Samsu U. Nurdin* , Devi Sabarina
, Devi Sabarina , Subeki
, Subeki and Sussi Astuti
and Sussi Astuti
Department of Agricultural Product Technology, Agriculture Faculty, Lampung University, Jl. Sumantri Brojonegoro, Bandar Lampung, +6235145, Indonesia.
Correspondind Author E-mail: samsu.udayana@fp.unila.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1707
Abstract
The study aimed to evaluate the effects of bay (B), pandan (P), citrus leaves (C) and their combinations against starch hydrolysis enzymes (α-glucosidase and α-amylase enzymes) and antioxidant activity and to examine the role of polyphenol compounds in enzyme inhibition and antioxidant activity. Three single leaves extracts and five of their combinations were applied to inhibit α-glucosidase hydrolyzing p-nitrophenyl-α-D-glucopyranosyde or α-amylase hydrolyzing starch solution as well as to scavenge free radicals. The leaf extracts and their combination showed inhibition activities against α-glucosidase and α-amylase enzymes with range of inhibition activities were between 17.63% to 26.04% and 20.14% to 35.30% respectively. There is no significantly differ among the extracts in modulation of α-glucosidase activity, but each extract exhibited different effect on α-amylase or antioxidant activities. Mixing P with B and C increases the inhibitory activity of the extract against α-amylase as seen that percent of inhibition of BPC is significantly higher than P, eventhough their total phenolic content was not different. The synergism or antagonism effect was not observed when the extracts were combined as the enzyme inhibition or antioxidant activities are not depend on the proportion of the extract in the mixtures. The role of polyphenol compounds on inhibition of the starch digestion enzymes and on antioxidant activity was not observed. Further study is required to fully elucidate the effect of the leaf or their combinations on diabetic animal models or diabetic patients.
Keywords
Antidiabetic; Antioxidant; Bay Leaf; Citru Leaf; Pandan Leaf; Phenolic Compound
Download this article as:| Copy the following to cite this article: Nurdin S. U, Sabarina D, Subeki S, Astuti S. Antidiabetic and Antioxidant Activities of Bay, Pandan, Citrus Leaves and Their Combination in Vitro. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Nurdin S. U, Sabarina D, Subeki S, Astuti S. Antidiabetic and Antioxidant Activities of Bay, Pandan, Citrus Leaves and Their Combination in Vitro. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2QtIF2h |
Introduction
Rice is staple food of most Asia countries including Indonesia. As rice is good source of starch, therefore, consumption or rice is suggested as risk factor of diabetes mellitus1 and 2. Reducing of starch digestibility of the rice is one of promissing strategies to reduce hyperglycemic effect of the rice3.
Starch, a polysaccharide composed of alpha 1,4-linked glucose units (amylose) and alpha 1,4-1,6-linked branched structure (amylopectin), is cleaved in the duodenal cavity involved several hydrolitic enzymes such as pancreatic alpha-amylase and brush border glycosidase4. Inhibition of these enzymes is not only considered as a strategy to reduce the digestibility of the starch but also a treatment of carbohydrate uptake disorder, such as diabetes and obesity5 and 6. Plants are an important source of phytochemical compounds those have inhibition activities against the enzymes, therefore, they have potentiality for therapeutic drug or functional food for the diseases7.
Rice is prepared by cooking (steaming or boiling) rice soaked in water. Addition of aromatic or flavouring ingredients such as Indonesian bay (Eugenia polyantha Wight), pandan (Pandanus amaryllifolius Roxb.) and citrus (Citrus hystrix) leaves is a common practice in Indonesian rice cooking. Whether the leaves have beneficial effects on rice starch digestibility when they are mixed with rice remain unexplored. However, the therapeutic benefits of these ingredients for diabetes have been reported.
Aqueous extracts of the bay leaves improve glucose and insulin metabolism in in vitro model8. Consumption 1 to 3 g a day of ground bay leaves by type 2 diabetes patients reduced serum glucose with significant decreases ranging from 21 to 26% after 30 d and improved lipid profile of the subjects9. Moreover, methanolic extract of bay leaf displayed scavenging activity against superoxide and hydroxyl radicals in a concentration-dependent manner10.
