Manuscript accepted on :13-May-2019
Published online on: 01-06-2019
Plagiarism Check: Yes
Reviewed by: Shipra Gupta
Second Review by: M. Mohan Varma
Badr E. El-Bialy*1, Neveen G. El-Boraey2, Ragaa A. Hamouda3 and Mohamed M. Abdel-Daim4
1Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, 32897, Egypt.
2Department of Microbial Biotechnology, Genetic Engineering and Biotechnology Research Institute (GEBRI), University of Sadat City, Sadat City, 32897, Egypt.
3Department of Biology, Faculty of sciences and Arts Khulais, University of Jeddah, Saudi Arabia Department of Microbial Biotechnology, Genetic Engineering and Research Institute, Sadat University, Sadat city, 32897, Egypt.
4Department of Pharmacology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt.
Corresponding Author E-mail: badr_elsaid10@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1670
Abstract
Carbon tetrachloride (CCl4) is used extensively as an industrial solvent and considered the best-characterized experimental animal model of xenobiotic-induced hepatic toxicity via reactive oxygen species (ROS) generation. This study was designed to evaluate the protective effects of Spirulina platensis (SP) versus Spirulina platensis supplemented with thiamine (SPt) against subacute CCl4 toxicity in rats. Rats were divided into six equal groups; Control vehicle (0.5 ml/rat 1:1 olive oil in water), SP (800 mg/kg b.wt.), SPt (800 mg/kg b.wt.), CCl4 (1ml/kg b.wt.), SP + CCl4 and SPt + CCl4. All treatments were orally and daily for a month except CCl4 was given three times weekly. CCl4 caused significant reduction in body weight gain, haemoglobin content and haematocrit percentage accompanied by leukocytosis, granulocytosis, monocytosis and lymphocytopenia. Moreover, there were significant increase in the levels of serum ALT, AST; total, direct and indirect bilirubin; urea and creatinine of CCL4- intoxicated rats. CCL4- induced significant increase of malondialdehyde levels with significant reduction of catalase activity in liver and kidney. In addition, hepatic and renal various histopathological alterations were recorded. SP and SPt ameliorated almost these changes while they couldn’t reverse the reduction of body weight gains and red blood indices. The more potent effects on measured parameters were elucidated by SPt. In conclusion SP and SPt could be used as natural antioxidant supplements to counteract the CCl4 adverse effects.
Keywords
Antioxidants; Biomarkers; CCl4; Haematology; Oxidative Stress; Spirulina
Download this article as:| Copy the following to cite this article: El-Bialy B. E, El-Boraey N. G, Hamouda R. A, Abdel-Daim M. M. Comparative Protective Effects of Spirulina and Spirulina Supplemented with Thiamine Against Subacute Carbon Tetrachloride Toxicity in Rats. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: El-Bialy B. E, El-Boraey N. G, Hamouda R. A, Abdel-Daim M. M. Comparative Protective Effects of Spirulina and Spirulina Supplemented with Thiamine Against Subacute Carbon Tetrachloride Toxicity in Rats. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2MivHWG |
Introduction
Reactive oxygen species are among the main causes of almost degenerative diseases. Oxidative stress induced when generated ROS and free radicals overwhelm the antioxidant protective capacity1.
Carbon tetrachloride is well known for causing oxidative stress, hepatotoxicity and nephrotoxicity in rats2,3. CCl4 intoxication augmented oxidative stress due to free radicals generation that is thought to be the reason behind tissue damage4.
Antioxidants possess protective and therapeutic values against various diseases by increasing the levels of body endogenous antioxidants and hence, decrease lipid peroxidation process5. Previous studies proved that natural substances from edible and medicinal plants exhibited strong antioxidant protective activity against CCl4-induced oxidative damage, because they contain various free radical scavengers such as phenolic and flavonoid compounds6.
Spirulina platensis is a microscopic blue-green alga (cyanophytes /cyanobacteria). Its nutritional values referred to its constituents of proteins of essential amino acids (55%-70%), carbohydrates (15%-25%), essential fatty acids (18%) minerals, vitamins and pigments like phycocyanin, carotenes, and chlorophyll7. The antioxidant properties of SP could be attributed mainly to polyunsaturated fatty acids, phycocyanin and phenolic contents8, in addition to some essential elements that have antioxidant effect like zinc and selenium9.
Previous studies cleared protective effects of SP against many toxicants. Simultaneous administration of SP to lead exposed animals significantly inhibited lipid peroxidation and restored the endogenous antioxidants to normal levels in liver, lung, heart, kidney and brain of rats10. SP has a protective effect against cardiotoxicity induced by doxorubicin in mice11. It has antioxidant properties to scavenge free radicals and to inhibit lipid peroxidation induced by cadmium in liver of rats12. SP supplementation could overcome deltamethrin induced hepatotoxicty, nephrotoxicity and neurotoxicity by abolishing oxidative tissue injuries13.
This study aimed to evaluate the potential protective effects of SP versus SPt against CCl4 induced haematological changes, hepatotoxicity and nephrotoxicity in rats.
Materials and Method
Chemicals and Diagnostic kits
Chemicals of Zarrouk’s medium, phosphate buffer saline (PBS), diethyl ether, CCl4 and chemicals of histopathology were purchased from El Nasr Chemical Company, Cairo, Egypt.
Diagnostic kits for assaying serum aminotransferases (alanine aminotransferase, ALT and aspartate aminotransferase, AST) were purchased from Spinreact Ctra. Sta. Coloma, Girona, Spain. Kits for assaying total bilirubin, direct bilirubin, urea and creatinine were purchased from the Diamond Diagnostic Company, Holliston, USA.
Diagnostic kits for assaying malondialdehyde (MDA) level as lipid peroxidation product and catalase (CAT) activity in hepatic and renal tissue homogenates were purchased from the Biodiagnostics Company, Dokki, Giza, Egypt
Constituents and Preparation of Zarrouk’s Medium
Zarrouk’s medium consisted of 1L distilled water, 1g/L NaCl, 0.2g/L MgSO4.7H2O, 0.01g/L FeSO4.7H2O, 0.04g/L CaCl2.2H2O, 0.08g/L Na2EDTA , 0.5g/L K2HPO4, 2.5g/L NaNO3, 1g/L K2SO4 and 16.8g/L NaHCO3 Plus A5 micronutrient 1ml micronutrients (H3BO3, MnCl2.4H2O, ZnSO4.4H2O, Na2MoO4, CuSO4.5H2O).
