Manuscript accepted on :18-June-2019
Published online on: 27-06-2019
Plagiarism Check: Yes
Reviewed by: Dini Sri Damayanti
Second Review by: Liudmila Spirina
Praveen Kumar S. E1 , Kurady Laxminarayana Bairy2, Veena Nayak*1, Shiva Kumar Reddy3
, Kurady Laxminarayana Bairy2, Veena Nayak*1, Shiva Kumar Reddy3 , Amruth Kiran4
, Amruth Kiran4 and Abhijna Ballal3
and Abhijna Ballal3
1Department of Pharmacology, Kasturba Medical College Manipal, Manipal Academy of Higher Education, Manipal, Karnataka - 576104, India.
2Department of Pharmacology, RAK College of Medical Sciences, RAK Medical and Health Sciences University, P.O.Box 11172, Ras Al Khaimah, UAE.
3Centre for Molecular Neurosciences, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka - 576104, India.
4Department of Pharmacology, Melaka Manipal Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka - 576104, India.
Corresponding Author E-mail: veena.nayak@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/1692
Abstract
Alzheimer’s disease (AD) is an age-related progressive disorder characterized by neurodegeneration and accumulation of abnormal proteins. Artesunate, an anti-malarial drug has recently been shown to have anti-inflammatory, antiviral, angiogenic and other pleiotropic effects. It has also improved cognitive decline induced by hepatic coma which is suggestive of its role in learning and memory. In view of this, the current study was planned to assess the effect of the combination of artesunate with rivastigmine and memantine in aluminium chloride induced neurotoxicity. The study was conducted on 24 male adult albino wistar rats which were divided into four groups (n=6). Group - I to IV received saline, aluminium chloride (AlCl3), AlCl3 + artesunate +rivastigmine, AlCl3 + artesunate + memantine for 60 days respectively. After the 60th day of treatment, all animals were subjected to a passive avoidance task. All the animals were then sacrificed to study the histopathological changes in the hippocampus. Artesunate in combination with rivastigmine and memantine showed significant improvement in memory impairment and reduced neuronal death. Neuronal viability was more prominent in combination treatments as shown by histopathological results. Artesunate in combination with rivastigmine and memantine could be useful in AD. Artesunate can be used as an adjuvant therapy because artesunate combined with standard drugs produced significant neuroprotective effect.
Keywords
Aluminium Chloride; Alzheimer Disease; Artesunate; Maze Learning
Download this article as:| Copy the following to cite this article: Kumar S. E. P, Bairy K. L, Nayak V, Reddy S. K, Kiran A, Ballal A. Amelioration of Aluminium Chloride (AlCl3) Induced Neurotoxicity by Combination of Rivastigmine and Memantine with Artesunate in Albino Wistar Rats. Biomed Pharmacol J 2019;12(2). |
| Copy the following to cite this URL: Kumar S. E. P, Bairy K. L, Nayak V, Reddy S. K, Kiran A, Ballal A. Amelioration of Aluminium Chloride (AlCl3) Induced Neurotoxicity by Combination of Rivastigmine and Memantine with Artesunate in Albino Wistar Rats. Biomed Pharmacol J 2019;12(2). Available from: https://bit.ly/2X7pVw2 |
Introduction
Alzheimer’s disease (AD) is a chronic progressive neurodegenerative disorder associated with memory loss and accumulation of abnormal protein.1 Thirty-six million people worldwide are affected with dementia and in that 75% are estimated to be living with AD, and it is predicted to affect 1 in 85 people by 2050.2 AD is most often seen in people over 65 years and even occurs at an earlier age because of lifestyle, stress, depression, frustration.
Elevated mood swings and declined memory performance especially short-term memory task like forgetting names, numbers, places, inability to achieve new memories, hostility; irritability are the common symptoms. As the disease progresses from mild to moderate, there is worsening of cognitive and learning processes, inability to calculate, loss in object-based memory and emotional disturbances.3 The pathological changes in AD include neuronal loss in basal fore brain cholinergic neurons projecting to hippocampus and cortex, the formation of hyper-phosphorylated tau protein and its accumulation into neurofibrillary tangles (NFT) also called as Tau proteins and later formation of Aβ plaques by the sequential proteolytic action of β-secretase.1,3 These modifications were significantly seen in the cholinergic system of hippocampus and cortex which is closely related to the memory impairment and cognition deficits in AD.
