Ni Putu Linda Laksmiani* and I. Putu Wiratama Nugraha
and I. Putu Wiratama Nugraha
Department of Pharmacy, Faculty of Mathematics and Natural Sciences, Udayana University, 80362, Iodonesia.
Corresponding Author E-mail: lindalaksmiani@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1703
Abstract
Excessive exposure of UV light increase melanin synthesis and cause hyperpigmentation of the skin. The pharmacological activity of secang (Caesalpinia sappan L.) with the main compound, brazilien and brazilin as antioxidants that have potency as free radicals scavenger and directly inhibit tyrosinase activity in the process of melanogenesis. This study aims to determine the inhibitory ability of secang ethanolic extract on tyrosinase enzymes in vitro and evaluate the affinity of brazilein and brazilin as skin depigmentation agents against melanogenesis target protein in silico using molecular docking. In vitro testing using tyrosinase inhibitor assay with L-DOPA as its substrate and calculated the percentage inhibition value and IC50. The IC50 of the extract than compared with the positive control, namely kojic acid and ascorbic acid. Insilico research was carried out using autodock 4.2 program by evaluating the binding energy between the active compound of brazilein and brazilin with melanogenesis protein. Inhibition of the tyrosinase enzyme is showed through the IC50 value from ethanolic extract, kojic acid and ascorbic acid respectively 104 μg/ mL, 44 μg/mL and 37 μg/mL. Binding energy of the molecular docking process between brazilein, brazilin, kojic acid and ascorbic acid with the target protein of melanogenesis enzymes (tyrosinase, tyrosinase related protein 1, and D-Dopachrome tauomerase) are -8.37; -6.56; -5.03; -5.35 kcal/mol in tyrosinase, -7.75; -6.40; -5.32; -5.8 kcal/mol in tyrosinase related proteins 1 and -9.93; -8.26; -5.8; -6.52 kcal/mol in D-Dopachrome tautomerase. Secang ethanolic extract could be developed into a skin lightening agent or depigmentation agent through inhibition of 3 target proteins that induce melanogenesis. Although invitro results show the inhibitory ability of the tyrosinase enzyme is lower than kojic acid and ascorbic acid but in silico, it is seen that brazilein and brazilin in secang ethanolic extract have a stronger affinity compared to kojic acid and ascorbic acid. For this reason, it is necessary to purify the extract into a fraction so that it can get more active ingredients of brazilein and brazilin, and in vitro testing for inhibition of the tyrosinase related protein 1 enzyme, and D-Dopachrome tautomerase.
Keywords
Depigmentation; Molecular Docking; Secang (Caesalpinia Sappan L.); Tyrosinase Inhibitor Assay
Download this article as:| Copy the following to cite this article: Laksmiani N. P. L, Nugraha I. P. W. Depigmentation Activity of Secang (Caesalpinia Sappan L.) Extract Through Tyrosinase, Tyrosinase Related Protein-1 and Dopachrome Tautomerase Inhibition. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Laksmiani N. P. L, Nugraha I. P. W. Depigmentation Activity of Secang (Caesalpinia Sappan L.) Extract Through Tyrosinase, Tyrosinase Related Protein-1 and Dopachrome Tautomerase Inhibition. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2EZJAmy |
Introduction
Production of melanin in the skin is a normally effort of the body to protect the skin from the adverse effects of ultraviolet (UV) radiation. However, excessive exposure to UV light increase melanin synthesis and cause hyperpigmentation of the skin.1 Hyperpigmentation is a skin disorder that due to an increase in the process of melanogenesis which causes darkening of the skin color and this condition is the most common skin complaint experienced by people in Asia.
Biosynthesis of melanin in the melanosome requires three enzymes, including tyrosinase, tyrosinase-related protein 1 (TRP 1) and tyrosinase-related protein 2 (TRP 2) or D-Dopachrome tautomerase. Tyrosinase acts as a catalyst in two different reactions, namely the tyrosine hydroxylation process to dihydroxy-phenylalanine (L-DOPA) and L-DOPA oxidation become dopaquinone. Dopaquinone was converted to dopachrome by autoxidation and then was converted to 5, 6 dihydroxy indole-2-carboxy acid (DHICA) using D-Dopachrome tauomerase enzyme (TRP 2).2 DHICA is then converted to Indole-5,6-quinone carboxylic acid by TRP 1 to form eumelanin.3,4,5 Therefore the inhibition of those melanogenesis enzymes are able to reduce the effects of darkening of the skin as depigmentation activity.
