Manuscript accepted on :12-June-2019
Published online on: 26-06-2019
Plagiarism Check: Yes
Reviewed by: Hendry Irawan
Second Review by: Shaimaa phd bio
Sandeep Kumar1, Ajay Kumar2 and Mohammad Mustufa Khan*3
1Department of Biochemistry, Hind Institute of Medical Science, Sitapur,Uttar Pradesh - 261303, India.
2Department of Biochemistry, Integral Institute of Medical Sciences and Research, Integral University, Lucknow, Uttar Pradesh - 226026, India.
3School of Biological Engineering and Sciences, Shobhit University, Gangoh, Saharanpur, Uttar Pradesh - 247341, India.
Corresponding Author E-mail: mustufakhan01084@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1728
Abstract
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by hyperglycemia due to insufficient secretion or action of insulin.Elevated oxidative stress and diminished antioxidants may play an important role to develop T2DM and its complications. Aldose reductase (AR) enzyme plays a key role in the reduction of glucose to sorbitol by Polyol pathway. To estimate the AR activity and malondialdehyde (MDA) levels and in patients with T2DM. In this case-control study, a total number of 60 subjects (30 T2DM and 30 age-matched controls) wererecruited.Fasting blood sugar (FBS), Post-Prandial blood sugar (PPBS), AR activity and MDA levels were estimated in all the subjects. The AR activity was estimated by nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidation method. The plasma MDA levels were estimated by the thiobarbituric acid reactive substance (TBARS) method. For Statistical analysis, all the data were compared between the two groups by using unpaired t-test. Pearson correlation coefficient was calculated among T2DM. A P value <0.05 was considered as statistically significant for all data analyzed. The mean of FBS, PPBS, AR activity, and MDA levels were found significantly high in T2DM as compared to controls (P<0.001, P<0.001, P<0.001, P<0.001, respectively). A significant positive correlation was found between FBS and PPBS among T2DM (r=0.71, P<0.01). However, There was no significant correlation found between AR activity and MDA level among T2DM (r=0.002, P>0.05). Results showed thatthe mean of FBS, PPBS, AR activity, and MDA levels were found significantly higher in T2DM than controls. There was no significant correlation found between AR activity and MDA level among T2DM.
Keywords
Aldose Reductase; Lipid Peroxidation; Malondialdehyde; Oxidative Stress; Polyol Pathway; Type 2 Diabetes Mellitus
Download this article as:| Copy the following to cite this article: Kumar S, Kumar A, Khan M. M. Estimation of Aldose Reductase Activity and Malondialdehyde Levels in Patients with Type 2 Diabetes Mellitus. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Kumar S, Kumar A, Khan M. M. Estimation of Aldose Reductase Activity and Malondialdehyde Levels in Patients with Type 2 Diabetes Mellitus. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2Fz61iP |
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disorder with characteristics of hyperglycemia due to insufficient secretion or action of insulin.1,2 In T2DM patients, an imbalance between reactive oxygen species (ROS) production and antioxidant level has been reported.3,4 Abnormally high levels of lipid peroxidation and the simultaneous decline of antioxidant defense mechanism can lead to damage of cellular organelles like mitochondria, Golgi bodies etc and lead to increased oxidative stress.5, 6 It is reported that the chronic rise of plasma glucose causes major complications of diabetes, including retinopathy, neuropathy, nephropathy, and macrovascular and microvascular damage.2,7 According to International Diabetes Federation (IDF) report, a total of 425 million adults have diabetes and 1 in 2 remains undiagnosed worldwide.8 There are more people with diabetes residing in urban (269.7 million) than in rural (145.1 million) areas.9 In India, there are 72.9 million people living with diabetes mellitus. The overall prevalence of diabetes is 8.8% in Indian adults.8 Anjana et al reported that the prevalence of diabetes is higher in low socio-economical status (SES) groups mainly in city dwellers or slum dwellers residing in the economically developed states.10