Though the precise etiology of the disease is not known, genetic and environmental factors have been attributed to the causation of the disease.4,5 Amongst the environmental factors, the aluminium (Al) toxicity has been correlated with the increasing incidence of Alzheimer’s disease. It is the most abundant non-essential element present in our environment, and it is the third most constituent about 8% of the earth crust, exceeded only by oxygen and silicon. Its exposure to humans is massive because of its presence in food, water, dust, air, beverages, flavored drinks, energy drinks, and medicines. In addition to this, usage of its compound in cooking with aluminium utensils, usage of aluminium rich bags and foils , also in paper making, fire retardant, water treatment, filters, food additives, colours, drug preparations, and also by the consumption of corn, shell fish, yellow cheese, dairy products, spices, salt, breads, pastries, tooth paste, cakes, sausages, sugar-rich foods and drinks, tea herbs and cosmetics. Another study reported that tea, the most widely consumed beverage in the world that represents a major dietary source of Al and its concentration in tea infusions ranges about 0.035 and 16.82 mg/l and oral bioavailability of Al from the beverage was 0.37%.4,5
Moreover, Al is used in the pharmaceutical industry for drug preparation such as antacids phosphate binders, and buffer formulations of aspirin, vaccines and allergen injections. People staying in proximity to cement factories are more prone to Al exposure.1,6
Aluminium exposed animals have shown the neuro fibrillary tangles formation, cholinergic neuronal terminal loss in hippocampus and cortex, β amyloid protein aggregation (AB), development of oxidative stress and neuronal apoptosis in the hippocampus which is a site for memory formation and synaptic plasticity occurs during learning which is similar to the pathogenesis of AD.
Artesunate is a well-known standard drug used in the treatment of chloroquine-resistant malaria and cerebral malaria because of its safety profile and efficacy. It is a semisynthetic derivative of artemisinin from Chinese herbal plant Artemisia annua which was used for fever and chills in China. Apart from its antimalarial activity, artesunate has pleiotropic effects such as anti-inflammatory7 anticancer, antiviral7 and anti-allergic in asthma, impeding angiogenesis.8 Besides these, artesunate has recently been shown to have improved hepatic coma induced cognitive decline,9 reduced blood-brain barrier (BBB) damage in sub arachnoid haemorrhage10 and decreases infarct volume, brain oedema, survival rate and neurological deficit score in animal model of middle cerebral artery occlusion (MCAO) in rats.11 In addition to this artesunate in combination with erythropoietin (EPO) synergistically has significantly improved neuro-inflammation in cerebral malaria.12 Artesunate also showed increased seizure threshold and decreased the incidence of seizure episodes.13
The current treatment strategies for AD include anticholinesterases like donepezil, rivastigmine and NMDA receptor antagonists like memantine, though they are highly efficacious drugs, their long-term safety is not known. In view of this, there is a need for better and well-tolerated drugs for AD. The previous studies conducted by the authors had shown that artesunate alone could restore the aluminium chloride induced neurotoxicity in rats. However, there were no studies with artesunate being used in combination with rivastigmine and memantine. This promoted us to frame the study to decipher the combination of artesunate with rivastigmine and with memantine to see whether artesunate produces a synergistic effect with available drugs.
Materials and Methods
Animals
Twenty-four healthy adult inbred Male Wistar albino rats (150 -250 g) from the central animal research facility, Manipal were used in this study. The animals were housed under conditions of 25±2º C, 45-55% relative humidity and standard light and dark cycle (07:00 am to 07:00 pm) and had free access to water and standard diet (fortified with minerals and vitamins). All the animals were kept in polycarbonate cage with sterile paddy husk. All the experiments were performed as per Committee for the purpose of control and supervision of experiments on animals (CPCSEA) guidelines after obtaining the approval from Institutional Animal Ethics Committee (IAEC/KMC/56/2016), Kasturba Medical College, Manipal.
Chemicals and Reagents
The chemicals and reagents were procured as follows: Aluminium chloride (AlCl3) from Merck life sciences private limited, Artesunate was procured from (Shaanxi top pharm chemical co., Ltd.), and Rivastigmine and Memantine from Sun pharma Laboratories limited. All other chemicals and solvents were of the highest analytical grade-available commercially.
Experimental Design
Twenty-four male albino Wistar rats were randomly selected and divided into four groups containing six animals each (Figure 1). After 60 days of drug administration, the rats were subjected to the passive avoidance test, then anesthetized and processed for extra cardiac perfusion, the brain was then shelled out and fixed in 10% formalin for histological examination.
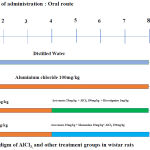 |
Figure 1: Experimental Paradiagm of AlCl3, and other treatment groups in wistar rats.
|
Behavioral Assessment
Passive Avoidance Test
Passive avoidance test is a behavioral model for testing fear motivated avoidance learning and memory in experimental rodents. In this study, passive avoidance test was performed according to the procedure of Rai et al. 2001.15-20
The experiment was divided into three parts. 1)Exploration 2) Learning. 3) Retention.
