Akshani Anjula Wijesooriya , Srianthie A. Deraniyagala
, Srianthie A. Deraniyagala and Chamari M. Hettiarachchi*
and Chamari M. Hettiarachchi*
Department of Chemistry, University of Colombo, Colombo 03, Sri Lanka.
Corresponding Author E-mail: chamarih@chem.cmb.ac.lk
DOI : https://dx.doi.org/10.13005/bpj/1673
Abstract
Plant based remedies are of much importance in healthcare due to low side effects. The effective constituents contained in seeds of a papaya have not been utilized efficiently in the production of medicines. The purpose of this study was driven towards determining the total phenolic content, total flavonoid content, antioxidant capacity, anti-inflammatory activity and antibacterial properties of the aqueous seeds extract of a Sri Lankan variety (Red Lady) of papaya (AESP). The AESP prepared according to the method of “Kasaya” in Ayurvedic medicine was used for the investigations according to standard procedures. Total phenolic content and the total flavonoid content of the AESP were 13.5±2.2 mg (pyrogallol equivalence)/g and 315.9±104.6 mg (quercetin equivalence)/g respectively. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of AESP ranged from 12.4% to 81.2% for concentrations of 1.0-20.0 mg/ml; ascorbic acid gave 31.7% - 91.9% activity. The AESP displayed strong reducing power compared to ascorbic acid in the reducing power assay. The hydroxyl radical scavenging activity of the AESP ranged from 6.6% to 69.1% for concentrations ranging from 20.0-95.0 mg/ml; ascorbic acid activity was 37.7%-74.9%. The nitric oxide radical scavenging activity of AESP was 23.9%-62.7% for concentrations of 2.0-20.0 µg/ml; ascorbic acid gave 26.8%-63.5% activity. AESP concentrations ranging from 75.0-150.0 µg/ml exhibited human red blood cell (HRBC) membrane stabilization protection of 15.5%-22.7% compared to 50.8%-58.4% for aspirin. The AESP showed antibacterial activity against Bacillus subtilis and Staphylococcus aureus bacterial species. AESP possess promising antioxidant, anti-inflammatory and antibacterial activity. The aqueous decoction of the discarded seeds of papaya would facilitate remedies for many diseases in which radicals are implicated as well as assist against certain bacterial infections and also has an anti-inflammatory potential.
Keywords
Anti-Inflammatory Activity; Antibacterial Activity; Antioxidant Activity; Carica Papaya
Download this article as:| Copy the following to cite this article: Wijesooriya A. A, Deraniyagala S. A, Hettiarachchi C. M. Antioxidant, Anti-Inflammatory and Antibacterial Activities of the Seeds of A Sri Lankan Variety of Carica Papaya. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Wijesooriya A. A, Deraniyagala S. A, Hettiarachchi C. M. Antioxidant, Anti-Inflammatory and Antibacterial Activities of the Seeds of A Sri Lankan Variety of Carica Papaya. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2W7O1FI |
Introduction
Despite the rapid development of modern medicine, plant based remedies still play an important role in healthcare industry.1 Due to the health risk, toxicity and side effects of synthetic drugs, there is much attention goes towards natural products. Secondary metabolites of plants are used in the production of valuable synthetic compounds such as pharmaceuticals, cosmetics and nutraceuticals.2 The combination of secondary products present in the plants result in beneficial medicinal effects. These important bioactive compounds are mostly alkaloids, steroids, tannins, flavonoids and phenolic compounds.2
In a healthy individual there is a balance between reactive oxygen species (ROS) and the inherent antioxidant capacity of the body. Overproduction of ROS in cells may cause damage to DNA, RNA and proteins leading to significant damage to cell structures.3 Oxidative stress occurs due to the overproduction of ROS and significant decrease in antioxidant defenses which may result in many harmful conditions in the body, such as chronic disease, cancer, Parkinson’s disease, diabetes, cardiovascular disease and atherosclerosis.3,4 Antioxidants are associated in reducing the risk of such diseases by stabilizing or deactivating free radicals. Therefore, much attention had been paid to the medicinal plants with antioxidant constituents because of their free radical scavenging abilities.5
Inflammation is defined as a bodily response to injury and infection.6 There are two types of inflammation as Acute and Chronic. Acute inflammation is of short duration and is resolved quickly. Chronic inflammation is a prolonged process in which tissue destruction and inflammation occur at the same time.7
Medicinal plants can be considered as a source of novel compounds with antimicrobial activity.8 Antimicrobial activity of plants can be determined by observing the growth response of various microorganisms to plant extract. Plants show antimicrobial activity because of the presence of bioactive compounds like glycosides, saponins, flavonoids and alkaloids.9
Carica papaya L. is native to southern Mexico and the Central American region. It is a popular fruit crop that belongs to family Caricaceae. Papaya has been used as a nutritious and medicinal plant.10 In Sri Lanka, papaya is mainly grown as a home garden crop as well as mixed culture field and three papaya varieties are grown primarily. They are Rathna, Red Lady, Sinta. The seeds of papaya are considered a waste which usually polluted our habitat. Previously carried out research in China has revealed that the seeds of papaya contain p-hydroxybenzoic acid and vanillic acid which showed significant antioxidant activities.11 This study is employed to determine the total phenolic content, total flavonoid content, antioxidant activity, anti-inflammatory activity and antibacterial activity of the aqueous seeds extract of red lady variety of Carica papaya.