Pandan leaf is a tropical plant which is used mostly as a flavoring agent for certain rice and bread recipe11. Water extract of pandan leaves reduced blood glucose level as well as improvement the insulin resistance of obese mice12. In healthy subjects, drinking pandan leaf tea effectively decreased postprandial blood sugar through inhibition of α-glucosidase enzyme and induction of insulin production in pancreatic cell13.
Citrus leaf is an aromatic Asian leaf most often used in Indonesian recipes including cooked rice recipes14. The leaf extracts exhibited anticancer activity through reduction of cancer cell line viability15. Fresh juice from Citrus fruits contains phenolics, tannins and flavonoids and exhibited anti-alpha amylase and alpha-glucisodase in vitro16.
Mechanism of the protective effect of dietary antioxidants has been hypothesized through inhibition of oxidation chain reactions17. Thus, consumption plant foods rich in antioxidant compounds could reduce incidence of chronic diseases, such as diabetes, through down regulation of oxidative stress17-20. In this study, we aimed to evaluate the effects of bay, pandan, citrus leaves and their combinations against starch hydrolysing enzymes (α-glucosidase and α-amylase enzymes) and antioxidant activity. Additionally, due to the leaves are rich in polyphenol16, 21, 22, the role of polyphenol compounds in enzyme inhibition and antioxidative effect was also elucidated.
Materials and Method
Plant Material
Bay, pandan and citrus leaves were collected from local market in Bandar Lampung, Indonesia. The leaves, immediately, after collection were thoroughly washed with water and dried in oven at 60°C. Dried leaves were powdered using grinder to produce coarsely powder.
Preparation of Extracts
The dried leaf powder of bay, pandan, citrus or their combination (10 g) were boiled in 100 mL water for 20 minutes. The extract was filtered (extract 1), and the residue was reboiled in 100 mL for 20 minutes, then filtered to get extract 2. Extract 1 and extract 2 were then mixed and considered as 100% extract. Proportion of each type of leaves in the combination was 50.0 % when the combination contained two types of plants, and 33.3 % when the combination contained three types of plants (Table 1).
Table 1: Proportion of leaves in dried leaf combination.
| Treatments | Proportion of each leaf in combinations (%) | ||
| Bay | Pandan | Citrus | |
| BPC | 33.3 | 33.3 | 33.3 |
| BC | 50.0 | 0.0 | 50.0 |
| PC | 0.0 | 50.0 | 50.0 |
| BP | 50.0 | 50.0 | 0.0 |
| B | 100.0 | 0.0 | 0.0 |
| P | 0.0 | 100.0 | 0.0 |
| C | 0.0 | 0.0 | 100 |
Assay of α-glucosidase Inhibitory Activity
The slightly modified method described by Rao et al.23 was applied to measure the effect of the leaf extracts on 𝛼-glucosidase activity using α-glucosidase crude enzyme (Shandong Longda Bio-Products Co., Ltd.). The substrate solution p-nitrophenyl glucopyranoside (pNPG) (Sigma Aldrich, Switzerland) was prepared in aquades (0.03012 g/100mL). Briefly, sample of 200 𝜇L leaf extracts were preincubated with 2 mL of α-glucosidase crude enzyme for 10 min at 37°C. The reaction was initiated by addition of 1 mL of pNPG substrate and incubated at 37°C for 30 min. The reaction was stopped by adding 2 mL of 2% Na2CO3 (Merck, Germany). The 𝛼-glucosidase activity was determined by measuring the yellow-colored paranitrophenol released from pNPG at 405 nm (Thermo Scientific Genesys 20, USA). Percentage inhibition is calculated as %Inhibition = [(Abs control – Abs extract)/ Abs control] × 100.
Assay of α-amylase Inhibitory Activity
α-amylase inhibitory activity assay was performed at 37°C using α-amylase crude enzyme (Shandong Longda Bio-Products Co., Ltd.). A mixture containing 1 ml α-amylase crude enzyme, 0.1 ml phosphate buffer 0.1 M and 0.2 extract (A) or water (B) was incubated for 10 min at 37°C. Then, 3 ml of 4% starch solution (wheat starch) was added to the mixture and incubated for 60 min at 37°C24. Reducing sugar released from the starch hydrolysis was measured using DNS method. α-amylase inhibitory activity was calculated by the following formula:
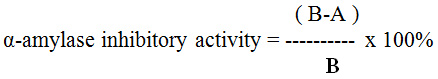
Where A and B represent sugar concentrations in the reaction mixture with and without an addition of leaf extract, respectively.