One liter of Zarrouk’s medium was prepared in a glass flask with adjusting PH of the medium to 9.5 (that suitable for growth of SP). Then this medium was sterilized in the autoclave under moist temperature at 121°C for 20 minutes after stoppering by aluminum foil. One liter is divided in flasks each contains 200 ml of the medium.
Spirulina collection and identification
Spirulina platensis (SP) was obtained from Al-Natron valley, Egypt. Its purification and identification were done according to methods described by Rippka14 and Vonshak15 respectively in microbiology lab, Genetic Engineering and Biotechnology Research Institute (GEBRI), University of Sadat City, Sadat City Egypt. S. platensis was isolated after repeated light migrations on its medium described by Zarrouk16. It was grown in Erlenmeyer flasks containing 200 ml Zarrouk’s medium. at 25±2°C, pH 9.5 with continuous illumination using cool white fluorescent light (2500 Lux) and twice daily shaking by hand for 15 days. Cells were collected by filtration using filter paper 8 mm pore size (Screen printing paper) and washed with buffer solution (pH 7), diluted to known volume and processed for further inoculation (kept as stock solution).
The cultured S. platensis was recultivated by adding 50 ml of stock solution after its vigorous shaking to flasks contained 200 ml Zarrouk’s medium or Zarrouk’s medium supplemented with thiamine (0.01g/L) and incubated under the same mentioned conditions for 21 days.
Spirulina algae (SP and SPt) were collected by filtration and then dried in oven at 75°C for 2-6 hrs and then grinded by mortar until became powder. The required daily dose from spirulina powder (SP or SPt) is dissolved in water to be in suspension form at the day of its administration to animals using Ultrasonic homogenizer sonicator (Biologics Inc. USA manufacturer and leading innovator).
Experimental animals
Sixty male Sprague-Dawley rats (120-150 g) were used in this study. They were purchased from AL-Zyade Experimental Animals Production Center, Giza, Egypt. All animals were placed in polypropylene cages with mesh wire tops. They kept under normal day and night cycle in a natural ventilated room with good hygienic conditions. Rats fed standard chow diet and clean tap water ad libitum. The rats were maintained for 2 weeks prior to the start of experiment for acclimatization. Animal rearing and handling guide lines were approved by the Research Ethical Committee of the Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt.
Experimental design
After acclimatization period, rats were divided into six equal groups and then weighed (Initial body weights) as following:
Group 1 (Control vehicle): Animals administered olive oil with water 1:1 orally (0.5 ml/rat/day) as vehicle of both Spirulina and CCl4.
Group 2 (SP): Animals administered S. platensis (800 mg/kg body weight).
Group 3 (SPt): Animals administered S. platensis supplemented with thiamine (800 mg/kg body weight).
Group 4 (CCl4): Animals administered CCl4 (1ml/kg body weight) in olive oil (1:1).
Group 5 (SP + CCl4): Animals were pretreated with SP an hour before CCl4 administration.
Group 6 (SPt + CCl4): Animals were pretreated with SPt an hour before CCl4 administration.
All treatments were orally and daily for a month except CCl4 was given three times weekly.
Samples Collection
At the end of the experiment (after one month), the animals were weighed (Final body weights) to calculate body weight gains, fasted overnight and then anaesthetized using diethyl ether and sacrificed for samples collection.
Blood samples were collected from the median canthus of the eye using heparinized capillary tube. Blood sample of each rat was received in two tubes (tube containing EDTA for haemogram and plain centrifuge tube for serum separation). Serum samples were stored at -20°C until biochemical assay.
After blood collection, rats were sacrificed. Liver and kidneys of each rat were collected. A part of each organ was homogenized in phosphate buffered saline and kept at -80°C after that rapid tissue biochemical investigations were performed (including lipid peroxidation and catalase activity). Another part was kept in 10% neutral buffer formalin solution for the histopathological examination.
Haematological Estimation
Haematological parameters were estimated automatically using H32 VET 3-Part Differential Hematology Analyzer (Avantor Performance Materials Inc. Company, Center Valley, USA)
Blood indices including red blood cells count (RBCs), haemoglobin concentration (HGB), hematocrit percentage (HCT%), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), total leukocytes (TLC), differential leukocyte percentages and platelets count.
Serum Biochemical Analysis
Serum biochemical parameters were estimated colourimetrically according to methods of Murray17 for ALT and AST activities respectively, Kaplan et al.18 for total and direct bilirubin, Fawcett and Soctt19 for urea level and Schirmeister20 for creatinine level.
Determination of lipid peroxidation product and catalase activity in liver and kidney tissue homogenates
Malondialdehyde was estimated according to the procedure described by Ohkawa et al.21 and CAT activity was determined according to and Aebi22, following instructions of kits.
Histopathological Examination
Liver and kidney samples intended for histopathological investigations were fixed in 10 % neutral buffer formalin for 72 hrs. Samples were trimmed and processed by dehydration in serial grades of ethanol in 50–100%, cleared in xylene, and then embedded in paraffin. Sections (4 μm thick) were prepared by a microtome and then stained with hematoxylin and eosin as a general staining for tissue examination. The liver sections were examined for pathological changes by a light microscope.
Statistical Analysis
Values are presented as mean ± standard error of mean (SEM). Statistical significance of toxic effects of CCl4 and the protective effects of SP and SPt were determined by one way ANOVA (Analysis of Variance) followed by Duncan’s multiple range test. All statistical analyses were performed using SPSS (Statistical Package for Social Sciences) Version 16 released on 2007. Statistical significances between different means were considered at P<0.05.
Results
Changes in Body Weight Gains
Changes in body weight gains are recorded in Table 1. Compared to control vehicle group, oral administration of SP caused significant reduction (P<0.05) in body weight gains, in contrary, oral SPt administration induced insignificant increase in body weight gains.
Table 1: Mean values of initial, final body weights (g) and body weight gains (g) of control and different treated groups.
| Initial body weights | Final body weights | Body weight gains | |
| Control vehicle | 161.6 ± 3.61a | 201.4 ± 4.41a | 39.8 ± 2.42a |
| SP | 159.8 ± 2.42a | 193.2 ± 3.31a | 33.4 ± 1.96b |
| SPt | 159.6 ± 3.69a | 203.4 ± 4.24a | 43.8 ± 1.02a |
| CCl4 | 161.4 ± 3.74a | 169.2 ± 3.02b | 7.80 ± 1.59c |
| SP + CCl4 | 159.6 ± 3.41a | 170.2 ± 3.72b | 10.60 ± 1.66c |
| SPt + CCl4 | 158 ± 2.02a | 166.4 ± 1.47b | 8.4 ± 0.93c |
Data are presented as means ± SEM (n = 8). Spirulina platensis (SP), Spirulina platensis supplemented with thiamine (SPt), Carbon tetrachloride (CCl4). Values having different superscripts within same column are significantly different (P<0.05).