Materials and Methods
Collection of Plant Material
Fresh fruits of the Red Lady variety of papaya were collected in February 2018 from Buddhangala in Ampara district, Sri Lanka. Fruits were identified and authenticated at the Department of Agriculture, Ampara, Sri Lanka.
Preparation of Water Extract
All fruits were washed with water to remove contaminants. The seeds were separated from fruits and washed with water. Then seeds were air dried for two weeks at room temperature. The dried seeds were ground into a powder using a domestic grinder and stored in air tight containers in a refrigerator.
Water extract of powdered papaya seeds samples was prepared according to a previously published Ayurvedic traditional kasaya preparation method.12 Dried powdered sample 60.0 g (equivalent to 12 “kalan”) was simmer boiled with 960 ml (4 patha) of distilled water to obtain 240 ml (1 patha) of decoction. The water extract was filtered through a fine silk cloth. The filtrate was freeze dried and the powdered samples were stored at -4ºC in air tight containers until required.12
Preparation of Sample Concentrations
A stock solution of known concentration was prepared for each assay separately by dissolving a known amount of freeze-dried sample in a known volume of solvent. A concentration series was prepared by diluting the stock solution.
Determination of Total Phenolic Content
Folin Ciocalteu Assay13
An aliquot (4 ml) of 2% NaHC was mixed with each concentration of the plant extract (200 µl) and incubated in darkness for 2 minutes. To this solution Folin-Ciocalteu reagent (200 µl) was added and incubated in darkness for 30 minutes. The absorbance was recorded at 750 nm. The blank was prepared by replacing the Folin-Ciocalteu reagent with distilled water (200 µl). The assay was carried out in triplicate for each concentration. The standard curve of pyrogallol was plotted using the same procedure with pyrogallol (200 µl) instead of plant extract. The total phenolic content was determined from the pyrogallol calibration curve. The total phenolic content was expressed as milligrams of pyrogallol equivalents (PGE) per g of dried sample.
Determination of Total Flavonoid Content
Colorimetric Assay14
An aliquot (0.5 ml of each concentration of plant extract, distilled water (2 ml), 5 % NaN (150 µl) were mixed and incubated in darkness for 5 minutes. To this solution 10% Al (150 µl) was added and kept in darkness for 6 minutes. Then 1 M NaOH (1 ml), distilled water (1 ml) were added to the reaction mixture. The absorbance was measured at 510 nm. The assay was carried out in triplicate for each concentration. The blank was prepared by replacing Al solution with distilled water. The standard curve quercetin was plotted using the same procedure with quercetin (200 µl) instead of plant extract. The total flavonoid content was determined from the quercetin calibration curve. The total flavonoid content was expressed as milligrams of quercetin equivalents (QE) per g of dried sample.
Determination of Antioxidant Activity
DPPH Free Radical Scavenging Assay15
A stock solution of 240.0 mg/l of DPPH solution was prepared and stored at 20ºC until required. The working DPPH solution was obtained by diluting DPPH solution with distilled methanol to attain an absorbance of about 0.98 ± 0.02 at 517 nm. An aliquot (3 ml) of working DPPH solution was mixed with each concentration of plant extract (100 µl). The reaction mixtures were shaken well and incubated in the dark for 15 minutes at room temperature. The absorbance was measured at 517 nm. The assay was carried out in triplicate for each concentration. The control was prepared by mixing working DPPH solution (3 ml) with distilled water (100 µl). The blank was prepared by mixing distilled methanol (3 ml) with distilled water (100 µl). The same procedure was carried out for standard concentration series using 100 µl of ascorbic acid instead of plant extract. The percentage of radical scavenging activity (RSA) was calculated using the following equation (1) where AS is the absorbance of the sample and AC is the absorbance of the control.