Antioxidant Activity Measurement
The antioxidant activity assay of the extracts was performed according to protocol describe by Xu et al.25 using DPPH (2,2-Diphenyl-1-Picrylhydrazyl) (Merck, Germany) radical scavenging activity methods. Leaf extract (0.25 mL) was mixed with 2 mL DPPH solution (3.3 mg of DPPH in 100 mL methanol) and 8 ml ethanol(JT Baker) , vortexes gently, then incubated at room temperature for 30 min in the dark, and the absorbance (A1) was measured at 517 nm (Thermo Scientific Genesys 20, USA). The absorbance (A0) of a control sample (distilled water instead of leaf extract) was also recorded at the same wavelength. Radical scavenging activity (%) was calculated by using the formula = [(A0 − A1)/A0] × 100, where A0 was the absorbance of control sample and A1 was the absorbance of extracts.
Total Phenolic Analysis
Total phenolic content of the extract was measured using the Folin–Ciocalteu (Merck, Germany) reagent26 with slight modification. 0.2 mL of leaf extract was mixed with 0.2 mL of aquades and 0.2 mL of Folin–Ciocalteu reagent (1 N). Then 4 mL of sodium carbonate (Merck, Germany) solution ( 2% ) was added and then allowed to stand for 30 min in the dark for incubation. The absorbance was measured at 760 nm in a spectrophotometer (Thermo Scientific Genesys 20, USA). A standar curve was prepared using Gallic acid (Tokyo Chemical Industry Co., Ltd) (0.00-0.01 mg/mL). The total phenolic contents were expressed in terms of gallic acid equivalents (GAE) (mg of per mL extract).
Statistical Analysis
Results are expressed as the mean of 3 replicates. Statistical analysis was carried out with a statistical program Minitab version 18. One way-Anova with Fisher test was used. Results were considered significant if p<0,05.
Results and Discussion
Alpha glucosidase enzyme is located in the brush border of the small intestine and is involved in starch digestion to release monosaccharide. Inhibition of the enzyme leads to retardation of starch digestion in small intestine. Study of α-glucosidase inhibitor activity of extracts of bay, pandan, citrus leaves or their combination might contribute to the understanding of their potentiality for diabetic management. Commercially, inhibitor of this enzyme is available for glucose-lowering medications for diabetic patients27.
The α-glucosidase inhibitory activity of the extracts of single or mixture of leaves is not depend on the type of extracts (Fig 1). Percent of inhibition of B, P and C or their combinations against α-glucosidase was around 32%. Synergism or antagonism effect was not observed when the extracts were combined as the inhibition activity is not changed when these leaves were mixed.
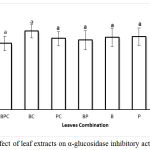 |
Figure 1: Effect of leaf extracts on α-glucosidase inhibitory activities.
|
Each value represents a mean ± SEM (n = 3); BPC, BC, PC and BP were combination B, P and C, B and C, P and C, B and P respectively. B, P and C were Bay, Pandan, and Citrus leaves extracts, respectively. The bars represent the mean of three replicates. Data points denoted by different superscripts (letters on the bar) differ significantly wit p <0,05.
It has been reported that the phenolic compounds of plant extracts have an ability to inhibit α-glucosidase28 and 29. B, P and C extracts were suggested containing different type polyphenol compounds with different affectivity against α-glucosidase. Although the total amount of the phenolic compounds in Indonesian bay leaves is the highest among other tested leaves (Fig.2), its inhibitory activity against α-glucosidase is similar as others (Fig. 1). Out results are in line with the previously reported results whereas not all phenolic compounds or fractions in plant extracts showed similar inhibition activity against α-glucosidase enzyme30 and 31. The phenolic compounds of Artemisia species extracts showed inhibitory activity against α-glucosidase enzyme (IC50 = 214.42-754.12 μg/mL) but not all individual phenolic compounds in the extract exhibited high inhibition. Extract of Artemisia containing high caffeoylquinic acids was the most pronounce31. The ethyl acetate extract of Clinopodium taxifolium (Kunth) Govaerts (Lamiaceae) showed stronger inhibitory activity against α-glucosidase than the methanolic and the hexanic extracts where ursolic acid contained in the three extracts was the individual phenolic compound that showed a strong inhibitory activity32. However, similar inhibition of 2 different fractions (P < 0.05) against α-glucosidase also has been observed for fraction of methanol (rich in phenolic and flavonoid compounds) and ethyl acetate (rich in proanthocyanidins) from Ehretia cymosa Thonn30. Therefore , it is suggested that phenolic compounds contained by B extract are less effective in inhibiting of α-glucosidase than the phenolic compounds in P or C extract, but their total phenolic concentration have no correlation with anti-α glucosidase activity (p = 0.654) (Table 2).