Rats orally administered CCl4 (1ml/kg body weight three times weekly for a month) without or with SP or SPt (800 mg/kg b.wt) showed significant reduction of body weight gains compared with that of control vehicle group at P<0.05.
Haematological Parameters
Red Blood Cell Indices
Red blood indices are recorded in Table 2. There were insignificant changes between control vehicle and all different groups in RBCs count and MCHC at P<0.05. In addition, there were insignificant changes between control vehicle group and groups administered SP and SPt in HGB concentration, HCT %, MCV and MCH. In the same manner, these parameters showed insignificant changes among the last three groups (CCl4, SP+CCl4 and SPt+CCl4) but showed significant reduction in these groups compared with those of control vehicle group at P<0.05.
Table 2: Mean values of red blood cell indices of control and treated rats.
| RBCs × 106/µL | HGB (g/dl) | HCT % | MCV (fl/cell) | MCH (pg/cell) | MCHC (g/dl) | |
| Control vehicle | 6.49 ± 0.14a | 12.5 ± 0.17a | 30.88 ± 0.31a | 47.68 ± 0.85a | 19.32 ± 0.61a | 40.5 ± 0.58a |
| SP | 6.59 ± 0.19a | 12.36 ± 0.22a | 31.06 ± 0.35a | 47.14 ± 0.57a | 18.76 ± 0.34a | 39.84 ± 0.75a |
| SPt | 6.66 ± 0.07a | 12.26 ± 0.11a | 30.76 ± 0.46a | 46.20 ± 0.56a | 18.68 ± 0.28a | 38.84 ± 0.19a |
| CCl4 | 6.47 ± 0.26a | 11.34 ± 0.34b | 28.4 ± 0.53b | 43.92 ± 0.79b | 17.48 ± 0.22b | 40.08 ± 0.49a |
| SP + CCl4 | 6.66 ± 0.24a | 11.26 ± 0.31b | 29.20 ± 0.52b | 43.9 ± 0.63b | 17.40 ± 0.31b | 39.02 ± 0.30a |
| SPt + CCl4 | 6.47 ± 0.34a | 11.02 ± 0.46b | 28.24 ± 0.85b | 43.68 ± 0.69b | 17.10 ± 0.34b | 39.14 ± 0.48a |
Data are presented as means ± SEM (n = 8). Spirulina platensis (SP), Spirulina platensis supplemented with thiamine (SPt), Carbon tetrachloride (CCl4). RBCs (red blood cells), HGB (haemoglobin), HCT (hematocrit), MCV (mean corpuscular volume), MCH (mean corpuscular haemoglobin) and MCHC (mean corpuscular haemoglobin concentration). Values having different superscripts within same column are significantly different (P<0.05).
Leukocytes Count and Differential Leukocyte Percentages
Total leukocytes and percentages of differential leukocytes are presented in Table 3. It was cleared that rats orally administered SP and SPt for a month caused elevation in TLC in relation to control vehicle group at P<0.05. Rats administered CCl4 alone or with SP or SPt showed significant elevation in TLC in comparing with control vehicle rats at P<0.05.
In regarding lymphocyte percentage, SPt, CCl4, SP+CCl4 and SPt+CCl4 induced significant reduction in lymphocyte percentage in comparing with that of control vehicle and SP groups.
At P<0.05, there were significant elevation in monocytes percentages at comparing SPt, CCl4, SP+CCl4, SPt+CCl4 groups with control vehicle and SP groups.
Granulocyte percentages were significantly elevated in CCl4, SP+CCl4 and SPt+CCl4 than those of control vehicle, SP and SPt groups at P<0.05.
Platelets Count
Table 3 cleared that, there were insignificant changes at P<0.05 in platelet count between control vehicle and other different groups.
Table 3: Mean values of leukocyte count, differential leukocyte percentages and platelets count of control and treated rats.
| TLC × 103/µL | Lymphocytes % | Monocytes % | Granulocytes % | Platelets × 103/µL | |
| Control vehicle | 12.31 ± 0.53c | 79.45 ± 2.50a | 6.36 ± 0.47c | 14.19 ± 2.56b | 618 ± 30.22a |
| SP | 16.90 ± 1.01b | 76.37 ± 2.98a | 7.93 ± 0.61c | 15.7 ± 2.98b | 610 ± 31.41a |
| SPt | 17.69 ± 0.79b | 61.02 ± 3.72b | 22.57 ± 1.57a | 16.41 ± 3.38b | 617± 30.85a |
| CCl4 | 24.27 ± 2.57a | 59.87 ± 4.16b | 12.52 ± 0.81b | 27.61 ± 3.43a | 594 ± 32.26a |
| SP + CCl4 | 25.27 ± 1.12a | 60.33 ± 3.19b | 12.77 ± 0.72b | 26.9 ± 3.64a | 551 ± 30.61a |
| SPt + CCl4 | 28.34 ± 1.36a | 52.01 ± 2.43b | 21.09 ± 0.91a | 26.9 ± 1.92a | 598 ± 42.98a |
Data are presented as means ± SEM (n = 8 Spirulina platensis (SP), Spirulina platensis supplemented with thiamine (SPt), Carbon tetrachloride (CCl4). TLC (Total leukocytes count) Values having different superscripts within same column are significantly different (P<0.05).
Serum Biochemical Findings
Serum Hepatic Biomarkers
The effects of CCl4 exposure alone or with SP or SPt on serum ALT and AST activities in rats are recorded in Table 4.