Diabetes and free radicalsare strongly associated4. Studies reported that T2DM revealed oxidative stress load, oxidative destruction of sub-cellular membrane lipids has been implicated along with other types of intracellular oxidative damage in the normal aging process and in the pathophysiology of a number of chronic illnesses.11,12 Glucose oxidation is the main source of free radicals generation.The polyol pathway leads to the reduction of glucose to sorbitol via aldose reductase (AR) enzyme in an nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) dependent manner. Sorbitol is then oxidized to fructose by the enzyme sorbitol dehydrogenase, with nicotinamide adenine dinucleotide (NAD+) reduced to nicotinamide adenine dinucleotide hydrogen (NADH). The main function of AR enzyme is to reduce toxic aldehydes formed by ROS or other substrates to inactive alcohols. Under normal conditions, AR has a low affinity for glucose, with a very small percentage of total glucose converted to sorbitol via this pathway. Under hyperglycemic conditions, there is an increase in the enzymatic activity and production of sorbitol, resulting in an overall decrease in NADPH. NADPH is an essential cofactor for the production of glutathione (GSH), a critical intracellular antioxidant.3,13-16 Increased glucose pumped by the polyol pathway does not produce ROS directly but contributes greatly to an overall redox imbalance in the cell that leads to oxidative stress17summarized in Figure 1.More than 30% of the glucose pumped into the polyol pathway causing a significant depletion of NADPH and as a result a significant decrease in the GSH level. Thus, AR activity diminishes the cellular antioxidant capacity during hyperglycemic conditions.3,13,17 It is reported that lipids are the primary targets of ROS.They may also react with transition metals like iron or copper to form stable aldehydes, such as active aldehydes malondialdehyde (MDA), which damage cell membranes.16,18 Peroxidation of lipids produces highly reactive aldehydes, such as MDA.18,19 MDA has been an established biomarker of free radical-mediated lipid damage and oxidative stress.20
Elevated oxidative stress and diminished anti-oxidants may increase the risk for T2DM and its complications. Various studies have reported the association of imbalance oxidants and anti-oxidants ratio with T2DM,4 Retinopathy,21 Neuropathy,22 Nephropathy,23 and CVD24summarized in Figure 1.
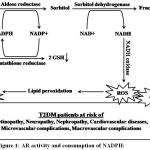 |
Figure 1: AR activity and consumption of NADPH:
|
Diagrammatic representation of the production of reactive oxygen species (ROS), advanced glycated products (AGEs) and malondialdehyde (MDA) in T2DM increase the risk for T2DM complications.
Thus, it is aimed to estimate the AR activity and MDA levels and in patients with T2DM.This study might be helpful for the better management and treatment of T2DM patients and delay and/or reduce its complications.
Materials and Method
Subject Selection
In this case-control study, a total of 60 subjects (30 T2DM and 30 age-matched controls) aged between 30-70 years was enrolled from outpatient Department of Medicine and Diabetes Clinic of IIMS&R, Integral University, Lucknow, Uttar Pradesh, India. The study was conducted out from January 2017 to June 2017. This study is approved by the institutional research and ethical committee and followed the ethical standards with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.25 Written informed consent was taken from each subject recruited for the study.
The inclusion of T2DM and control subjects: The person having FBS (≥126mg/dl) and PPBS (≥200mg/dl) considered as cases. The person having FBS (<125mg/dl) and PPBS (<200mg/dl) was considered as controls.26
Subjects with ischemic heart disease, angina, Myocardial Infarction (MI), electrocardiogram abnormalities, anemia (Hb of 8.0 g/dl or less), those with other concurrent sicknesses like the chronic liver disease, chronic kidney disease, hypothyroidism or those on drugs like antihypertensive, antioxidants and diuretics, and pregnant women were excluded for both case and control groups. Detailed medical history was taken from each subject.
