean ± SD) | |||
| Positive Control | 10 µl | 20 µl | 40 µl | |
| Sodium hypochlorite mint 3% | 24 ± 0.16 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Sodium hypochlorite mint 5% | 38 ± 0.15 | 8 ± 0.19 | 9 ± 0.17 | 13 ± 0.19 |
| Sodium hypochlorite plain 3% | 38 ± 0.13 | 11 ± 0.13 | 11 ± 0.18 | 12 ± 0.12 |
| Sodium hypochlorite plain 5.2% | 38 ± 0.12 | 10 ± 0.12 | 10 ± 0.19 | 12 ± 0.17 |
Dye exclusion assay revealed that when the endodontic irrigants were tested for activity against buccal mucosal fibroblast, there was not much difference in the viability of the cells initially; but when the concentration of the irrigant and time duration was increased, the viability of the cells decreased to a greater extent. This indicate that the irrigants were not cytotoxic to fibroblast cells (Fig. 2).
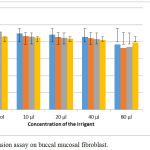 |
Figure 2: Dye exclusion assay on buccal mucosal fibroblast.
|
For enzyme based study, all the irrigants NaOCl mint 3%, NaOCl mint 5%, NaOCl plain 3%, and NaOCl plain 5.2% were tested against 3T3 cells by MTT. The results revealed that there was a decrease in the level of the enzyme dehydrogenase for all the irrigants tested with increase in time duration and concentration of the extract. Statistical analysis by ANOVA was not found to be significant for any of the irrigants with p values > 0.2 for all irrigants by MTT (Fig. 3).
 |
Figure 3: Cytotoxicity of irrigants on 3T3 cell line by MTT.
|
Upon exposure to all four irrigants at different concentrations for a time duration of 24 hours, the glutathione level was found to increase gradually on 3T3 cells. There was a steady increase in the glutathione level when the concentration was increased from 10 µl to 80 µl. Among the four irrigants tested, NaOCl plain 5.2% increased to a maximum level. Statistical analysis by ANOVA showed a p-value of 0.2675 for NaOCl mint 3 % and NaOCl mint 5%. Whereas the other irrigants NaOCL plain 3% and NaOCl plain 5.2% too had a greater p-value of 0.3470 and 0.3338, respectively and were not significant (Fig 4).
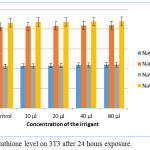 |
Figure 4: Glutathione level on 3T3 after 24 hours exposure.
|
Similar to glutathione, SOD level also increased. Among the four irrigants tested NaOCl mint 5% and NaOCl plain 5.2% increased SOD to a greater extent than the other two irrigants tested (Fig. 5).
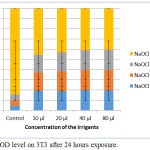 |
Figure 5: SOD level on 3T3 after 24 hours exposure.
|
The results were not significant by ANOVA. All the four irrigants viz., NaOCl mint 3%, NaOCl mint 5%, NaOCl plain 3%, and NaOCl plain 5.2% had comparatively similar role in CAT on treatment against 3T3 cells, as glutathione and SOD. The CAT level increased when the concentration of the irrigants and exposure time was increased. Similar to other statistical results, in CAT too the results were not significant with p >0.2 for all four irrigants tested (Fig. 6).
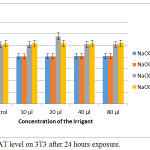 |
Figure 6: CAT level on 3T3 after 24 hours exposure.
|
Discussion
Control of endodontic microbiota is the basis of endodontic treatment. Many irrigation solutions have been used to treat the infected root canal (Ayhan et al. 1999, Bystrom et al. 1985). E. faecalis had been chosen as a testing organism after root canal treatment. E. Faecalis is commonly induces the root canal failure because of its ability to survive in the root canal. According to Yap et al. NaOCl (0.9%) are highly effective against E. faecalis (Yap et al. 2014).