Table 4: Mean values of serum hepatic biomarkers of control and different treated groups.
| ALT (U/L) | AST (U/L) | T Bilirubin (mg/dl) | D Bilirubin (mg/dl) | IND Bilirubin (mg/dl) | |
| Control vehicle | 83.71 ± 1.61d | 100.43 ± 1.09d | 0.511 ± 0.008d | 0.134 ± 0.003c | 0.377 ± 0.009d |
| SP | 82.00 ± 1.76d | 98.71 ± 1.70d | 0.569 ± 0.023d | 0.147 ± 0.004c | 0.421 ± 0.023d |
| SPt | 81.71 ± 1.63d | 97.43 ± 2.15d | 0.549 ± 0.017d | 0.139 ± 0.006c | 0.410 ± 0.016d |
| CCl4 | 204.14 ± 3.28a | 217.57 ± 2.26a | 1.011±0.024a | 0.183 ±0.004a | 0.829 ± 0.023a |
| SP + CCl4 | 119.57 ± 0.57b | 129.71 ± 1.08b | 0.857 ± 0.029b | 0.167 ± 0.005b | 0.690 ± 0.027b |
| SPt + CCl4 | 101.71 ± 1.57c | 113.29 ± 1.11c | 0.708 ± 0.022c | 0.166 ± 0.005b | 0.543 ± 0.019c |
Data are presented as means ± SEM (n = 8). Spirulina platensis (SP), Spirulina platensis supplemented with thiamine (SPt), Carbon tetrachloride (CCl4), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (T bilirubin), direct bilirubin (D bilirubin), indirect bilirubin (IND bilirubin). Values having different superscripts within same column are significantly different (P<0.05).
There were insignificant changes in serum ALT and AST activities at P<0.05 between control vehicle, SP and SPt groups. The activities of serum ALT and AST significantly elevated in CCl4 intoxicated group compared to control vehicle one. Administration of either SP or SPt with CCl4 in groups 5 and 6 induced significant reduction in ALT and AST activities compared with CCl4 intoxicated rats at P<0.05 and these values were still significantly elevated than those of control vehicle.
From the recorded data, it was noticed that values of serum ALT and AST activities showed significant (P<0.05) reduction in SPt+CCl4 group than those of SP+CCl4 group.
Total, direct and indirect bilirubin of different groups were presented in Table 4. These parameters showed insignificant changes at P<0.05 between control vehicle, SP and SPt groups. Intoxicated rats with CCl4 for one month revealed significant increase (P<0.05) in total, direct and indirect bilirubin in comparing to control vehicle rats. Daily administration of SP and SPt simultaneously with CCl4 induced significant reduction in total, direct and indirect bilirubin but still significantly higher than those of control vehicle group. SPt+CCl4 group showed significant reduction at P<0.05 in total and indirect bilirubin but insignificant reduction of direct bilirubin in relation to SP+CCl4 group.
Serum Renal Biomarkers (Urea and Creatinine)
As recorded in Table 5, serum urea and creatinine showed no significant difference at P<0.05 between control vehicle, SP and SPt groups. In regarding CCl4 intoxicated rats, the mean values of urea and creatinine significantly increased than those of control vehicle group. Coadministration of either SP or SPt with CCl4 induced significant reduction in serum urea and creatinine levels compared with rats administered CCl4 alone at P<0.05 and these values were still significantly elevated than those of control vehicle rats in SP+CCl4 group only and returned around normal ranges in SPt+CCl4 group.
Table 5: Mean values of serum urea and creatinine of control and treated rats.
| Urea (mg/dl) | Creatinine (mg/dl) | |
| Control vehicle | 25.43 ± 0.54c | 0.689 ± 0.008c |
| SP | 25.6 ± 1.21c | 0.569 ± 0.01c |
| SPt | 25.2 ± 0.86c | 0.585 ± 0.01c |
| CCl4 | 38.1 ± 1.45a | 1.123 ± 0.09a |
| SP + CCl4 | 31.4 ± 1.12b | 0.864 ± 0.02b |
| SPt + CCl4 | 27.8 ± 1.39c | 0.701 ± 0.03c |
Data are presented as means ± SEM (n = 8). Spirulina platensis (SP), Spirulina platensis supplemented with thiamine (SPt), Carbon tetrachloride (CCl4). Values having different superscripts within same column are significantly different (P<0.05).
From the recorded data, It was noticed that there were significant changes between SPt+CCl4 and SP+CCl4 groups in both serum urea and creatinine levels at P<0.05. These parameters showed reduction in SPt+CCl4 than those of SP+CCl4 group.
Tissue Biochemical Findings
Malondialdehyde (MDA) level in liver and kidney tissues homogenate
Malondialdehyde contents in liver and kidney tissue homogenates as one of lipid peroxide markers showed insignificant difference in control vehicle, SP and SPt groups at P<0.05 (Table 6). Also from the recorded data, there were significant elevation in MDA level of both liver and kidney in CCl4 intoxicated rats versus those of control vehicle rats. Administration of either SP or SPt with CCl4 caused significant reduction in MDA contents in hepatic and renal tissues in comparing with CCl4 intoxicated group but still significantly elevated than those of control vehicle group at P<0.05.
Catalase activity of liver and kidney tissues homogenate
Table 6 showed CAT antioxidant enzyme activity of control vehicle and different treated groups. The control vehicle, SP and SPt groups showed insignificant variation in CAT activity of hepatic and renal tissues at P<0.05. Intoxicated rats with CCl4 showed significant reduction in catalase activity of liver and kidney in comparing with control vehicle group. Daily administration of SP or SPt to intoxicated rats induced significant elevation in catalase activity in comparing with CCl4 group but still significantly lower than those of control vehicle group in liver homogenate and around the normal ranges in kidney homogenate.
Table 6: Mean values of MDA levels (nmol/g tissue) and Catalase activities (U/g tissue) in liver and kidney tissues of control and treated rats.
| MDA (mmol/g tissue) | CAT (U/g tissue) | |||
| Liver | Kidney | Liver | Kidney | |
| Control vehicle | 30.33 ± 0.79c | 44.64 ± 2.15c | 5.28 ± 0.15a | 4.08 ± 0.18a |
| SP | 29.78 ± 0.99c | 42.54 ± 2.66c | 5.39 ± 0.13a | 4.31 ± 0.18a |
| SPt | 29.03 ± 1.50c | 43.20 ± 1.88c | 5.24 ± 0.23a | 4.24 ± 0.18a |
| CCl4 | 55.33 ± 2.22a | 67.18 ± 1.90a | 2.94 ± 0.19c | 2.89 ± 0.11 b |
| SP + CCl4 | 42.63 ± 0.73b | 52.58± 3.04b | 4.09 ± 0.07b | 3.86 ± 0.18a |
| SPt + CCl4 | 42.29 ± 1.55b | 53.34 ± 2.99b | 4.06 ± 0.17b | 3.94 ± 0.04a |
Data are presented as means ± SEM (n = 8). Spirulina platensis (SP), Spirulina platensis supplemented with thiamine (SPt), Carbon tetrachloride (CCl4), MDA (malondialdehyde), CAT (catalase). Values having different superscripts within same column are significantly different (P<0.05).