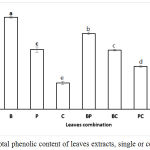 |
Figure 2: Total phenolic content of leaves extracts, single or combination.
|
BPC, BC, PC and BP were combination B, P and C, B and C, P and C, B and P respectively. B, P and CT were Bay, Pandan, and Citrus leaves extracts, respectively. The bars represent the mean of three replicates. Data points denoted by different superscripts (letters on the bar) differ significantly wit p <0,05. Total phenol content of each combination was calculated based on content of single extracts.
Table 2: Correlation coefficients between total phenolic with antiglucosidase, antiamylase and antioxidant activities of the extracts.
| Activities | Cell Contents | Total Phenol |
| Antiglucosidase | Pearson correlation | 0,104 |
| P-Value | 0,654 | |
| Antiamylase | Pearson correlation | -0,112 |
| P-Value | 0,630 | |
| Antioxidant | Pearson correlation | -0,527 |
| P-Value | 0,014 |
*Correlation is considered significant when p <0.05.
Alpha amylase is a carbohydrate hydrolyzing enzyme secreted by salivary glands and pancreas that can hydrolyze starch into oligosaccharide and simple sugars. Inhibition of this enzymes inhibits starch digestion and reduce the rate of glucose absorption in small intestine. Our result shows that extracts of B and C exhibit α-amylase inhibition higher than P but the difference is statistically not significant (Fig. 3). As the total phenolic concentration of the three extracts was difference (Fig 2), it is suggested that the effect of the extract on α-amylase activity merely depend on phenolic composition than on the total phenolic concentration. Phenolic compounds extracted from different plant species or cultivar has different composition and anti α-amylase activity33. Less than 15% of 126 extracts gained from 17 plants posed inhibition activity against α-amylase with varying degree, whereas 3 of them, those contain different compounds, had inhibition level more than 50%34. Chemical analysis of 2 Oat varieties (Amlal and F11-5) revealed the phenolic composition of their extracts was different, where IC50 for amylase inhibition of F11-5 was higher than Amlal, 1027.14 μg/mL and 723.91 μg/mL respectively33.
Inhibition pattern of the extracts (single or mixture) against α-amylase enzyme (Fig 3) was not similar as the pattern of the extracts against α-glucosidase (Fig 1). There is no significantly differ among the extracts in modulation of α-glucosidase activity, but each extract exhibited different effect on α-amylase activity. Mixing P with B and C increases the inhibitory activity of the extract against α-amylase as seen that percent of inhibition of BPC is significantly higher than P, eventhough their total phenolic content was not different. P and BPC extracts reduce the α-amylase activity by 16 % and 26 %, respectively. The increasing of inhibition activity of P due to mixing with B and C (BPC) was not due to increasing of total phenolic concentration (Fig 2) as no correlation between its phenolic concentration and inhibition activity was observed (p=0.630; Table 2). It is suggested that synergism effect was occurred when P was mixed with B and C as shown that mixture of plant extracts show superior effect when compared to single extract at the equivalent concentration35. Previously Lau et al.36 identified a synergism effect of a mixture of extracts of Astragalus membranaceus and Rehmanniae glutinosa roots in wound-healing of a diabetic foot ulcer animal model.
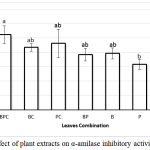 |
Figure 3: Effect of plant extracts on α-amilase inhibitory activities.
|
Each value represents a mean ± SEM (n = 3); BPC, BC, PC and BP were combination B, P and C, B and C, P and C, B and P respectively. B, P and CT were Bay, Pandan, and Citrus leaves extracts, respectively. The bars represent the mean of three replicates. Data points denoted by different superscripts (letters on the bar) differ significantly wit p <0,05.