Earlier studies have proved that, 2% concentration of CHX is highly effective in eliminating E. faecalis from superficial layers of the dentinal tubules (Basrani et al. 2002, Gomes et al. 2003, Vahdaty et al. 1993). Evans et al. in their study proved that 1–2% CHX when combined with calcium hydroxide kills E. faecalis more effectively (Evans et al. 2003). During root canal treatment the main use of an irrigant is to reduce the bacterial load. For the removal of the smear layer, EDTA is also used but it cannot do it completely without NaOCl. It has been found that saline, water, CHX or iodine compounds have no specific effect on the smear layer.
Bui et al., in their study using scanning electron microscope proved that effect of irrigating root canals with a combination of CHX and NaOCl on root dentin found that there were no differences in the amount of debris remaining between the experimental groups and negative control group although there were fewer patent tubules. They also showed that CHX does not have tissue dissolving property. (Bui et al. 2008).
Antimicrobial solution may not eradicate all microbes from the root canal area (Estrela et al. 2001). If the canal is not refilled with antimicrobial solutions more often or between each visit, the microorganism multiplies rapidly within 4 days (Bystrom & Sundqvist 1983). It was proved that high pH of NaOCl has a role to play in the cytoplasmatic membrane integrity due to biosynthetic alterations in cell metabolism, enzymatic inhibition and phospholipids destruction (Bystrom & Sundqvist 1983).
Grawehr et al. studied the interactions of NaOCl with EDTA and showed that EDTA retained its calcium-complexing ability along with NaOCl. However, tissue dissolving capacity of NaOCl was lost. Hence, both should be used separately (Grawehr et al. 2003). During root canal treatment the most important irrigating solution is Sodium NaOCl and it should be used throughout the process of instrumentation. It is found that at lower concentrations it dissolves organic tissue whereas, at higher concentrations, it kills bacteria.
A study had shown that the optimal effect of the antimicrobial agent was due to dense microbial population (Torabinejad et al. 2003). However, another study showed doxycycline in REDTA BioPure MTAD, is a bacteriostatic antibiotic which does not kill bacteria and prevents the susceptible bacterial multiplication. Biofilm-supplemented periapical infection cannot be controlled by the host defense mechanism where it is important to treat them by chemical and mechanical debridement procedures (Torabinejad et al. 2003, Chai et al. 2013).
Reduction of cellular GSH is due to the toxic effect of HEMA, TEGDMA, and UDMA. Volk et al. (2006) conducted a study and proved this could be the reason to detoxify the ROS produced by the toxins in human gingival fibroblast cell cultures. Another study showed that TEGDMA was cytotoxic in vitro cultures due to a decrease in intracellular GSH (Martins et al. 2012). The “first line” enzymic antioxidant is SOD. Generally, O2 dismutation shows a major role in reducing the ROS (Fridorich 1986). CAT, is a heme enzyme which utilizes NADPH for its function. Conversion of H2O2 into O2 is carried out by CAT which also regulates intracellular H2O2 (Chaves et al. 2010). In an earlier study found that a Methyl Methacrylate and Methacrylic acid induced dose-dependent cytotoxicity in human primary cell cultures. In the current study, it can be concluded that cytotoxicity and viability of the cell are based on ROS formation that too more often induced by monomers (Eckhardt et al. 2009, Kong et al. 2009).
Conclusion
The results of this study indicated that endodontic irrigants were potentially controlling the Enterococcus faecalis and non-toxic/reduced viability of 3T3 cells by MTT which could be due to the oxidative stress and loss of cellular integrity probably due to the liberation of ROS evidenced by the alteration of antioxidant enzymes Glutathione, SOD and CAT.
Conflict of Interest
There is no conflict of interest.