Histopathological Findings
Liver histopathological changes of different groups (Figure 1) revealed no histopathological changes in control vehicle, SP and SPt groups (A, B and C). The livers of intoxicated rats with CCl4 (D) showed severe peripheral fatty changes (+++), interlobular fibrosis (+++) and congestion. Leukocytes infiltration and necrosis were also recorded in intoxicated rats. Peripheral fatty changes and interlobular fibrosis became moderate (++) with congestion in group treated with SP with CCl4 (E) and mild (+) in group treated with SPt with CCl4 (F).
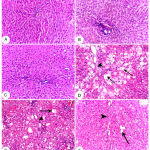 |
Figure 1: Liver histopathology, Rat.
|
A, B and C) represent control vehicle, Spirulina (SP) and Spirulina supplemented with thiamine (SPt) treated groups, respectively, which showing normal hepatic histoarchitectures. D, represent CCl4 intoxicated group: showing extensive fatty change in peripheral hepatocytes (arrows) +++ and interlobular fibrosis (arrow head) +++ and congestion. E, represent SP+ CCl4 group: showing moderate fatty change in peripheral hepatocytes (arrow) ++ and moderate interlobular fibrosis (arrowhead) ++ and congestion. F, represent SPt + CCl4: showing mild fatty change in peripheral hepatocytes (arrow) + and mild interlobular fibrosis (arrowhead) +. H&E stain, X20.
Kidney histopathological changes of different groups (Figure 2) revealed normal renal histoarchitectures in control vehicle, SP and SPt groups (A, B and C). The kidneys of intoxicated rats with CCl4 (D) showed severe hydropic degeneration. Mild congestion and mild intratubular edema in group treated with SP with CCl4 (E) and mild intratubular edema in group treated with SPt with CCl4 (F).
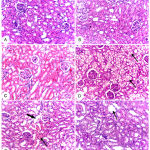 |
Figure 2: Kidney histopathology, Rat.
|
A, B and C) represent control vehicle, Spirulina (SP) and Spirulina supplemented with thiamine (SPt) treated groups, respectively, which showing normal renal histoarchitectures. D, represent CCl4 intoxicated group: showing extensive hydropic degeneration in epithelial lining of renal tubules (arrows). E, represent SP+ CCl4 group: showing mild congestion (thin arrow) and mild intratubular edema (thick arrow). F, represent SPt + CCl4: showing mild intratubular edema (arrow). H&E stain, X20.
Discussion
This study was planned to investigate the comparative heptoprotective and nephroprotective effects of Spirulina (SP) and Spirulina supplemented with thiamine during growth (SPt) via their antioxidant mechanisms against CCl4 subacute toxicity in rats.
The recorded results showed reduction in body weight gains in groups of rats intoxicated with CCl4 alone or in combining with SP or SPt in comparing with control vehicle rats. This means that administration of SP or SPt to the intoxicated rats couldn’t counteract the lowering effects of CCl4 on body weight gains.
Reduction in body weight gains of rats was recorded after one month of CCl4 administration23. CCl4 caused loss of appetite due to its adverse effects on gastrointestinal tract including diarrhea, irritation, nausea, and abdominal pain24. The reduction in body weight gains also could give indirect indication of disturbance in hepatic functions that affect food efficiency ratio23.
Although administration of SP daily for 30 days caused significant reduction in body weight gains in G2, the administration of SPt showed increase in body weight gains in G3 in relation to control vehicle group at P<0.05.
Supplemented diet with SP resulted in a significant reduction of body weight in obese and ischaemic heart patients with significant improvement of lipid profil25. However at feeding of rats on diet containing SP at 10, 20 and 30% (w/w) did not have adverse effect on body weight gains in male and female rats26.
Spirulina is a highly nutritious microalga rich in protein, essential fatty acids, minerals, and vitamins and low in calories. It is used as food supplement to fight starvation and malnutrition and on the other hand to aid in weight loss. It is thought to be an appetite suppressant as it contains the amino acid L-phenylalanine that stimulates the secretion of cholecystokinin, an important satiety hormone in humans, helps in suppressing appetite center in the brain and reduction in feed intake27,28. Also SP helps in fat mobilization and hypolipidemic effects that are critical in weight loss by activation of lipase enzymes29.
In contrast SPt showed slight increase in body weight gains in relation to control rats. Thiamine has biotic and abiotic effects by acting as a coenzyme of the tricarboxylic acid cycle and catabolism of glucose with progressive generation of ATP that is used in other metabolic pathways and hence promoting growth rate30,31.
At Reading the red blood cells indices, RBCs count and MCHC showed insignificant changes between different groups. While HGB concentration, HCT%, MCV and MCH showed significant reduction in the last three groups (CCl4, SP+CCl4 and SPt+CCl4) compared to control vehicle group. From the listed data, it is cleared that CCl4 caused microcytic hypochromic anaemia that was not corrected by daily concomitant administration of SP and SPt to rats.
Carbon tetrachloride caused anaemia in rats previously approved by32,33. Saba et al. approved that CCl4 increased erythrocyte fragility and caused microcytic hypochromic anaemia32. Abdel-Wahhab, et al. found that CCl4 significantly affect the quantity and function of HGB molecule whereas total HGB and Oxy-HGB contents decreased significantly than that of control rats34. It was found that CCl4 induced insignificant changes in RBCs count, PCV% and MCV but caused significant decrease in HGB concentration in relation to control group2.
In the present study the reduction in body weight gains and anaemia occurred concomitantly in CCl4, SP+CCl4 and SPt+CCl4 groups; this means that SP and SPt couldn’t correct the depleting effects of CCl4 frequent doses for a month.
The recorded data of leukocytes count and differential leukocyte percentages showed significant leukocytosis in CCl4, SP+CCl4 and SPt+CCl4 groups in comparing with control vehicle group and this leukocytosis is associated with lymphocytopenia, monocytosis and granulocytosis (related to neutrophilia). Our results are in agree with2,32,33,35.