The presence of pigmentation disorders is considered to disturb beauty, so one of the actions that must be taken for preventing the hyperpigmentation in the skin is by using skin lightening cosmetic or cosmeceuticals products. The skin whitening agent has been widely used in the cosmetics and clinical treatment world. These whitening products are used both as skin lightening or skin depigment. Substances which are commonly used as skin whitening such as hydroquinone, azelic acid, mercury and kojic acid.6,7 However, some of these compounds have dangerous side effects related to carcinogenesis and mutagenesis.8 Kojic acid at high concentrations induce hepatocarcinogenesis.9 Cosmetics with hydroquinone content will accumulate in the skin which can cause mutations and DNA damage.
Therefore it is necessary to develop depigmentation agents from nature. One of medicinal plants that potentially to manage hyperpigmentation is secang (Caesalpinia sappan L.). Brazilein and brazilin are major active compound on secang that proven have many pharmacological activity such as antioxidant, antiproliferation and induce apoptosis on some cancer cell.10 Those active compound are classified into homoisoflavonoid. Flavonoid have been evaluated could deactivates the tyrosinase enzyme and mediates the relationship between antioxidant properties and antimelanogenesis.11 The characteristics of flavonoids to form chelates with copper ions, inhibit tyrosinase by producing interactions between flavonoids with copper ions and the catalytic domain of tyrosinase.12 This research was conducted to investigate the depigmentation activity of secang ethanolic extract through melanogenesis enzym inhibition using tyrosinase inhibitor assay and in silico test with autodock 4.2 to evaluate the affinity of brazilein and brazilin contained from secang ethanolic extract with tyrosinase, TRP-1 and TRP-2 enzym compared ascorbic acid and kojic acid as depigmentation agent.
Materials and Method
Sample Collection
Sample of this research is heartwood of secang (Caesalpinia sappan L.). Heartwood of secang were obtained from Center for Research and Development of Medicinal Plants and Traditional Medicine, Tawangmangu Karanganyar, Jawa Tengah and determined directly by this department.
Chemical Materials
Ethanol 70% was purchased from Kurniajaya, Surabaya, Indonesia, tyrosinase from mushroom (Sigma), L-DOPA (Sigma), potassium dihydrogen phosphate (Merck), dipotassium hydrogen phospate (Merck), L-ascorbic acid (Sigma-Aldrich), kojic acid (Sigma-Aldrich).
Extraction of Secang Heartwood
Dried heartwood of secang was powdered and extracted by maceration with ethanol 70%. The ratio of powder sample and solvent 1:10 (for 48 h). The ethanolic extract was then concentrated under reduce pressure by rotary evaporator.
Tyrosinase Inhibitor Assay
Preparation of phosphate buffer pH 6.5; 2.5 mM L-DOPA solution in phosphate buffer, tyrosinase solution 240 units / mL in phosphate buffer. The maximum wavelength measurements were carried out with 2700 μL of 50 mM phosphate buffer solution (pH 6.5) and 800 μL of L-DOPA solution pipetted into the test tube. The solution was incubated at room temperature for 10 minutes. Then 500 µL of the tyrosinase solution was added to the test tube and incubated again at room temperature for 25 minutes. Uptake was measured by a UV-Vis spectrophotometer to obtain maximum absorption. Testing the inhibition of secang ethanolic extract against the enzyme tyrosinase was carried out by comparing the inhibitory value of kojic acid and ascorbic acid. The measurements were carried out using a UV-Vis spectrophotometer. For each test compound made in a different test tube.
The non-inhibiting test solution was made with a phosphate buffer solution and L-DOPA was pipetted and put into a test tube, then incubated at room temperature for 10 minutes. After the tyrosinase solution was added to the test tube, it was homogenized using a vortex mixer and re-incubated for 25 minutes at room temperature. Then the absorption was measured with a UV-Vis spectrophotometer at the maximum wavelength of measurement. The test solution with inhibitors was made with 2500 μL phosphate buffer solution, L-DOPA and 200 μL of inhibitor agent. All of solution put into a test tube, then incubated at room temperature for 10 minutes. After that, a tyrosinase solution was added to the test tube, homogenized with a vortex mixer and incubated again for 25 minutes at room temperature. Then the absorption is measured at the maximum measurement wavelength.