References
- Anuradha B, Rajamoni I, Lalitha MK, Sriram T (2014) A new irrigant against faecalisin the root canal disinfection. Biosciences Biotechnology Research Asia 11,121–7.
- Arias-Moliz M.T., Ordinola-Zapata R., Baca P., Ruiz-Linares M., Ferrer-Luque C.M. (2014) Antimicrobial activity of a sodium hypochlorite/etidronic acid irrigant solution. Journal of Endodontics40, 1999–2002.
- Ayhan H, Sultan N, Cirak M, Ruhi MZ, Bodur H (1999) Antimicrobial effects of various endodontic irrigants on selected microorganisms. International Endodontic Journal 32, 99 –
- Basrani B, Santos JM, Tjaderhane L, Grad H, Gorduysus O, Huang J (2002) Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 94, 240 – 24
- Berutti E, Marini R (1997) Penetration ability of different irrigants into dentinal tubules. Journal of Endodontic 23, 727 – 728.
- Bufflier P, Suchett-Kaye G, Morrier JJ, Benay G, Decoret D, Bonin P, Renard F, Barsotti D (1997) In vitro evaluation of the antibacterial effects of intracanal micro plasma system treatment. Journal of Endodontic 23, 28 – 31.
- Bui TB, Baumgartner JC, Mitchell JC (2008) Evaluation of the interaction between sodium hypochlorite and chlorhexidine gluconate and its effect on root dentin. Journal of Endodontics 34, 181–185.
- Bystrom A, Claesson R, Sundqvist G (1985) The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endodontic and Dental Traumatology 1(5), 170 – 175.
- Bystrom A, Sundqvist G. (1983) Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surgery, Oral Medicine and Oral Pathology 55(3), 307- 312.
- Chai WL, Hamimah H, Abdullha M. (2013) Evaluation of antibacterial efficacy of antibiotics and calcium hydroxide against Enterococcus faecalisbiofilm in dentin. Sains Malasiana 42, 73–80.
- Chaves CA, Machado AL, Carlos IZ, Giampaolo ET, Pavarina AC, Vergani CE (2010) Cytotoxicity of monomers, plasticizer and degradation by-products released from dental hard chairside reline resins. Dental Materials 26(10), 1017 – 1023.
- Cohen S, Burns RG. (2006) Pathways of the pulp. 9th ed, USA, Mosby. Elsivier.
- Eckhardt A, Gerstmayr N, Hiller KA, Bolay C, Waha C, Spagnuolo G (2009) TEGDMA-induced oxidative DNA damage and activation of ATM and MAP kinases. Biomaterials 30(11), 2006 – 2014.
- Estrela C, Estrela CRA, Bammann LL, Pecora JD (2001) Two methods to evaluate the antimicrobial action of calcium hydroxide paste. Journal of Endodontics 27(12), 720 – 723.
- Evans MD, Baumgartner JC, Khemaleelakul SU, Xia T (2003) Efficacy of calcium hydroxide: Chlorhexidine paste as an intracanal medication in bovine dentin. Journal of Endodontics 29, 338 – 33
- Fridorich I. (1986) Superoxide dismutase. Advances in Enymology 58, 61 – 97.
- Gijo John, Pavan Kumar K, Sujatha Gopal S, Surya Kumari, Bala Kasi Reddy (2015) Enterococcus faecalis, a nightmare to endodontist: A systematic review. African Journal of Microbiology Research 9(13), 898-908.
- Gomes BP, Souza SF, Ferraz CC, Teixeira FB, Zaia AA, Valdrighi L (2003) Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalisin bovine root dentine in vitro. International Endodontic Journal 36, 267.
- Grawehr M, Sener B, Waltimo T, Zehnder M (2003) Interactions of ethylenediamine tetraacetic acid with sodium hypochlorite in aqueous solutions. International Endodontic Journal 36, 411 – 417.
- Harrison JW (1984) Irrigation of the root canal system. Dental Clinics of North America 28, 797 – 808.