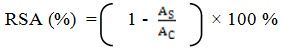
Reducing Power Assay15
An aliquot (2 ml) of plant extract was mixed with phosphate buffer (2 ml, 0.2 M, pH 6.6) and potassium ferricayanide (2 ml, 10.0 mg/ml). This mixture was incubated at 50ºC for 20 minutes. To this solution trichloroacetic acid (2 ml, 100.0 mg/l) was added and the mixture was centrifuged at 3000 r/min for 10 minutes to collect the upper layer of the solution. A volume of 2 ml of each reaction mixture was mixed with distilled water (2 ml) and freshly prepared Fe solution (0.4 ml, 0.1% w/v). After 10 minutes, the absorbance of the reaction mixture was measured at 700 nm. Higher absorbance of the reaction mixture indicates a higher reducing power. The assay was carried out in triplicate for each concentration. The same procedure was carried out for standard concentration series using 2 ml of ascorbic acid instead of plant extract. Distilled water was taken as the blank.
Hydroxyl Radical Scavenging Assay15
A volume of 100 µl of each concentration of the plant extract was mixed with 500 µl of 2-deoxyribose (2.8 mM) in phosphate buffer (50.0 mM , pH 7.4), 200 µl of premixed Fe (100 mM) and ethylenediaminetetraacetic acid (EDTA) (100 mM) solution (1:1; v/v), (100 µl, 200 mM). In order to trigger the reaction, ascorbic acid (100 µl, 300 mM) was added and incubated for 1 hour at 37ºC. A volume of 0.5 ml of the reaction mixture was added to trichloroacetic acid (1 ml, 2.8%; w/v), then thiobarbituric acid (1 ml, 1%) was added to the same reaction mixture. The reaction mixture was heated for 15 minutes on a boiling water bath. The reaction mixture was allowed to cool down to the room temperature and the absorbance was measured at 532 nm. The assay was carried out in triplicate for each concentration. The same procedure was carried out for standard concentration series using 100.0 µl of ascorbic acid instead of plant extract. The control was prepared by using a mixture of 2-deoxyribose in phosphate buffer, premixed Fe and EDTA, and ascorbic acid. Distilled water was used as the blank. Hydroxyl radical scavenging activity was calculated using the equation 1.
Determination of Anti-inflammatory Activity
Nitric Oxide Radical Scavenging Assay16
An aliquot of 0.5 ml of Sodium nitroprusside (SNP) in phosphate buffer saline (PBS) was added to each concentration of plant extract (1 ml). The reaction mixtures were incubated at 25 ºC for 180 minutes. Griess reagent was prepared by mixing equal volumes of 1% sulphanilamide and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride (NDD) immediately before use. After incubation freshly prepared Griess reagent (1 ml) was added into each of the reaction mixture and the absorbance was measured at 546 nm. The assay was carried out in triplicate for each concentration. The same procedure was carried out for standard concentration series using 1 ml of ascorbic acid instead of plant extract. The control was prepared by using a mixture of SNP, PBS and Griess reagent. Distilled water was used as the blank. The nitric oxide radical scavenging activity was calculated using the equation 1.
Human Red Blood Cell (HRBC) Membrane Stabilization Assay17
The blood sample was collected from a healthy human volunteer who had not taken any anti-inflammatory drugs for two weeks prior to the experiment. A volume of 5 ml of blood was collected and centrifuged at 5000 r/min for 20 minutes. Supernatant was discarded and the pellet was washed three times with a volume of normal saline equal to the pellet volume. Centrifugation was carried out until yellowish color of the supernatant become clear.
The reaction mixture was prepared by mixing 1 ml of each concentration of plant extract and 0.1 ml of red blood cell suspension. The reaction mixture was incubated at 56 ºC for 30 minutes. The tubes were cooled and the reaction mixtures were centrifuged at 3000 r/min for 10 minutes. The absorbance of the supernatant was measured at 540 nm. Distilled water was used as the blank. The control was prepared using red blood cell suspension. The assay was carried out in triplicate for each concentration. The same procedure was carried out for standard concentration series using 1 ml of aspirin instead of plant extract. The percentage of protection was calculated using the following equation (2) where AS is the absorbance of the sample and AC is the absorbance of the control.