Molecular Docking
Target Protein Preparation
Protein preparation was carried out using the Chimera 1.10.1 program by separating the 3-dimensional structure of the tyrosinase with protein data based (PDB ID: 2Y9X), tyrosinase-related protein 1 (TRP 1) (PDB ID: 5M8M) and tyrosinase-related protein 2 (TRP 2) (PDB ID: 3KAN) from its native ligand. The first step of protein preparation is removal of water molecules (H2O) in the target protein. Then the native ligand on the target protein is then eliminated by the Chimera 1.10.1 program which aims to provide pocket (cavity), so that the pocket coordinates and binding site center as docking material can be known. Separate native ligands are used for method validation.
Brazilien, Brazilin, Ascorbic Acid and Kojic Acid Optimization
The 3-dimensional structure of compound such as brazilein, brazilin, ascorbat acid and kojic acid optimized using the Hyperchem 8 program complete with hydrogen atoms. Optimization of the 3-dimensional structure of compound by using AM1 semi-empirical computational methods and single-point calculations and geometry optimization. Single point calculations are used to find out the total energy of compound configurations, while geometry optimization is used to find stable configuration values with lower values than single point calculations.
Molecular Docking Method Validation
Validation of molecular docking method is done by redocking the native ligand of each target protein to the target protein that has previously been removed by the native ligand. Redocking is held using the AutoDock Tools application with the Autodock 4.2 and Autogrid programs. The validation parameter of molecular docking method is the value of root mean square distance (RMSD). This RMSD is a deviation from the position of the native ligand bond with protein after being docking to the actual position of the native ligand bond. The method is said to be valid if the value of RMSD is ≤ 3.0 Å.13 After the method used is valid, docking of the test compound on the target protein can be done.
Docking Brazilien, Brazilin, Ascorbic Acid and Kojic Acid to Tyrosinase, Tyrosinase-Related Protein 1 (TRP 1) and Tyrosinase-Related Protein 2 (TRP 2) or D-Dopachrome Tautomerase As Target Protein
The optimization result of brazilein, brazilin, ascorbat acid and kojic acid are then docking to the target protein that has been prepared (the native ligand is omitted). Docking is done using the Autodock 4.2 program. The results of the analysis will show the compound with conformation with the lowest binding energy to bind to the target protein.
Data AnalysisTyrosinase Inhibitor Assay
In tyrosinase inhibitor assay method, the calculation of % tyrosinase inhibition due to treatment is by comparing the difference in absorbance of the non inhibiting solution with the absorbance of inhibiting test compound solution to the absorbance of the non inhibiting solution multiplied by 100% (Equation (1). To determine whether or not there is a tyrosinase inhibition activity, a computerized linear regression analysis is performed to obtain the IC50 value.
![]()
Where Abs non is absorbance of non inhibiting solution (consist of L-DOPA and tyrosinase) and Abs test is absorbance of inhibiting test compound solutioan (consist of L-DOPA, test extract or compound and tyrosinase).
Molecular Docking Analysis
Molecular docking results are binding energy and the type of hydrogen bond formed between compounds and target proteins. The type of hydrogen bond formed between compounds and target protein is used to analyze the mechanism of interaction formed. While the binding energy obtained was used for the analysis of the affinity of compounds on the target protein. Binding energy values indicate the bond strength between compounds and proteins. Lower the binding energy value means stronger and more stable bond, so that the affinity or ability of the compound bind to the target protein is getting higher. If the binding energy between compounds and melanogenesis enzyme target protein (tyrosinase, TRP 1, and TRP 2) gets lower, stronger interaction between compounds and melanogenesis enzyme target protein, so that compounds have potential as a melanogenesis enzyme inhibitor and depigmentation activity.