The significant increase in WBCs recorded in this study in CCl4 groups may refer to the release of marginal or peripheral neutrophils into the circulation which produced the recorded neutrophilia in those rats under the influence of stress hormones as cortisol and catecholamine36. lymphopenia (through shifting haematopoiesis to granulocytic lineages) leading to increased ratio between neutrophils and lymphocytes under stress was recorded by Huff et al.37 After CCl4 intoxication, rats became under stress of adverse effects of the toxin that referred to its irritant effect during administration and/or its toxic effects after absorption.
Spirulina and SPt in all treated groups increased leukocytes count. Spirulina had immuno-modulatory functions against lead acetate immunosuppression38.
In this study, there were insignificant reduction in platelet counts in CCl4 intoxicated groups in relation to control vehicle group as recorded by Essawy et al.39
Obtained results of the present study revealed that administration of CCl4 1 ml/kg b.wt, three times a week for a month induced hepatotoxicity in albino rats that approved by significant increases in serum ALT and AST activities with increment in total, direct and indirect bilirubin in relation to those of control vehicle rats. The administration of SP and SPt concomitantly with CCl4 induced significant reduction in these parameters in comparing with CCl4 intoxicated rats and these reductions almost clear in SPt group.
Carbon tetrachloride proved to have hepatotoxic effects even after a single or repeated dose. It caused a significant increase in plasma AST, ALT, ALP and LDH activities and total bilirubin concentration2,,23,40. Serum aminotransferases were increased as a result of hepatic cells injury and increased cellular membranes permeability and enzymes leakage41.
Carbon tetrachloride induced renal injury indicated by significant increase in serum levels of urea and creatinine in comparing with control vehicle group. This results in agree with Zahran et al.35 but disagree with Fortea et al.2 SP and SPt administered with CCl4 could ameliorate these changes as serum urea and creatinine levels significantly decreased than those of CCl4 intoxicated rats and returned around control levels in SPt+CCl4 group.
Nephrotoxicity induced by CC14 was attributed to its damage effect on nephron structural integrity in the form of degenerative changes in glomerulus and vacuolization of renal tubules42.
All toxic effects of CC14 are elucidated after its metabolic activation in the endoplasmic reticulum by cytochrome P450 enzymes (mainly by CYP2E1) to highly reactive trichloromethyl radical (CCl3•), that rapidly reacts with oxygen to form the highly reactive trichloromethyl peroxyl radical (CCl3OO•). These radicals induce membrane lipid peroxidation and disturb calcium homeostasis to produce cellular injury43.
Malondialdehyde (MDA) is among the many secondary products of lipid peroxidation process and used as biomarker for the assessment the degree of lipid peroxidation. From the recorded data, there were significant elevation in MDA level in both liver and kidney tissues homogenate in CCl4 intoxicated rats in comparing with control vehicle rats and these findings agree with Laouar et al. and Suzuki et al.40,44 CCl4 can induce hepatic and renal injuries via lipid peroxidation by free radical metabolic products. Lipid peroxidation is in turn considered as an indicator of structural and functional alterations of cellular and organelles membranes with failure of antioxidant defense mechanisms to stop the formation of excessive free radicals45. Whereas co-administration of either SP or SPt with CCl4 significantly lowered MDA contents in hepatic and renal tissues compared to CCl4-exposed rats. The inhibition of lipid peroxidation by S. platensis may be attributable to free radical scavenging activity of its antioxidant components12.
Catalase (CAT) is an enzymatic antioxidant can catalyze the breakdown of hydrogen peroxide into oxygen and water46. Our recorded findings revealed that intoxicated rats with CCl4 showed significant reduction in catalase activity of both liver and kidney in comparing with control vehicle group. These results were in agreement with earlier findings of 42,47. Daily treatment with SP or SPt induced significant elevation in catalase activity in comparing with CCl4 group but still significantly lower than those of control vehicle group in liver tissue and around the normal ranges in kidney tissue.
Natural occurring plants and algae have medicinal and nutritional values referring to their content of many phytochemicals. In this study the improvement effects of SP and SPt against CCl4 referred to Spirulina antioxidant properties. It has scavenging activity to free radicals and inhibition of lipid peroxidation due to its content of Phycocyanin48. Spriulina acts as chemopreventive agent, which enhance the antioxidant detoxification system49.
Addition of SP in basal diet improved lipids profile, liver and kidney functions altered by CCl423. Also oral daily administration of SP resulted in significant decrease in the elevated liver enzymes, significant increase in the antioxidant enzymes, decrease in MDA and TNF-α levels and the lipid profile in hepatotoxicity induced by CCl4 in rats47.
Spirulina ameliorates methotrexate hepatotoxicity via antioxidant, immune stimulation, and proinflammatory cytokines and apoptotic proteins modulation50.
Considering pathological alterations of rats’ livers in this study showed many degenerative changes of the hepatic tissue including fatty change, leucocytes infiltration, congestion of blood vessels, necrosis and fibrosis. Similar results were obtained by those of51,52. Also CCl4 induced pathological alterations in kidneys represented by congestion and severe hydropic degeneration as recorded by Venkatanarayana, et al.42
The pathogenesis of various liver injuries including hepatic fibrosis and collagen deposition was associated with oxygen-derived free radicals and lipid peroxidation process53. Moreover upon liver injury, hepatic stellate cells (HSCs) become activated, converting into myofibroblast-like cells and produce extracellular matrix (ECM), playing a major role in hepatic fibrosis54.
In previous unpublished study to the authors of this paper, it was found that SPt contain higher concentrations of all pigments as (chlorophyll, carotenoids, phycocyanin, phycoerythrin, allophycocyanin, phycobiliprotein) and these phytopigments have antioxidative effect. Vadiraja et al. and liu et al. demonstrated that C. phycocyanin, one of the major biliproteins of S. platensis can significantly reduce CCl4 induced acute liver injury in rats, possibly due to lower levels of reactive metabolites of CCl4 by inhibiting some of the cytochrome P450 mediated reactions involved in the formation of CCl4 reactive metabolites in addition to its free radical scavenging ability55,56.
Conclusion
SP and SPt could mitigate the toxic effects of CCl4 on liver and kidney indicated by improvement of hepatic markers, renal markers, tissue antioxidant status and pathological pictures. The more potent effects were referred to SPt than SP. From listed data we also concluded that in the time of counteracting some adverse effects of CCl4, on blood leukogram some parameters didn’t reversed as body weight gains and red blood indices.
Conflict of Interest
The authors declare no conflict of interest associated with this work.