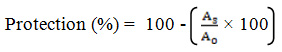
Determination of Antibacterial Activity
The AESP was tested against pre identified three bacterial strains; Escherichia coli (ATCC25922), Staphylococcus aureus (MRSAATCC33591) and Bacillus subtilis (ATCC11778) which were obtained from the pharmacy laboratory of the Faculty of Science, University of Colombo, Sri Lanka. Selected bacteria strains were cultured in prepared Lysogeny Broth (LB) agar plates and incubated overnight at 37ºC. The desired concentration of each bacterial inoculum was obtained by comparing the turbidity with 0.5 McFarland standard.18,19
Agar well diffusion method20
A volume of 200 µl of the above bacterial inoculum was added to the LB agar plate and spread evenly using a glass spreader. Agar plates were incubated at 37ºC for 30 minutes. On each spread plate five wells were made using the sterilized cork borer. An aliquot of 50 µl plant extract (100 mg/ml) was added to three wells. For other two wells, 50 µl of positive control (ciprofloxacin, 0.25 mg/ml) and negative control (distilled water) were added separately. All plates were sealed and incubated at 37ºC overnight in the incubator. The average diameter of the resultant inhibition zone was measured.
Result and Discussion
The main objective of this research study was to investigate the antioxidant, anti-inflammatory and antibacterial properties of the aqueous extract of the seeds of a red lady variety of Carica papaya. AESP was prepared according to the method of kasaya preparation in Ayurvedic medicine.
Total Phenolic Content
Phenolic compounds are the major group of compounds acting as primary antioxidants or free radical terminators. Plants polyphenols can act as reducing agents, hydrogen atom donators and singlet oxygen scavenger Phenolic compounds of plants are associated with health benefit in humans. Those benefits include prevention of obesity and diabetes, reduction in the risk of cardiovascular diseases and improved functioning of the immune system.15,22 Total phenolic content was estimated using the Folin-Ciocalteu method. The phenolic compounds reduce the phosphotungstic phosphomolybdenum complex to Mo(V) species resulting the change of yellow color of the solution to blue color.15 The AESP exhibited 13.5 ± 2.2 mg (PGE)/g of total phenolic content.
Total Flavonoid Content
Flavonoids are the most common and popular group of polyphenolic compound Flavonoids may act as antioxidants by scavenging radicals such as superoxide anion, lipid peroxyl radicals, hydroxyl radicals, singlet oxygen quenching and metal ion chelation.15,23Total flavonoid content was determined by Aluminium chloride colorimetric assay. Aluminium chloride forms acid stable complexes with flavonoids because of that the color of the reaction mixture turns to brown after the addition of AlCL3.23 The AESP showed 315.9 ± 104.6 mg (QE)/g of total flavonoid content.
DPPH Radical Scavenging Activity
The DPPH assay is based on the ability of the plant extract to donate electron or hydrogen atom to the DPPH radical.24 Donation of electron from plant extract to DPPH radical causes purple color of the test sample to turn into yellow color depending on the scavenging ability of the antioxidant compounds. The degree of color change is proportional to the concentration of the antioxidants.15 Figure 1 shows the percentage of DPPH radical scavenging activity (SA) vs. concentration of AESP and percentage of DPPH radical scavenging activity vs. concentration of ascorbic acid.
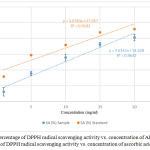 |
Figure 1: Percentage of DPPH radical scavenging activity vs. concentration of AESP (●) Percentage of DPPH radical scavenging activity vs. concentration of ascorbic acid (●).
|
Table 1: Absorbance values for AESP and ascorbic acid concentration series in ferric reducing power assay.