Results and Discussion
Secang Ethanolic Extract Inhibit Tyrosinase Activity Using Tyrosinase Inhibitor Assay
Tyrosinase is an enzyme monooxygenase which acts as a catalyst in the hydroxylation reaction of monophenol to form diphenol (monophenolase) and oxidation of diphenol to quinone (diphenolase). Tyrosinase plays an important role in melanin formation during the process of melanogenesis because tyrosinase is able to hydroxylate L-tyrosine (monophenol) to L-DOPA (diphenol) and oxidize L-DOPA to dopaquinone (quinone compound). Dopaquinone formed will react spontaneously to form dopakrom. Its role in the process of melanogenesis occurs because tyrosinase has a copper (Cu) group which is an active site that can bind to the substrate in the process of forming melanin.14,15 In vitro tyrosinase inhibition was carried out based on measurements of orange to red compounds, dopachrome which is the result of L-DOPA oxidation by tyrosinase.16 Colour of dopachrome product depends on concentration of L-DOPA. The presence of inhibitors causes the production of dopachrome reduced which is marked by decreasing an orange intensity. Based on the lock and key theory, enzymes only bind to a specific substrate so that the substrate used must be appropriate because it will affect the reaction results. There are two substrate which play an important role in the formation of dopacrome, namely L-tyrosine and L-DOPA.17,18 L-DOPA is chosen as substrate because based on the reaction, dopachrome products absorption values could be measured by UV-VIS spectrophotometry method. This whole process involves an enzymatic reaction where all reactions run at optimum conditions. One of the optimum conditions that necessary to be considered is wavelength of dopachrome.19 Absorbance reading of dopachrome product is carried out at the maximum wavelength of it at 480 nm shown in Figure 1. At the maximum wavelength, the molar absorptivity of the dopachrome product is constant, the error of absorbance reading is relatively small and much sensitive.
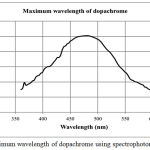 |
Figure 1: Maximum wavelength of dopachrome using spectrophotometry visible.
|
Dopachrome was formed from tyrosinase enzyme catalysis against L-DOPA.
Potency of secang (Caesalpinia sappan L.) ethanolic extract has been evaluated in this study using tirosinase inhibitor assay. The value of tyrosinase enzyme inhibition activity was obtained by calculating the dopachrome inhibition formed using equation 1 to get the inhibition percentage. Percentage of inhibition was compared with the concentration of extract so that the linear regression equation was obtained and the IC50 value was calculated. Tyrosinase enzyme inhibition activity is indicated by IC50 value, which is the concentration that inhibit 50% of the tyrosinase enzyme. Secang ethanolic extract compared with kojic acid and ascorbic acid as a positive control. Kojic acid is a fungal metabolite that acts as a chelator and has been shown to be a skin lightening agent in cosmetics as well as vitamin c.The results of the inhibitory tyrosinase assay of secang ethanolic extract, kojic acid and ascorbic acid in vitro are presented in Figure 2. The measurement of IC50 from the extract was carried out by making a series of extract concentrations of 5, 10, 25, 50, 100, 150 and 200 μg/mL. Where as for kojic acid and ascorbic acid using series levels of 2.5, 5, 10, 20, 40, 60 and 80 μg/mL. This concentration series will be used to make linear equations to determine IC50 extract values, kojic acid and ascorbic acid. In the linear equation obtained shows a dose dependent phenomenon, the inhibitory effect increases with increasing concentration. Increasing inhibitory effect is indicated by the increasing percentage of inhibition on the tyrosinase enzyme activity by giving higher concentration. With the increasing concentration of secang ethanolic extract, the absorbance value becomes smaller, which means smaller amount of dopachrome is formed due to the enzyme tyrosinase resistance and can no longer be a catalyst. The IC50 value of the ethanolic extract was 104 μg/mL seen in Table I. While kojic acid and ascorbic acid have IC50 values of 44 μg/mL and 37 μg/mL respectively. Ethanol extract has a greater IC50 value compared to kojic acid and ascorbic acid. It happen caused by secang ethanolic extract still in the form of crude extract. Therefore, fractionation of extract needs to be done so the inhibition of the catalytic mechanism of enzyme tyrosinase becomes higher and the IC50 of this fraction as much as kojic acid and ascorbic acid.
Table 1: The IC50 of tyrosinase inhibitory effect of secang ethanolic extract.
| Name | IC50 Value |
| Positive Control (Standard) | |
| Kojic acid | 44 μg/mL |
| Ascorbic acid | 37 μg/mL |
| Extract | |
| Secang ethanolic extract | 104 μg/mL |
 |
Figure 2: Percentage inhibition of tyrosinase activity caused by treatment of kojic acid (A); ascorbic acid (B) and secang ethanolic extract (C). Results are shown as mean ± SD (n=3).