Acknowledgements
The authors gratefully acknowledge and thank Prof. Dr. Abd Ellatif Elbaishy (Professor of Pathology, Faculty of Medicine, Banha University and Dr. Anis Zaid (Assistant Professor of Pathology Faculty of Veterinary Medicine, University of Sadat City) for their valuable help in finishing pathological work.
References
- Galicia-Moreno M and Guti´errez-Reyes G. The role of oxidative stress in the development of alcoholic liver disease. Gastroenterol Mex., 2014; 79(2): 135-144.
- Fortea J.I, Fernández-Mena C, Puerto M, Ripoll C, Almagro J, Bañares J, Bellón J.M, Bañares R and Vaquero J. Comparison of Two Protocols of Carbon Tetrachloride-Induced Cirrhosis in Rats – Improving Yield and Reproducibility. Scientific Reports., 2018; 8:9163.
- Safhi M. Nephroprotective Effect of Zingerone against CCl4-Induced Renal Toxicity in Swiss Albino Mice. Molecular Mechanism. Oxidative Medicine and Cellular Longevity., 2018; 2:1-7.
- Recknagel R.O, Glende E.A, Dolak J.A and Waller R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacology & therapeutics., 1989; 43:139-154.
- Bansal A.K, Bansal M, Soni G and Bhatnagar D. Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact., 2005; 156(2–3): 101-111.
- Cheng N and Ren N. Properties of vanillin in carbon tetrachloride treated rats. Eur J Pharm., 2011; 668: 133-139.
- Agrawal R, Soni K, Tomar J.S and Saxena S. Hepatoprotective activity of Spirulina species. International Journal of Scientific & Engineering Research., 2013; 4(10):1093-1101.
- Kumari J, Babitha B, Jaffar S, Guruprasad M, Ibrahim M, and Khan S. potential health benefits of Spirulina platensis. An international journal of advances in pharmaceutical sciences., 2011; 2: 2-3.
- Kuriakose G and Kurup M. Hepatoprotective effect of Spirulina lonar on paracetamol induced liver damage in rats. Asian J. Exp. Biol. Sci., 2010; 1: 614-623.
- Upasani C.D and Balaraman R. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phytother Res.,2003; 17(4):330-4.
- Khan M, Shobha C.J, Rao U.M, Sundaram C.M, Singh S, Mohan J.I, Kuppusamy P and Kutala K.V. Protective effect of Spirulina against doxorubicin-induced cardio toxicity. Phytother Res., 2005; 19: 1030–1037.
- Karadeniz A, Cemek M and Simsek N. The effects of Panax ginseng and Spirulina platensis on hepatotoxicity induced by cadmium in rats. Ecotoxicol Environ Safety., 2009; 72: 231-235.
- Abdel-Daim M, El-Bialy B.E, Rahman H.G, Radi A.M, Hefny H.A and Hassan A.M. Antagonistic effects of Spirulina platensis against subacute deltamethrin toxicity in mice: Biochemical and histopathological studies. Biomed Pharmacother., 2016; 77(2016): 79-85
- Rippka R. Isolation and Purification of Cyanobacteria. In: Methods in Enzymology: Cyanobacteria, Glazer, A.N. (Ed.). Academic Press, San Diego, CA., 1988; 167: 3-28.
- Vonshak A. Spirulina platensis Arthrospira:Physiology, Cell-Biology and Biotechnology. CRC Press,USA., 1997; 1-16.
- Zarrouk C. Contribution a l’etude d’une cyanophycee: Influence de divers facteurs physiques et chimiques sur la croissance et photosynthese de Spirulina maxima (Setch et Gardner) Geitler. PhD Thesis, University of Paris, France. 1966; pp: 4-5.
- Murray R. Alanine aminotransferase and Aspartate aminotransferase.In: Clinical Chemistry, Eds., Kaplan A, AL Peace, the C. V. Mosby Co., St Louis, Toronto, Princeton. 1984; 1088-1090 (ALT), 1112-1116 (AST).
- Kaplan A, Rubaltelli F.F, Hammerman C, Vilei M.T, Leiter C and Abramov A. Bilirubin. In: Clinical Chemistry: Theory, Analysis and Corelation, Kaplan, L.A. and A.J. Pesce (Eds.). The C.V. Mosby Co., St. Louis. Toronto, Princeton. 1984; 436, 650, 1238-1241.
- Fawcett JK and Soctt JE. A rapid and precise method for the determination of urea. J. Clin. Path., 1960; 13: 156–159.
- Schirmeister J. Determination of creatinine level. Dtsch. Med. Wschr., 1964 89: 1940–1947.
- Ohkawa H, Ohishi N and Yagi K.. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry.,1979; 95: 351–358.
- Aebi H. Catalase in vitro. Methods in Enzymology., 1984; 105: 121–126.
- EL-Sayeda G.E, EL-Sahar and Abor M.M. Abed EL- Rahman. Hepatotoprotective Activity of Different Doses of Spirulina against CCl4 Induced Liver Damage in Rats. J Am Sci., 2012; 8(8):916-923. 15
- The International Technical Information Institute,Toxic and Hazardous Industrial Chemicals Safety Manual; Tokyo, Japan.,1988; p. 109.
- Ramamoorthy A and Premakumari S. Effect of supplementation of Spirulina on hypercholesterolemic patients. J Food Sci Technol., 1996; 33:124–128.
- Salazar, M, Chamorro, G.A, Salazar, S and Steele, C.E. Effect of Spirulina maxima consumption on reproduction and peri- and postnatal development in rats. Food Chem Toxicol., 1996; 34: 353–359.
- Ballinger A.B.1 and Clark M.L. L-phenylalanine releases cholecystokinin (CCK) and is associated with reduced food intake in humans. Evidence for a physiological role of CCK in control of eating. Metabolism., 1994; 43(6):735-8.
- Harrison L. Master Your Metabolism: The All-Natural (All-Herbal) Way to Lose Weight: The all-natural (herbal way) to lose weight, puplished by Sourcebooks, Inc. America. Chapter 7 Losing weight with living juices. 2003; p: 167.
- Torres-Durán P.V, Miranda-Zamora R, Paredes-Carbajal M.C, Mascher D, Blé-Castillo J, DíazZagoya J.C and Juárez-Oropeza M.A. Studies on the preventive effect of Spirulina maxima on fatty liver development induced by carbon tetrachloride, in the rat. J Ethnopharmacol., 1999; 64: 141– 147.