- Harrison JW, Svec TA, Baumgartner JC. (1978) Analysis of clinical cytotoxicity of endodontic irrigants. Journal of Endodontics 4, 6 – 11.
- Herzog DB, Hosny NA, Niazi SA, Koller G, Cook RJ, Foschi F, Watson TF, Mannocci F, Festy F (2017) Rapid Bacterial Detection during Endodontic Treatment. Journal of Dental Research 96(6), 626-632.
- I Fridovich. (1989) Superoxide dismutases. An adaptation to a paramagnetic gas. Journal of Biological Chemistry 264,
- Kong N, Jiang T, Zhou Z, Fu J (2009) Cytotoxicity of polymerized resin cements on human dental pulp cells in vitro. Dental Materials 25(11), 1371 – 1375.
- Kuruvilla JR, Kamath MP (1998) Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined as endodontic irrigants. Journal of Endodontic 24, 472 – 476.
- Li-Wan Lee, Ya-LingLee, Sheng-Huang Hsiao, Hung-Pin Lin (2017) Bacteria in the apical root canals of teeth with apical periodontitis. Journal of the Formosan Medical Association 116(6), 448-456.
- Lowry et al., (1951) Protein measurement with Folin-Phenol reagent. Journal of Biological Chemistry 19(3), 275.
- Luddin N, Ahmed HM (2013) The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. Journal of Conservative Dentistry 16, 9–16.
- Martins CA, Leyhausen G, Geurtsen W, Volk J (2012) Intracellular glutathione: a main factor in TEGDMA-induced cytotoxicity? Dental Materials 28(4), 442 – 448.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, Journal of Immunological Methods 65, 55-63.
- Patricia P. Wright, Bill Kahler, and Laurence J. Walsh (2017) Alkaline Sodium Hypochlorite Irrigant and Its Chemical Interactions. Materials (Basel). 10(10), 1147.
- Qian-Qian Wang, Cheng-Fei Zhang, Chun-Hung Chu, and Xiao-Fei Zhu (2012) Prevalence of Enterococcus faecalis in saliva and filled root canals of teeth associated with apical periodontitis. International Journal of Oral Science 4(1), 19–23.
- Rossi Fedele G, Scott W, Spratt D, Gulabivala K, Roberts AP (2006) Incidence and behaviour of Tn916-like elements within tetracycline-resistant bacteria isolated from root canals. Oral Microbiology and Immunology 21, 218 – 22.
- Sadia Tabassum and Farhan Raza Khan (2016) Failure of endodontic treatment: The usual suspects. European Journal of Dentistry 10(1), 144–147.
- Svec TA, Harrison JW (1977) Chemomechanical removal of pulpal and dentinal debris with sodium hypochlorite and hydrogen peroxide vs normal saline solution. Journal of Endodontic3, 49 – 53.
- F. (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Analytical Biochemistry 27(3), 502 – 522.
- Torabinejad M, Shabahang S, Aprecio RM, Kettering JD (2003) The antimicrobial effect of MTAD: an in vitro investigation. Journal of Endodontics 29, 400 – 403.
- Vahdaty A, Pitt Ford TR, Wilson RF (1993) Efficacy of chlorhexidine in disinfecting dentinal tubules in vitro. Endodontic and Dental Traumatology9, 243 – 24
- Volk J, Engelmann J, Leyhausen G, Geurtsen W (2006) Effects of three resin monomers on the cellular glutathione concentration of cultured human gingival fibroblasts. Dental Materials 22(6), 499 – 505.
- Yap B, Zilm PS, Briggs N, Rogers AH, Cathro PC (2014) The effect of sodium hypochlorite on Enterococcus faecaliswhen grown on dentine as a single- and multi-species biofilm. Australian Endodontic Journal 40, 101–10.
- Zehender M (2006) Root canal irrigants. Journal of Endodontics 32, 389 – 90.