|
Brazilein and Brazilin, Major Compound In Secang Ethanolic Extract Have the Potential Activity to Inhibit Tyrosinase, Tyrosinase-Related Protein 1 (TRP 1) and Tyrosinase-Related Protein 2 (TRP 2) or D-Dopachrome Tautomerase as Target Protein by Molecular Docking
Process of separating target protein from tyrosinase enzyme with native ligand was successfully carried out which resulted a target protein structure file without native ligand and native ligand solely in pdb format. In the process of molecular docking method validation, 10 conformations were obtained and one conformation from each brazilein and brazilin as active compound in secang extract with the lowest RMSD value was selected. The results of the validation of the molecular docking method indicate that the value of RMSD ≤ 3 Å (Table II) means the method used has met the conditions of use and will give a valid data. This is because the native ligand coordinates are close or almost the same as the initial position on the active side of the protein. RMSD value ≤ 3 Å is generally used as a reference for success in docking methods.13,20,21
Table 2: RMSD value of molecular docking validation method.and binding energy of active compound with target proteins using molecular docking.
| No | Target proteins | RMSD (Å) | Ligand | Binding energy (kcal/mol) | Hydrogen bond | (Ligand-protein) group |
| 1 | Tyrosinase (2Y9X) | 2.06 | Native ligand | -4.79 | HIS61 | OA1-HE2 |
| Brazilein | -8.37 | HIS296 | O-HE2 | |||
| Brazilin | -6.56 | HIS244 | O-HE2 | |||
| Kojic acid | -5.03 | HIS259 HIS296 | O-HE2
O-HE2 |
|||
| Ascorbic acid | -5.35 | HIS259 HIS296 | O-HE2
O-HE2 |
|||
| 2 | Tyrosinase related protein 1/TRP 1 (5M8M) | 2.33 | Native ligand | -5.37 | SER394
HIS404 HIS192 |
O5-HG
O6-HE O6-HE2 |
| Brazilein | -7.75 | ARG321 | O-HH11 | |||
| Brazilin | -5.39 | HIS192
HIS404 |
O6-HE
O6-HE2 |
|||
| Kojic acid | -5.32 | HIS215 | O-HE2 | |||
| Ascorbic acid | -5.82 | HIS377
HIS404 SER394 |
O-HE2
O-HE2 O-HG |
|||
| 3 | Dopachrome tautomerase/TRP 2 (3KAN) | 2.46 | Native ligand | -6.49 | PRO1
ILE64 |
N1-HN1
N3-HN |
| Brazilein | -9.93 | LYS32
ASN38 PRO1 |
O-HZ2@B
O-HD22 O-HN1 |
|||
| Brazilin | -8.26 | LYS109 | O-HZ2 | |||
| Kojic acid | -5.8 | PRO1
ASN38 LYS32 |
O-HN1
O-HD22 O-HZ3@B |
|||
| Ascorbic acid | -6.52 | PRO1
LYS109 ILE64 ASN38 |
O-HN1
O-HNZ2 O-HN O-HD22 |
 |
Table 3: Interaction between of brazilein, brazilin, kojic acid and ascorbic acid with target protein melanogenesis enzyme with amino acid residue on target protein.
|
Based on the docking results shown in table II, brazilein and brazilin with amino acids in the target protein of the enzyme melanogenesis form hydrogen bonds. In addition to the formation of hydrogen bonds, molecular docking also produces binding energy between the test compound and the target protein. This binding energy is generated from the scoring function of values produced from protein complexes with ligands or test compounds. Where in conformity search, molecular docking will predict the position of a ligand [I] on a target protein [E] and will form a complex [E + I] = [EI]. This bond energy is also called Gibbs energy (ΔG). This energy is related to the affinity of ligands for proteins.22 The binding energy obtained by using the autodock 4.2 program by summing the final intermolecular energy, final total internal energy, and tertional free energy then reduced by unbound system’s energy. The potential affinity of brazilin and brazilein for target proteins could be seen by comparing the binding energy formed between the active content and the target protein against the native ligand binding energy with the target protein. Molecular docking brazilein and brazilin with the target protein of melanogenesis enzymes (tyrosinase, tyrosinase related protein 1, and D-Dopachrome tautomerase) using the Auto Dock Tools application equipped with the Autodock 4.2 program and Autogrid producing RMSD parameters, hydrogen bonds and binding energy values. Analysis of binding energy in the results of molecular docking of brazilein and brazilin with target proteins produces a fairly low binding energy.