It has been shown that potency of α-glucosidase and α-amylase inhibition of plant extracts are related to the presence of phenolic compounds28,33,37 those have antioxidant activity33. Therefore, in the current study we investigated the antioxidant powers of the extracts with DPPH radical scavenging assay. Figure 4 shown a variation in DPPH radical scavenging activities of the extracts ranging from 49% to around 77 %. The C extract exhibited a stronger DPPH scavenging ability than the P extract, though the difference is not statistically significant (76% and 74 %, respectively). On the contrary the B extract had the weakest scavenging activity (49%). A Mixture of the C and B extracts was proven to be more effective in the enhancement of the antioxidant activity of the B extract than any other combinations with the scavenging activity of 77% (Fig. 4).
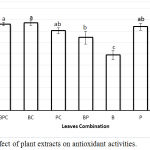 |
Figure 4: Effect of plant extracts on antioxidant activities.
|
Each value represents a mean ± SEM (n = 3); BPC, BC, PC and BP were combination B, P and C, B and C, P and C, B and P respectively. B, P and C were Bay, Pandan, and Citrus leaves extracts, respectively.. The bars represent the mean of three replicates. Data points denoted by different superscripts (letters on the bar) differ significantly wit p <0,05.
The antioxidant activity patterns of the extract combinations does not depend on the antioxidant activity of the single extract or their total phenolic content. For example, the total phenolic compound of the C extract was lower than of the P extract. However, both extracts exhibited a similar antioxidant activity. Additionally, the BC extract mixture showed higher antioxidant activity than the BP extract mixture (21.68 and 19.18 %, respectively), though the total phenolic compound of the BP mixture is higher than the BC mixture (7.57 and 5.90 mg/dL respectively). Therefore, accumulation rather than synergism effect on antioxidant activity was detected when the extracts were mixed. Negative correlation (-0.527) between total phenol concentration of the extracts with antioxidant activity was observed (p = 0.014), in which plant extracts with higher concentration of total phenol have lower antioxidant activity (Fig. 2 and Fig. 4). Similar findings have been reported showing that total phenolic compounds in the extracts of ginger, curcuma, cinnamon38 and Korean propolis39 have negative correlation with their antioxidant activity. Antioxidant activity of the plant extracts may not always positively correspond to the total phenol concentration, but may be determined by the composition of phenolic compounds39.
Furthermore, the molecular interactions between major phenolic compounds in plant extracts determine the antioxidant capacity of the extracts40. A mixture of plant extracts with high total phenol compounds will have low antioxidant capacity when major phenolic compounds in the extracts have antagonistic interactions. A mixture of chlorogenic acid and caffeic acid is an example of this description whereas a combination of gallic acid and caffeic acid that showed synergistic interaction, has high antioxidant capacity41. Therefore, antioxidant activity of mixture of phenolic-rich plant extracts may not always the sum of antioxidant activity of individual extract or individual phenolic compound present in the extracts40.
Conclusion
The leaf extracts and their combination showed inhibition activities against α-glucosidase and α-amylase enzymes and scavenging activity against free radicals. The synergism or antagonism effect was not observed when the extracts were combined as the enzyme inhibition and scavenging activities are not depend on the proportion of the extract in the mixtures. Additionally, the role of polyphenol compounds on inhibition of the starch digestion enzymes or scavenging of free radicals was not observed. Further study is required to fully elucidate the effect of the leaf or their combinations on diabetic animal models or diabetic patients.
Authors’ Contributions
All the authors have contributed equally to the manuscript.
Aknowledgements
This work was funded by research grant to Research Centre for Nutrition, Health and Herbal University of Lampung 2015/2016 from Research and Community Bureau, University of Lampung, Indonesia. We would also like to thank Nurlinawati PhD for reviewing the draft of this paper.
Conflict of Interest
There are no conflict of interest.
References
- Sowmya N, Lakshmipriya N, Arumugam K, Venkatachalam S, Vijayalakshmi P, Ruchi V, et al. Comparison of dietary profile of a rural south Indian population with the current dietary recommendations for prevention of non-communicable diseases (CURES 147). Indian J Med Res 2006; 144(1):112-119.
- Golozar A, Khalili D, Etemadi A, Poustchi H, Fazeltabar A, Hosseini F, et al. White rice intake and incidence of type-2 diabetes: analysis of two prospective cohort studies from Iran. BMC Public Health 2017; 17(1):133.
- Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr 2013; 97(3):505-16.