| Concentration (mg/ml) | Absorbance values for AESP at 700 nm | Absorbance values for the standard at 700 nm |
| 1.0 | 0.06 ± 0.05 | 0.36 ± 0.005 |
| 5.0 | 0.30 ± 0.18 | 0.48 ± 0.005 |
| 10.0 | 0.38 ± 0.19 | 0.66 ± 0.050 |
| 15.0 | 0.68 ± 0.38 | 0.85 ± 0.016 |
| 20.0 | 1.14 ± 0.25 | 0.98 ± 0.008 |
Hydroxyl Radical Scavenging Activity
Hydroxyl radical is the most reactive oxygen species that causes serve damage to adjacent biomolecules. It reacts with cell membrane phospholipids, polyunsaturated fatty acids which cause damage to cell.24 Hydroxyl radical scavenging activity of the plant extract was measured by using deoxyribose method. It is based on the generation of hydroxyl radicals from a Fenton reaction between ferrous ions and hydrogen peroxide.25 The hydroxyl radicals formed by the Fenton reaction react with deoxyribose to form malonaldehyde which gives a pink chromogen when heated with thiobarbituric acid at low pH conditions.15,25 Hydroxyl radical scavenger compounds present in the plant extract compete with deoxyribose and scavenge the hydroxyl radical which leads to the decrease in the pink color intensity.15 Figure 2 shows the percentage of hydroxyl radical SA vs. concentration of AESP and percentage hydroxyl radical SA vs. concentration of ascorbic acid.
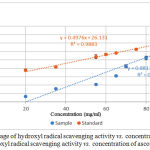 |
Figure 2: Percentage of hydroxyl radical scavenging activity vs. concentration of AESP (●) Percentage hydroxyl radical scavenging activity vs. concentration of ascorbic acid (●).
|
Hydroxyl radical SA of the AESP was 6.6% – 69.1% whereas ascorbic acid gave values 37.7% – 74.9% for concentrations of 20.0 – 95.0 mg/ml.
Nitric Oxide Radical Scavenging Activity
Nitric oxide is generated by endothelial cells, macrophages and neurons.26 Inflammation, cancer and pathogenic conditions occur due to the overproduction of nitric oxide free radical.17 NO scavenging activity was estimated by the use of Griess reaction. Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates NO which interacts with oxygen to produce nitrite ions which can be estimated by the use of the Griess reagent.25 Plant extracts contain scavengers of NO radical that will compete with oxygen leading to reduce the production of nitrite ions which decrease the formation of pink azo dye.17 NO scavenging capacity is determined by the decrease in the absorbance and reduction in color intensity of the reaction mixture at 546 nm induced by antioxidants. Figure 3 shows the percentage of NO radical SA vs. concentration of AESP and percentage of NO radical SA vs. concentration of ascorbic acid.
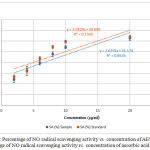 |
Figure 3: Percentage of NO radical scavenging activity vs. concentration of AESP (●) Percentage of NO radical scavenging activity vs. concentration of ascorbic acid (●).
|
In the present study NO radical SA of the AESP was 23.9% – 69.4% whereas ascorbic acid exhibited 26.8% – 79.6% for concentrations of 2.0 – 60.0 µg/ml.
HRBC Membrane Stabilization Assay
The HRBC membrane stabilization method was used to study the in-vitro anti-inflammatory activity. The erythrocyte membrane is analogues to the lysosomal membrane.17 During the inflammation, many disorders are produced due to the release of lysosomal enzymes. The extracellular activity of these enzymes is related to acute or chronic inflammation. The non-steroidal drugs inhibit these lysosomal enzymes and stabilize the lysosomal membrane.27 Inhibition of the release of lysosomal content of neutrophils at the site of inflammation is possible because of the resemblance of red blood cell membrane with lysosomal membrane. The prevention of HRBC membrane lysis is taken as a measure of anti-inflammatory activity.17,27 Figure 4 shows the percentage of protection vs. concentration of AESP and percentage of protection vs concentration of aspirin.
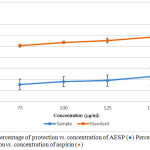 |
Figure 4: Percentage of protection vs. concentration of AESP (●) Percentage of protection vs. concentration of aspirin (●).Click here to view figure |
HRBC assay gave protection of 15.5% – 22.7% for concentrations ranging from 75.0 – 150.0 µg/ml whereas aspirin protection (%) varied from 50.8% – 58.4%.
Antibacterial Activity
The antibacterial activity of the plant extract was determined using agar well diffusion method. Table 2 shows the average diameter of inhibition zones during agar well method.