Binding energy in the molecular docking process can illustrate how the interaction and affinity between a test compound, brazilein and brazilin with the target protein. Affinity is the ability of a compound to bind to a target protein. The conformation was chosen with the lowest binding energy value because the lower binding energy value between brazilein and brazilin with the target protein, the stronger the bond formed. This is because the lower binding energy, the smaller the energy needed to form the bond and also greater energy needed by the compound to break the bond, so that the compound is more stable and produces greater affinity. Observation of the interaction of ligands (compounds) with proteins includes hydrogen bonds formed for conformation with the lowest bond energy. The structure of the compound docking with the target protein must be on the same active side as native ligand with a validated method. When the active site of binding between brazilein, brazilin and native ligand is the same, it will produce the same activity as the activity that is native ligand to the target protein. Based on Table II, the results of brazilein and brazilin docking with the target protein of melanogenesis enzymes produce negative (-) bond energy values. exothermally. This means that the formation of a bond between brazilein and brazilin with each target protein takes place spontaneously.
The interaction of hydrogen bonds can be seen by using various forms, for example in the form of lines, balls and cylinders to facilitate the observation process. The interaction of hydrogen bonds between native ligand, brazilein and brazilin with each target protein using a cylindrical have been demonstrated in Table III. Table II and Table III show that brazilein and brazilin have potency to inhibit the enzyme melanogenesis with a stronger affinity and a more stable bond compared to the native ligand, kojic acid and ascorbic acid as agents proven to be skin lightening. The melanogenesis enzyme is the three main enzymes that are known to have an important role in the process of skin pigmentation, especially in the eumelanin formation pathway which causes the skin to become darker. Tyrosinase acts as a catalyst in two different reactions, namely the tyrosine hydroxylation process to dihydroxy-phenylalanine (L-DOPA) and L-DOPA oxidation to dopaquinone.23,24 In the eumelanin pathway, dopaquinone is converted into dopachrome through autoxidation. D-Dopachrome tauomerase catalyze dopachrome become 5,6 dihydroxy indole-2-carboxy acid (DHICA). D-Dopachrome tauomerase is also often known as tyrosinase related protein 2. DHICA is then converted into Indole-5,6-quinone carboxylic acid by tyrosinase related protein 1 to form eumelanin (brown pigment).25 Inhibiting of these three enzymes, it will cause depigmentation activity. Based on the results of in silico molecular docking, brazilein and brazilin have depigmentation activities through the mechanism of inhibition of melanogenesis enzymes namely tyrosinase, tyrosinase related protein 1, and D-Dopachrome tauomerase on the eumelanin formation pathway, so that eumelanin will be less formed and the skin will brighter. Further research needs to be done by testing the purified fraction of secang ethanolic extract so that the amount of brazilein and brazilin becomes more numerous and the IC50 value in inhibiting the enzyme tyrosinase becomes lower and almost the same as kojic acid or vitamin C.
Conclusion
Secang ethanolic extract can be developed as a skin lightening agent because it is able to inhibit the enzyme tyrosinase in vitro. Brazilein and brazilin as the major compound of secang extract have a strong affinity and inhibit the three melanogenesis enzymes namely tyrosinase, TRP 1 and TRP 2 in silico through hydrogen bond formation and have lower binding energy than native ligand. Furthermore, fractionation of extracts is needed because it is a promising candidate for development as a skin lightening agent.
Acknowledgements
This research was funded by the ministry of research, technology and universities in granting regional superior product development programs (PPPUD).
Conflict of Interest
There is no conflict of Interest.
References
- Arung E. T, Furuta, S, Ishikawa H, Kusuma I. W, Shimizu K and Kondo R. 2011. Anti-melanogenesis properties of quercetin- and its derivative-rich extract from Allium cepa. Food Chemistry. 124: 1024–1028.
- Videira I.F.S, Moura D.F.L and Magina S. 2013. Mechanisms Regulating Melanogenesis. An Bras Dermatol. 88:76-83.
- Cayce K. A, McMichael A. J and Feldman S. R. 2004. Hyperpigmentation: An Overview of The Common Afflictions, Dermatol Nursing. 16: 401-416.
- Costin G. E, Valencia J. C, Wakamatsu K, Ito S, Solano F, Milac A. L, Vieira W. D, Yamaguchi Y, Rouzaud F, Petrescu A. J, Lamoreux M. L and Hearing V. J. 2005. Mutations in dopachrome tautomerase (Dct) affect eumelanin/pheomelanin synthesis, but do not affect intracellular trafficking of the mutant protein. J. 391: 249–259.