Laboratory Investigations
About 3ml of venous blood was collected in EDTA vial from each subject for estimation of AR activity and MDA levels. The AR activity was estimated by the NADPH oxidation method.27 A 10% erythrocyte suspension (hemolysate) was made by adding 50 mM sodium phosphate buffer, pH 7.4, containing 150 mM NaCl. The suspension was lysed by repeated freezing and thawing (three cycles) and centrifuged to remove insoluble material if any. The assay mixture in 1 ml contained 50 µmol potassium phosphate buffer pH 6.2, 0.4 mmol lithium sulfate, 5 µmol 2-mercaptoethanol, 10 µmol DL-glyceraldehyde, 0.1 µmol NADPH and enzyme preparation (hemolysate). The assay mixture was incubated at 37°C and initiated by the addition of NADPH at 37°C. The change in the absorbance at 340 nm due to NADPH oxidation. Absorbance was measured at 340 nm by UV-Visible double beam spectrophotometer (Systronics-2205, Systronic India Ltd. Gujarat, India). One unit was defined as micromoles NADPH oxidized/g Hb/min. The plasma MDA levels were estimated by thiobarbituric acid reactive substance (TBARS) method.28 Plasma was deproteinized and the precipitate is treated with thiobarbituric acid (TBA) at 90°C 1 hour. The pink color product formed at the end of the reaction which measured at 535 nm by UV-Visible double beam spectrophotometer (Systronics-2205, Systronic India Ltd. Gujarat, India).
Statistical Analysis
Data analysis was performed using the IBM SPSS software version 20.0 (Armonk, NY, USA). All the data were compared between the two groups by using analysis of variance (ANOVA) or unpaired t-test. Values were represented as mean ± SD (Standard Deviation). Pearson correlation coefficient was calculated among T2DM. A P value <0.05 was considered as statistically significant for all data analyzed.
Results
The mean age of T2DM and controls were found 55.7±5.85 and 57.03±4.15 years, respectively (P=0.31). The mean level of FBS and PPBS were found significantly higher in T2DM than controls (P<0.001, P<0.001, respectively). Similarly, the mean activity of AR was found significantly high in the T2DM as compared to controls (P<0.001). In addition, the mean level of MDA was also found significantly high in the T2DM as compared to controls (P<0.001) shown in Table 1.
Table 1: Clinical characteristics of T2DM and control.
| Parameters | T2DM (n=30) | Control (n=30) | P value |
| Age (years) | 55.7±5.85 | 57.03±4.15 | 0.31 |
| FBS (mg/dl | 231.0±82.0 | 83.0±9.3 | <0.0001* |
| PPBS (mg/dl) | 329.0±108 | 125.0±11.2 | <0.0001* |
| AR (U/g Hb/min) | 4.65±1.62 | 2.22±0.62 | <0.0001* |
| MDA (μmol/l) | 3.74±0.86 | 2.09±0.76 | <0.0001* |
Values are expressed as Mean ± Standard Deviation *Significant considered as P<0.05.
FBS: Fasting Blood Sugar, PPBS: Post-Prandial Blood Sugar, AR: Aldose Reductase, MDA: Malondialdehyde
A significant positive correlation was found between FBS and PPBS among T2DM (r=0.71, P<0.01). However, There was no significant correlation found between AR activity and MDA level among T2DM (r=0.002, P>0.05) shown in Table 2.
Table 2: Correlation between variables in T2DM.
| FBS (mg/dl) | PPBS (mg/dl) | AR (U/g Hb/min) | MDA (μmol/l) | |
| Age (years) | -0.113 | -0.035 | 0.245 | -0.085 |
| FBS (mg/dl) | 1 | 0.714** | 0.123 | 0.064 |
| PPBS (mg/dl) | – | 1 | 0.044 | 0.013 |
| AR (U/g Hb/min) | – | – | 1 | 0.002 |
| MDA (μmol/l) | – | – | – | 1 |
*Correlation is significant at the 0.05 level (2-tailed).
**Correlation is significant at the 0.01 level (2-tailed).
FBS: Fasting Blood Sugar, PPBS: Post-Prandial Blood Sugar, AR: Aldose Reductase, MDA: Malondialdehyde.