Table 2: The average diameter of inhibition zones during agar well method.
| Bacteria Strain | Positive control (ciprofloxacin) (mm) | Negative control (distilled water) (mm) | Mean diameter of the resultant inhibition zone with AESP (mm) |
| Bacillus subtilis | 27.1 ± 1.9 | 0.0 | 10.0 ± 1.4 |
| Staphylococcus aureus | 17.8 ± 2.2 | 0.0 | 3.3 ± 1.8 |
| Escherichia coli | 29.5 ± 1.5 | 0.0 | 0.0 |
The AESP gave positive results against B. subtilis and S. aureus bacterial species. The AESP did not show antibacterial activity towards E. coli bacteria species. E. coli is gram negative bacteria species and has impenetrable cell membrane Hence it results in high resistance towards antibiotics.
Conclusion
Our results showed that the AESP prepared according to the method of kasaya preparation has promising antioxidant potential as determined by DPPH radical scavenging assay, reducing power assay and hydroxyl radical scavenging assay. It also showed antibacterial activity against Staphylococcus aureus and Bacillus subtilis bacteria species. The aqueous seeds extract also showed anti-inflammatory activity as determined by NO radical scavenging assay and human red blood cell membrane stabilization assay. Finally, it is important to note that non edible parts(seeds) discarded as waste of red lady variety of Sri Lankan papaya can be used for medicinal purposes.
Acknowledgements
I would like to express my sincere gratitude to Mr. R. M. C. I. Rajapaksha, Technical assistant, Department of Agriculture, Ampara, Sri Lanka for providing necessary information and guidance during sample collection period. I own special thanks to Mr. Jayantha, Technical officer, Department of Biochemistry, Faculty of Medicine, University of Colombo, Sri Lanka.
Conflict of Interest
There is no conflict of interest.
References
- Elgorashi E. E, Taylor J. L. S, Maes A, de Kimpe N, van Staden J, Verschaeve L and Jäger A. K. The Use of Plants in Traditional Medicine: Potential Genotoxic Risks. South African J. Bot., 2002; 68 (3): 408–410.
- Maridass M and Britto A. J. De. Origins of Plant Derived Medicines. Ethnobot Leaflets., 2008; 12(2): 373–387.
- Ramakrishna H, Murthy S. S, Mamatharani D. R and Murthy P. G. Hydroxy Radical and DPPH Scavenging Activity of Crude Protein Extract of Leucas Linifolia: A Folk Medicinal Plant. Asian J. Plant Sci. Res., 2012; 2 (1): 30–35.
- Miyamoto A, Nakano S, Nagai K, Kishikawa N, Ohyama K, Aoyama T, Matsumoto Y and Kuroda N. Development of an Evaluation Method for Hydroxyl Radical Scavenging Activities Using Sequential Injection Analysis with Chemiluminescence Detection. Anal. Sci., 2017; 33 (6): 697–701.
- Dissanayake D. M. R. H, Deraniyagala S. A, Hettiarachchi, C. M and Thiripuranathar G. The Study of Antioxidant and Antibacterial Properties of Skin , Seeds and Leaves of The Sri Lankan Variety of Pumpkin . IOSR J. Pharm., 2018; 8 (2): 43–48.
- Phanse M. A, Patil M. J, Chaudhari K. A. P. D and Patel B. In-Vivo and in-Vitro Screening of Medicinal Plants for Their Anti-Inflammatory Activity: An Overview. J. Appl. Pharm. Sci., 2012; 2 (6): 19–33.
- Patel M, Murugananthan S and Gowda S. In Vivo Animal Models in Preclinical Evaluation of Anti- Inflammatory Activity-A Review. Int. J. Pharm. Res. Allied Sci., 2012; 1 (2): 01–05.
- Ponce A. G, Roura S. I, del Valle C. E and Moreira M. R. Antimicrobial and Antioxidant Activities of Edible Coatings Enriched with Natural Plant Extracts: In Vitro and in Vivo Studies. Postharvest Biol. Technol., 2008; 49 (2): 294–300.
- Nascimento G. G. F, Locatelli J, Freitas P. C and Silva G. L. Antibacterial Activity of Plant Extracts and Phytochemicals on Antibiotic-Resistant Bacteria. Brazilian J. Microbiol., 2000; 31 (4): 247–256.
- Fatima U and Shahid S. Pharmacological Activities of Carica Papaya Linn. J. Basic Appl. Sci., 2018; 14(2): 210–216.