- Ebanks J. P, Wickett R. R and Boissy R. E. 2009. Mechanisms Regulating Skin Pigmentation: The Rise and Fall of Complexion Coloration, International Journal of Molecular Sciences. 10: 4066-4087.
- Alam N, Yoon K. N, Shin P. G, Cheong J. C, Yoo Y. B and Lee T. S. 2011. Antioxidant, Phenolic Compounds Concentration, Xanthine Oxidase and Tyrosinase Inhibitory Activities of Pleurotus cornucopiae, Australian Journal of Basic and Applied Sciences. 5: 229-239.
- Choi M and Shin H. 2016. Anti-Melanogenesis Effect of Quercetin. Cosmetics. 3: 1-16.
- Lin J, Chiang H, Lin Y and Wen K. 2008. Natural Products with Skin–Whitening Effects. Journal of Food and Drug Analysis. 16: 1-10.
- Chusiri Y. 2011. Non-genotoxic mode of action and possible threshold for hepatocarcinogenicity of Kojic acid in F344 Food and Chemical Toxicology. 49: 471-476.
- Laksmiani L, Susidarti R. A and Meiyanto E. 2015. Brazilein increase the sensitivity of doxorubicin on MCF-7 resistant doxorubicin (MCF-7/DOX) cells through inhibition of HER-2 activation. Int J Pharm Pharm Sci. 7(2): 525-528.
- Choi M and Shin H. 2016. Anti-Melanogenesis Effect of Quercetin. Cosmetics. 3: 1-16
- Kim Y. J and Uyama H. 2005. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cellular and Molecular Life Sciences. 62: 1707–1723.
- Jain A. J and Nicholls A. 2008. Recommendations for evaluational methods. Comput. Aided Mol, 22: 133-139.
- Ramsden C. A and Riley P. A. 2014. Tyrosinase: the four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg Med Chem. 22 (8): 2388-2295.
- Silva A. P, Silva N. F, Andrade E. H. A, Gratieri T, Setzer W. N, Maia G. S and Silva J. K. R. 2017. Tyrosinase inhibitory activity, molecular docking studies and antioxidant potential of chemotypes of Lippia origanoides (verbenaceae) essential oils. PLoS ONE. 12: 1-17.
- Endang L and Holzgrabe U. 2014. Bioactive compounds in bengkoang (Pachyrhizus erosus) as antioxidant and tyrosinase inhibiting agents. Indonesian J. Pharm. 25 (2): 68-75.
- Thanigaimalai P, Manickam M and Namasivayam V. 2017. Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. Journal of Enzym Inhibition and Medicinal Chemistry. 32(1): 403-425.
- Chan Y. Y, Kim K. H, Cheah S. H. 2011. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J Ethnopharmacol. 137 (3): 1183-1188.
- Zuo A, Dong H. H, Yu Y.Y, Shu Q. L, Zheng L. X, Yu X. Y and Cao S. W. 2018. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups 2018. Chin Med. 13:51.
- Feinstein W. P and Brylinski M. 2015. Calculating an Optimal Box Size for Ligan Docking and Virtual Screening Against Experimental and Predicted Binding Pockets. Journal of Cheminformatics. 7: 1-10.
- Ferreira G. L, Santos R. N. D, Oliva G and Andricopulo A. D. 2015. Molecular Docking and Structure-Based Drug Design Strategies. Molecules. 20: 13384-13421.
- Kitchen D. B, Decornez H, Furr J. R and Bajorath J. 2004. Docking and Scoring in Virtual Screening in Drug Discovery, Methods and Applications. Rev. Drug Discov. 3: 935-949.
- Fais A., Corda M, Era B, Fadda M. B, Matos M. J, Quezada E, Santana L Picciau C, Podda G and Delogu G. Tyrosinase Inhibitor Activity of Coumarin-Resveratrol Hybrids. Molecules. 14: 2514-2520.
- Fu R, Zhang Y, Guo Y and Chen F. 2014. Antioxidant and tyrosinase inhibition activities of the ethanol-insoluble fraction of water extract of Sapium sebiferum (L.) Roxb. Leaves. South African Journal of Botany. 93: 98–104.
- Gillbro J. M and Olsson M. J. 2010. The Melanogenesis and Mechanisms of Skin-Lightening Agents – Existing and New Approaches. International Journal of Cosmetic Science. 33: 210–221.