Discussion
The mean level of FBS and PPBS were found significantly higher in T2DM than controls (P<0.001). Previous studies have reported that the mean level of FBS and PPBS have been observed significantly higher in T2DM as compared to controls in all age groups.29-31
The mean activity of AR was found significantly high in the T2DM as compared to controls (P<0.001). It is reported that the AR activity increased in T2DM as compared to controls which are the leading cause of diabetic retinopathy in T2DM.15,27,32 The study has been reported that when intracellular glucose levels are high polyol pathway for glucose metabolism is activated by AR activity. It is observed that the activity of AR is significantly higher in diabetic patients with complications like retinopathy as compared to controls.33 Increased AR activity has been correlated with cataract formation and retinopathy.32
In addition, the mean level of MDA was also found significantly high in the T2DM as compared to controls (P<0.001). Previous studies have reported that the MDA levels have been found significantly increased in T2DM as compared to controls.34-36
A significant positive correlation was found between FBS and PPBS among T2DM (r=0.71, P<0.01). Previous studies have reported that the FBS and PPBS have been shown strong positive correlation in T2DM.29-31 However, There was no significant correlation found between AR activity and MDA level among T2DM (P>0.05). In the animal model, Lee et al reported that the flux of glucose through the polyol pathway is the major cause of hyperglycemic oxidative stress in the tissue.37 The decrease in the reduced glutathione (GSH) levels with a rise in the MDA level in the transgenic mice lens, but not in the non-transgenic mice. A study suggested that auto-oxidation of glucose and non-enzymatic glycation do not contribute significantly to oxidative stress in diabetic lenses. AR reduction of glucose to sorbitol probably contributes to oxidative stress by depleting its cofactor NADPH, which is also required for the regeneration of GSH. Sorbitol dehydrogenase, the second enzyme in the polyol pathway that converts sorbitol to fructose, also contributes to oxidative stress, most likely because depletion of its cofactor NAD/ leads to more glucose being channeled through the polyol pathway.3,13,17,37 The MDA plays only a minor role in the development of cataract. However, chronic oxidative stress generated by the polyol pathway is likely to be an important contributing factor in the slow-developing diabetic cataract and other diabetic complications.16,18,37 In hyperglycemia conditions, the rate of reduction of glucose to sorbitol increases with increasing glucose levels in tissues that do not require insulin for glucose uptake mainly in lens due to the AR activation.15 Several studies suggested that ROS, MDA and AR activity is generated in the significant amount in T2DM, which play a key role in the pathogenesisof T2DM and its complications.3,4,17
Genetic predisposition may influence the development of T2DM complications. The C(-104)T polymorphism in the regulatory region of the AR gene have observed the risk for diabetic retinopathy inT2DM.38 In diabetic mice model, the human AR expression regresses atherosclerosis may be due to interfering in plaque macrophage inflammation reduction.39
In the therapeutic approach, some studies proposed that the AR inhibitorsmayprevent the glucose flux by the polyol pathway in hyperglycemic conditions. The AR inhibitors may delay or reduce the development of T2DM and its complications.40,41
Limitations
This study has some limitations: firstly, the sample size is less and this study should be replicated in large sample size. Secondly, we have studied AR activity and MDA levels. Some other enzymes of the polyol pathway such as sorbitol dehydrogenase and glutathione levels should be included to clarify the consumption of NADPH and reduction of GSH simultaneously.
Conclusion
Results showed that the mean of FBS, PPBS, AR activity, and MDA levels were found significantly higher in T2DM than controls. There was no significant correlation found between AR activity and MDA level among T2DM. However, elevated AR activity and oxidative stress play a key role in the pathogenesis of T2DM and its complications.
Acknowledgements
We are grateful to the residents of Medicine department and Biochemistry department including the technical staffs for their help and support in carrying out my thesis work. We also acknowledge Dr. Mohammad Zafar Idris, Dean/Director, and Dr. Roshan Alam, Professor & Head, Department of Biochemistry, Integral Institute of Medical Sciences & Research, Integral University, Lucknow, Uttar Pradesh, India for the invaluable help and encouragement to carry out research work without any hindrance.