- Schneider DA and RL Gourse. Relationship between Growth Rate and ATP Concentration in Escherichia coli: A bioassay for available cellular ATP. The journal of biological chemistry., 2004; 279(9): 8262–8268.
- Fattal-Valevski A.Thiamine (vitamin B1). J Evid Based Complement Altern Med., 2011; 16: 12–20.
- Saba A.B, Oyagbemi A.A and Azeez O.I. Amelioration of carbon tetrachloride-induced hepatotoxicity and haemotoxicity by aqueous leaf extract of Cnidoscolus aconitifolius in rats. J. Physiol., 2010; 25(2): 139 – 147.
- Sule O.J, Abdu A.R and Kiridi K. Effect of Carica papaya (L) Leaves on Haematological Parameters in CCl4-induced Wistar Albino Rats. British Journal of Medicine & Medical Research., 2016; 16(3): 1-6.
- Abdel-Wahhab M.A, abdel-wahhab K, Mannaa F. The Protective Effects of Whey Protein And Spirulina Against CCl4-Induced Erythrocyte Damage In Rats. Journal of Applied Sciences Research., 2013; 9(3):2063-2071.
- Zahran F, Gabr S.A, Abd El-Moneim A.E, Sharoud M.N, Hassanin W.F and Mesalam N.M. Prophylactic effect of camel milk on physiological and biochemical changes in CCl4-intoxicated rats. Journal of Experimental Biology and Agricultural Sciences., 2018; 6(1) : 211 – 219.
- Swenson M.J. Physiological properties and cellular and chemical constituent of blood. In: Dukes’ Physiology of domestic animals. Comstock Publishing Associates, Ithaca and London., 1993; 29-32.
- Huff G.R, W.E, Balog J.M, Rath N.C, Anthony N.B and Nestor K.E. Stress Response Differences and Disease Susceptibility Reflected by Heterophil to Lymphocyte Ratio in Turkeys Selected for Increased Body Weight. Poultry Science., 2005; 84:709 – 717.
- Ismail S.A.A. Ameliorative Potential of Spirulina platensis against Lead Acetate Induced Immuno-Suppression and Kidney Apoptosis in Rats. Ann Clin Pathol., 2017; 5(5): 1120.
- Essawy A, Hamed S.S, Abdel-Moneim A.M, Abou-Gabal A.A and Alzergy A.A. Role of black seeds (Nigella sativa) in ameliorating carbon tetrachloride induced haematotoxicity in Swiss albino mice. Journal of Medicinal Plants Research., 2010; 4(19):1977-1986.
- Laouar A, Klibet F, Bourogaa E, Benamara A, Boumendjel A, Chefrour A and Messarah M. Potential antioxidant properties and hepatoprotective effects of Juniperus phoenicea berries against CCl4 induced hepatic damage in rats. Asian Pacific Journal of Tropical Medicine., 2017; 10(3): 263–269.
- Paduraru I, Saramet A, Danila GH, Nichifor M, Jerca L and Iacobovici A. Antioxidant action of a new flavonic derivative in acute carbon tetrachloride intoxication. Eur J Drug Metab. Pharmacokinet., 1996; 21: 1-6.
- Venkatanarayana G, Sudhakara G, Sivajyothi P and Indira P. Protective effects of curcumin and vitamin E on carbon tetrachloride-induced nephrotoxicity in rats. EXCU Journal., 2012; 11: 641-650.
- Arun M and Asha Preliminary V.V. Studies on antihepatotoxic effect of Physalisperuviana Linn. (Solanaceae) against carbon tetrachloride induced acute liver injury in rats. J Ethnopharmacol., 2007; 111(1): 110-114.
- Suzuki K, Nakagawa K, Yamamoto T, Miyazawa T, Kimura F, Kamei M and Miyazawa T. Carbon tetrachloride-induced hepatic and renal damages in rat: inhibitory effects of cacao polyphenol. Bioscience, Biotechnology, and Biochemistry., 2015; 79(10):1669–1675.
- Wua C.R, Huang M.Y, Lin Y.T, Ju H.Y and Ching H. Antioxidant properties of Cortex fraxini and its simple coumarins, Food Chem., 2007; 104: 1464–1471.
- Kaushal J, Mehandia S, Singh G, Raina A and Arya S.K. Catalase enzyme: Application in bioremediation and food industry. Biocatalysis and Agricultural Biotechnology., 2018; 16:102-109.
- Kamel H.H, Mogoda O.S, Ahmed W.M.S and Mohamed A.H. Evaluation of the Hepatoprotective And Antioxidant Effects of Spirulina in Wistar Albino Rats. J. Comp. Path &Clinic Path., 2016; 29(1): 11-30.17
- Hirata T, Tanaki M, Ooike M, Tsunomura T and Sagakuchi M. Antioxidant activities of phycocyanobillin prepared from Spirulina platensis. Appl. Phycol., 2000; 12: 435-439.
- Aboul-Enein A.M, El-Baz F.K, El-Baroty G.S, Youssef A.M and Abd El-Baky H.H. Antioxidant activity of algal extracts on lipid peroxidation. Med. Sci. 2003; 3: 87-98.
- Khafagaa A.F and El-Sayed Y.S. Spirulina ameliorates methotrexate hepatotoxicity via antioxidant, immune stimulation, and proinflammatory cytokines and apoptotic proteins modulation Life Sciences., 2018; 196:9–17.
- Sreelatha S, Padma P.R and Umadevi M. Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food ChemToxicol., 2009; 47:702-8.
- Wang G, Li Z, Li H, Li L, Li J and Yu C. Metabolic Profile Changes of CCl4-Liver Fibrosis and Inhibitory Effects of Jiaqi Ganxian Granule. , 2016; 21,698 (11 pages).
- Poli G. Pathogenesis of liver fibrosis: role of oxidative stress, Aspects Med., 2000; 21: 49–98.
- Friedman S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. Biol. Chem., 2000; 275: 2247–2250.
- Vadiraja H.B, Gaikwad N.W and Madyashta K.M. Hepatoprotective effect of C-phycocyanin: protection for carbon tetrachloride and R-(+)- pulegone-mediated. Biochem Biophys Res Commun., 1998; 249 (2): 428-431.
- Liu Q, Huang Y, Zhang R, Cai T and Cai Y. Medical application of Spirulina platensis derived C-phycocyanin, Evid. Based Complement. Alternat. Med., 2016; 2016: 7803846.