- Zhou K, Wang H, Mei W, Li X, Luo Y and Dai H. Antioxidant Activity of Papaya Seed Extracts. Molecules., 2011; 16 (8): 6179–6192.
- Perera P. R. D, Ekanayaka S and Ranaweera K. K. D. S. R. In vitro study on antiglycation activity, antioxidant activity and phenolic content of Osbeckia octandra L. leaf decoction. J. Pharmacogn. Phytochem., 2013; 2 (4): 158–161.
- Weerasinghe W. P. N and Deraniyagala S. A. Antioxidant activity of some Sri Lankan endemic medicinal plants. Pharm. J., 2016; 6 (1): 9-14.
- Viera V. B, Piovesan N, Rodrigues J. B, Mello R. O, Prestes R. C, V dos Santos R. C R, de A Vaucher R, Hautrive T. P and Kubota E. H. Extraction of phenolic compounds and evaluation of the antioxidant and antimicrobial capacity of red onion skin (Allium cepa L.). Int. Food Res. J., 2017; 24 (3): 990-999.
- Saeed N, Khan M. R and Shabbir M. Antioxidant Activity , Total Phenolic and Total Flavonoid Contents of Whole Plant Extracts Torilis Leptophylla L. Complement. Altern. Med., 2012; 12 (4): 221-232.
- Boora F, Chirisa E and Mukanganyama S. Evaluation of Nitrire Radical Scavenging Properties of Selected Zimbabwean Plant Extracts and Their Phytoconstituents. J. Food. Process., 2014: 1-7.
- Kuganesan A, Thiripuranathar G, Navaratne A. N and Paranamana P. A. S. Antioxidant and anti-inflammatory activities of peels, pulps and seed kernels of three common Mango (Mangifera indica L.) Varieties in Sri Lanka. Int. J. Pharmaseutical Sci. Res., 2017; 8 (1): 70–78.
- Zapata A and Ramirez-arcos S. A Comparative Study of McFarland Turbidity Standards and the Densimat Photometer to Determine Bacterial Cell Density. Curr. Microbio., 2016; 70 (4): 907-909.
- Sen A and Batra A. Evaluation of Antimicrobial Activity of Different Solvent Extracts of Medicinal Plant: Melia Azedarach L. Int. J. Curr. Pharm. Res., 2012; 4 (2): 67–63.
- Abbas S. Z, Hussain K, Hussain Z, Ali R and Abbas T. Anti-Bacterial Activity of Different Soaps Available in Local Market of Rawalpindi ( Pakistan ) against Daily Encountered Bacteria Pharmaceutica Analytica Acta. Pharm. Anal. Acta., 2016; 7 (11): 10–12.
- Almey A. A. A, Khan C. A. J, Zahir I. S, Suleiman K. M, Aisyah M. R and Rahim K. K. Total phenolic content and primary antioxidant activity of methanolic and ethanolic extracts of aromatic plants’ leaves. Int. Food. Res. J., 2010; 17 (4): 1077-1084
- Bag G. C, Devi P. G and Bhaigyabati T. Research Article Assessment of Total Flavonoid Content and Antioxidant Activity of Methanolic Rhizome Extract of Three Hedychium Species of Manipur Valley 1. Int. J. Pharm. Sci. Rev. Res., 2015; 30 (28): 154–159.
- Pękal A and Pyrzynska K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods., 2014; 7 (9): 1776–1782.
- Jayasundara C. W, Deraniyagala S. A, Hettiarachchi C. M and Thiripuranathar G. Antioxidant and Antibacterial Activities of Leaves, Skin, Flesh and Seeds of Sri Lankan Variety of Cucurbita moschata. Int. J. Ayurveda Pharma Res., 2018; 6 (3): 1–7.
- Gülçin İ. Antioxidant Activity of Food Constituents: An Overview. Arch. Toxicol. 2012; 86 (3): 345–391.
- Parul R, Kundu S. K and Saha P. In Vitro Nitric Oxide Scavenging Activity Of Methanol Extracts Of Three Bangladeshi Medicinal Plants. Pharma Innov. 2012; 1 (12): 83–88.
- Nirmalraj S, Ravikumar M, Mahendraku M, Bharath B and Perinbam K. Antibacterial and Anti-Inflammatory Activity of Justicia Gendarussa Burm. F. Leaves. J. Plant Sci., 2015; 10 (2): 70–74.