Conflict of Interest
Author S. Kumar, A. Kumar, M. M. Khan declare that they have no conflict of interest.
Funding Source
This study was not funded by any funding agency or company.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
References
- Cersosimo E, Triplitt C, Solis-Herrera C, Mandarino LJ, DeFronzo RA. Pathogenesis of Type 2 Diabetes Mellitus. [Updated 2018 Feb 27]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. [Available from: https://www.ncbi.nlm.nih.gov/books/NBK279115/].
- ADA: American Diabetes Association. Standards of medical care in diabetes-2016: Summary of revisions. Diabetes Care. 2019;42(Supplement 1):S13-S28. DOI:10.2337/dc19-S002.
- Ullah A, Khan A, Khan I. Diabetes mellitus and oxidative stress––A concise review. Saudi Pharmaceutical Journal. 2016;24:547–553.
- Volpe CMO, Villar-Delfinol PH, Anjos PMFD, Nogueira-Machado JA.Cellular death, reactive oxygen species (ROS) and diabetic complications.Cell Death and Disease.2018; 9:119. DOI 10.1038/s41419-017-0135-z.
- Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biology. 2015;4:180-183.
- Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Kurutas Nutrition Journal. 2016;15:71. DOI 10.1186/s12937-016-0186-5.
- Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum?Indian J EndocrinolMetab. 2016; 20(4): 546–551.
- IDF: International Diabetes Federation. IDF Diabetes Atlas, Eighth Edition. Brussels, Belgium: International Diabetes Federation; 2017. [Available at https://www.idf.org/our-network/regions-members/south-east-asia/members/94-india.html].
- WHO: World Health Organization. World Health Day 2016. Diabetes scale up prevention, strengthen care and enhance surveillance. Technical paper on diabetes in India.World Health Organization Country for India 2016. [Available at http://www.searo.who.int/india/mediacentre/events/world_health_day/whd-technical-paper-4-april.pdf?ua=1].
- Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das NH et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017. [Available at http://dx.doi.org/10.1016/S2213-8587(17)30174-2].
- Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6(3):456-480.
- Meo SD, Reed TT, Venditti P, Victor VM. Role of ROS and RNS Sources in Physiological and Pathological Conditions.Oxidative Medicine and Cellular Longevity. 2016: 2016(12450490:44. DOI:10.1155/2016/1245049.
- Lorenzi M. The Polyol Pathway as a Mechanism for Diabetic Retinopathy: Attractive, Elusive, and Resilient. Experimental Diabetes Research. 2007;2007(61038):10. DOI:10.1155/2007/61038.
- Ramana KV. Aldose Reductase: New Insights for an Old Enzyme. Biomol Concepts. 2011; 2(1-2): 103–114.
- Tang WH, Martin KA, Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol. 2012;3:87. DOI: 10.3389/fphar.2012.00087.
- Phaniendra A, Jestadi DB, Periyasamy L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind J ClinBiochem. 2015;30(1):11–26.
- Yan LJ. Redox imbalance stress in diabetes mellitus: Role of the polyol pathway. Animal Model Exp Med. 2018; 1(1): 7–13.
- Ayala A, Munoz MF, Arguelles S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Medicine and Cellular Longevity. 2014;2014(360438):31. DOI:10.1155/2014/360438.
- Barrera G, Pizzimenti S, Daga M, Dianzani C, Arcaro A, Cetrangolo GP et al. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants. 2018; 7(8):102. DOI:10.3390/antiox7080102.
- Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of Oxidative Stress during Diabetes Mellitus.Journal of Biomarkers. 2013;2013(378790):8. Doi:10.1155/2013/378790
- Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61(1):29-38.
- Dewanjee S, Das S, Das AK, Bhattacharjee N, Dihingia A, Dua TK et al. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur j Pharmacol. 2018;833:472-523.