- Gray GM. Starch digestion and absorption in nonruminants. J Nutr 1992. 122(1):172-7
- Sales PM, Souza PM, Simeoni LA, Silveira D. α-Amylase inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharm Sci 2012; 15(1):141-83.
- Liu T, Yip YM, Song L, Feng S, Liu Y, Lai F, et al. Inhibiting enzymatic starch digestion by the phenolic compound diboside A: A mechanistic and in silico Food Research International 2013; 54(1):595-600.
- Governa P, Baini G, Borgonetti V, Cettolin G, Giachetti D, Magnano AR, et al. Phytotherapy in the Management of Diabetes: A Review. Molecules 2018; 23(1). pii: E105..
- Broadhurst CL, Polansky MM, Anderson RA. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J Agric Food Chem 2000. 48(3):849-52.
- Khan A, Zaman G, Anderson RA. Bay leaves improve glucose and lipid profile of people with type 2 diabetes. J Clin Biochem Nutr 2009; 44(1):52-6.
- Devi SL, Kannappan S, Anuradha CV. Evaluation of in vitro antioxidant activity of Indian bay leaf, Cinnamomum tamala (Buch. -Ham.) T. Nees & Eberm using rat brain synaptosomes as model system. Indian J Exp Biol 2007; 45(9):778-84.
- Faras AF, Wadkar SS, Ghosh JS. Effect of leaf extract of Pandanus amaryllifolius (Roxb.) on growth of Escherichia coli and Micrococcus (Staphylococcus) aureus. International Food Research Journal 2014; 21(1): 421-423.
- Saenthaweesuk S, Naowaboot J, Somparn Pandanus amaryllifolius leaf extract increases insulin sensitivity in high-fat diet-induced obese mice. Asian Pacific Journal of Tropical Biomedicine 2016; 6(10): 866-871.
- Chiabchalard A, Nooron N. Antihyperglycemic effects of Pandanus amaryllifolius Roxb. leaf extract. Pharmacogn Mag 2015; 11(41):117-22.
- Tips Memasak Nasi Lebih Enak dan Wangi Secara Alami. 2014. https://www.vemale.com/tips-dapur/52809-tips-memasak-nasi-lebih-enak-dan-wangi-secara-alami.html. Downloaded 10th August 2017.
- Tunjung WAS, Cinatl Jr J, Michaelis M, Smales CM. Anti-Cancer Effect of Kaffir Lime (Citrus hystrix DC) Leaf Extract in Cervical Cancer and Neuroblastoma Cell Lines. Procedia Chemistry 2015; 14:465-468.
- Abirami A, Nagarani G, Siddhuraju P. In Vitro Antioxidant, Anti-Diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and maxima fruits. Food Science and Human Wellness 2014; 3(1):16-25.
- Dal S, Sigrist S. The Protective Effect of Antioxidants Consumption on Diabetes and Vascular Complications. Diseases 2016; 4(3). pii: E24. doi: 10.3390/diseases4030024
- Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X, et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016; 21(10). pii: E1374.
- Nazarian-Samani Z, Sewell RDE, Lorigooini Z, Rafieian-Kopaei M. Medicinal Plants with Multiple Effects on Diabetes Mellitus and Its Complications: a Systematic Review. Curr Diab Rep 2018; 18(10):72.
- Al-Waili N, Al-Waili H, Al-Waili T, Salom K. Natural antioxidants in the treatment and prevention of diabetic nephropathy; a potential approach that warrants clinical trials. Redox Rep 2017; 22(3):99-118.
- Lelono RA, Tachibana S, Itoh K. In vitro antioxidative activities and polyphenol content of Eugenia polyantha Wight grown in Indonesia. Pak J Biol Sci 2009; 12(24):1564-70.
- Ghasemzadeh A, Jaafar HZ. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Complement Altern Med 2013; 13:341.
- Rao RR, Tiwari AK, Reddy PP, Babu KS, Ali AZ, Madhusudana K, et al. New furanoflavonoids, intestinal α-glucosidase inhibitory and free radical [DPPH) scavenging, activity from antihyperglycemic root extract of Derris indica (Lam). Bioorg Med Chem 2009; 17:5170–5175.
- Hasenah, A, Houghton PJ, Soumyanath A. α-amylase inhibitory activity of some malaysian plants used to treat diabetes; with particular reference to phyllanthus amarus. Journal of ethnopharmacology 2006. 107(3):449– 455
- Xu JG, Hu QP, Antioxidant and DNA-protective activities of chlorogenic acid isomers. Journal of Agricultural and Food Chemistry 2012; 60: 11625–11630.