- Sifuentes-Franco S, Padilla-Tejeda DE, Carrillo-Ibarra S, Miranda-Diaz AG. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy.International Journal of Endocrinology. 2018;2018(1875870):13. DOI:10.1155/2018/1875870.
- De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front Endocrinol. 2018;9:2. DOI: 10.3389/fendo.2018.00002.
- World Medical Association (WMA). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194.
- ADA: American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes- 2018. Diabetes Care. 2018;41(Suppl. 1):S13–S27. DOI:10.2337/dc18-S002.
- Reddy GB, Satyanarayana A, Balakrishna N, Ayyagari R, Padma M, Viswanath K et al. Erythrocyte aldose reductase activity and sorbitol levels in diabetic retinopathy. Mol Vis. 2008; 14: 593–601.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351-8.
- Khan MM, Sonkar GK, Alam R, Mehrotra S, Khan MS, Sonkar SK et al. Effect of age and body mass index on various clinical and anthropometric parameters of type 2 diabetic patients: a case-control study. Int J Health Sci Res. 2016; 6(11):132-142.
- Tiwari D, Kumar N, Alam R, Khan MM. Evaluation of serum magnesium and zinc levels in patients with Type 2 diabetes mellitus. International Journal of Clinical Biochemistry and Research. 2017;4(3):245-248.
- Khan S, Alam R, Khan MM. Association of Fructosamine and HbA1c in Newly Diagnosed Type 2 Diabetes Mellitus Patients.Indian Journal of Basic and Applied Medical Research. 2018:7(2):32-42.
- Nishimura C, Hotta Y, Gui T, Seko A, Fujimaki T, Ishikawa T et al. The level of erythrocyte aldose reductase is associated with the severity of diabetic retinopathy. Diabetes Res ClinPract. 1997;37:173–177.
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414:813-20.
- Moreto F, de Oliveira EP, Manda RM, Burini RC.The Higher Plasma Malondialdehyde Concentrations Are Determined by Metabolic Syndrome-Related Glucolipotoxicity. Oxidative Medicine and Cellular Longevity. 2014;2014(505368):7. DOI:10.1155/2014/505368.
- Casoinic F, Sampelean D, Buzoianu AD, Hancu N, Baston D. Serum Levels of Oxidative Stress Markers in Patients with Type 2 Diabetes Mellitus and Non-alcoholic Steatohepatitis. Rom J Intern Med. 2016;54(4):228-236.
- Ganjifrockwalaa FA, Josepha JT, George G. Journal of Endocrinology, Metabolism and Diabetes of South Africa. 2017; 22(2):21–25.
- Lee AYW, Chung SSM. Contributions of polyol pathway to oxidative stress in diabetic cataract.The FASEB Journal. 1999;13:23-30.
- Wihandani DM, Suastika K, Bagiada NA, Malik SG. Polymorphisms of Aldose Reductase (ALR2) Regulatory Gene are Risk Factors for Diabetic Retinopathy in Type-2 Diabetes Mellitus Patients in Bali, Indonesia. The Open Ophthalmology Journal. 2018;12:281-288. DOI: 10.2174/1874364101812010281.
- Yuan C, Hu J, Parathath S, Graer L, Cassella CB, Bagdasaraov S et al. Human Aldose Reductase Expression Prevents Atherosclerosis Regression in Diabetic Mice.2018;67(9):1880-1891.
- Gabbay KH. Aldose reductase inhibition in the treatment of diabetic neuropathy: where are we in 2004? CurrDiab Rep. 2004;4(6):405-8.
- Zhu C. Chapter 2: Aldose Reductase Inhibitors as Potential Therapeutic Drugs of Diabetic Complications. Published by IntechOpen Limited, London, UK January 2013. DOI:10.5772/54642 [Available at https://www.intechopen.com/books/diabetes-mellitus-insights-and-perspectives/aldose-reductase-inhibitors-as-potential-therapeutic-drugs-of-diabetic-complications].