- Ismail, J., Runtuwene, M.R.J., Fatimah, F. Penentuan total fenolik dan uji aktivitas antioksidan pada biji dan kulit buah pinang Yaki (Areca vestiaria Giseke). Jurnal Ilmiah Sains 2012; 12(2):84-88.
- Kerru N, Singh-Pillay A, Awolade P, Singh P. Current anti-diabetic agents and their molecular targets: A review. Eur J Med Chem 2018; 25;152:436-488.
- Kalita D, Holm DG, LaBarbera DV, Petrash JM, Jayanty SS. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS One 2018. 25;13(1):e0191025.
- Bothon FTD, Debiton E, Avlessi F, Forestler C, Teulade JC, Sohounhloue DKC. In vitro biological effects of two anti-diabetic medicinal plants used in Benin as folk medicine. BMC Complement Altern Med 2013; 13:51..
- Ogundajo A, Ashafa AT. Phytochemical Compositions and In vitro Assessments of Antioxidant and Antidiabetic Potentials of Fractions from Ehretia cymosa Thonn. Pharmacogn Mag 2017; 13(Suppl 3):S470-S480.
- Olennikov DN, Chirikova NK, Kashchenko NI, Nikolaev VM, Kim SW, Vennos C. Bioactive Phenolics of the Genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS Profile of the Siberian Species and Their Inhibitory Potential Against α-Amylase and α-Glucosidase. Front Pharmacol 2018; 9:756.
- Morocho V, Valle A, García J, Gilardoni G, Cartuche L, Suárez AI. α-Glucosidase Inhibition and Antibacterial Activity of Secondary Metabolites from the Ecuadorian Species Clinopodium taxifolium (Kunth) Govaerts. Molecules 2018. 23(1). pii: E146.
- Marmouzi I, Karym EM, Saidi N, Meddah B, Kharbach M, Masrar A, et al. In Vitro and In Vivo Antioxidant and Anti-Hyperglycemic Activities of Moroccan Oat Cultivars. Antioxidants (Basel) 2017; 6(4). pii: E102.
- Sudha P, Zinjarde SS, Bhargava SY, Kumar AR. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complementary and Alternative Medicine 2011; 11:5.
- Che CT, Wang ZJ, Chow MSS, Lam CWK. Herb-Herb Combination for Therapeutic Enhancement and Advancement: Theory, Practice and Future Perspectives. Molecules 2013; 18, 5125-5141.
- Lau KM, Lai KK, Liu CL, Tam JC, To MH, Kwok HF, et al. Synergistic interaction between Astragali Radix and Rehmanniae Radix in a Chinese herbal formula to promote diabetic wound healing. J Ethnopharmacol 2012; 141(1):250-6.
- Ombra MN, d’Acierno A, Nazzaro F, Spigno P, Riccardi R, Zaccardelli M, et al. Alpha-amylase, α-glucosidase and lipase inhibiting activities of polyphenol-rich extracts from six common bean cultivars of Southern Italy, before and after cooking. Int J Food Sci Nutr 2018; 16:1-11.
- Nurdin, S.U., Sukohar, A., Ramadhani, O.S. Antiglucosidase and Antioxsidant Activities of Ginger, Cinnamon, Turmeric and Their Combination. Internasional Journal of Pharmacy & Pharmaceutical Research 2017; 10(1):296-306.
- Wang X, Sankarapandian K, Cheng Y, Woo SO, Kwon HW, Perumalsamy H, et al. Relationship between total phenolic contents and biological properties of propolis from 20 different regions in South Korea. BMC Complement Altern Med 2016; 16: 65.
- Palafox-Carlos H, Gil-Chávez J , Sotelo-Mundo RR, Namiesnik J, Gorinstein S, González-Aguilar Antioxidant Interactions between Major Phenolic Compounds Found in ‘Ataulfo’ Mango Pulp: Chlorogenic, Gallic, Protocatechuic and Vanillic Acids. Molecules 2012; 17(11): 12657-12664.
- Hajimehdipoor H, Shahrestani R, Shekarchi M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Research Journal of Pharmacognosy (RJP) 2014; 1(3): 35-40.