During the exploration, trial animals were allowed to explore the apparatus both in light and dark compartment for 3 minutes and three trials with an intertrial interval of 5 minutes. In each trial three parameters were noted a) time is taken to enter the dark compartment (Latency) b) time spent in the dark compartment and c) a number of crossings between light and dark compartment. After the exploration trial, during the learning phase, animals were kept in the light compartment facing away from the entrance of the dark compartment. When the animal enters the dark compartment, the door between two compartments was closed, and three-foot shocks (50 Hz, 1.5 mA, and 1 s duration) were given at 5-second intervals. The door was immediately opened, and the rat was then replaced to its home cage. A retention test was done 24hrs after the electric shock in which animals were placed similar to exploration trial and were allowed to explore the apparatus for 3 minutes of 3 trials. Three parameters were recorded the same as during exploration trial; the latency time was noted as 3 minutes for those animals that did not enter the dark compartment. The rats which did not enter to the dark compartment indicated the positive memory retention.15,16,17,18,19,20
Histo-Pathological Examinations
All the animals were sacrificed immediately after behavioral studies, and then the brain was perfused with cold phosphate buffered saline and 10% formalin and then brain was stored in 10% formalin. The brain tissue was processed with different grades of alcohol and xylene and then kept in wax to infiltrate into the tissues and then paraffin blocks were made. The 5μ thickness paraffin sections were taken from the paraffin blocks, and then sections were processed with different grades of alcohol, xylene and then stained with hematoxylin and eosin and examined the histopathological changes using a light microscope under 40x/10x magnification.
Statistical Analysis
Statistical analysis was done by using graph pad prism version 5.0. All the groups were statistically analyzed by using the one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to compare between the different groups. The experimental data was represented as Mean ± SD with six rats in each group. P value <0.05 was considered to be statistically significant.
Results
In our previous study, artesunate alone had reversed the aluminium chloride induced neurotoxicity in both behavioural and histopathological studies. In the present study Artesunate and rivastigmine, memantine combination ameliorates learning and memory impairment. There were no significant changes during the explorative phase between the different groups of animals with regards to the latency, time spent in dark compartment and number of crossings (Figure 2). After 24 hours of three-foot shocks at the 5-second interval in the dark compartment, the rats were assessed for retention memory.
In the retention phase, AlCl3 treated a group of rats took lesser latency time (14.83 ± 16.90s) when compared to the control group (128 ± 28.40) to enter the dark compartment. This suggests that AlCl3 treated rats failed to learn or retain their memory, So there was a more significant difference (p<0.01 NC vs. AlCl3) as shown in (figure 2A). Artesunate and rivastigmine combination (102.3 ± 60.78s) and artesunate and memantine combination took more time (116.7 ± 59.36s) to reach the dark compartment. However both the combination drugs took more time to enter the dark compartment by remembering the electric shock during the learning phase, there were significant differences compared to AlCl3 (p<0.05, AlCl3 vs. Artesunate and Rivastigmine), (p< 0.01, AlCl3 vs. Artesunate and Memantine) as shown in (figure 2A).
Where as in time spent in the dark compartment (Figure 2B). 24hrs after the electric shock(learning) during the retention phase, aluminium chloride treated rats spent more time (118.42 ± 54.44s) in dark compartment compared to normal control (2.5 ± 2.8s). Whereas artesunate and rivastigmine (6.5s ± 5.9s), Artesunate and memantine (4.3 ± 7.09s) spent lesser time in the dark compartment by remembering electric shock during the learning phase compared to AlCl3. There was significant differences compared to AlCl3 (p<0.001 NC vs. AlCl3), (p<0.001 AlCl3 vs Artesunate and Rivastigmine), (p< 0.001 AlCl3 vs Artesunate and Memantine.) as seen in (figure 2B). In our next observation we observed the number of crossings between light and dark compartment, 24 hrs aftershock during retention phase there were a number of crossings in Aluminum chloride (2.83 ± 1.16) group compared to normal control (1.16 ± 0.75) but there was significant difference (p < 0.05, NC vs. AlCl3) among both the groups.whereas number of crossings in artesunate and rivastigmine (1.16 ± 0.98), Artesunate and memantine (1.0 ±.1.09) were less compared to aluminium chloride as shown in (figure 2C). They also showed a significant difference (p<0.05, AlCl3 vs. Artesunate and Rivastigmine), (p< 0.05, AlCl3 vs. Artesunate and Memantine.) among the groups compared to aluminium chloride.
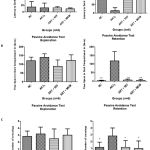 |
Figure 2: Represents the behavioural outcome of the study.
|
2-A represents the time taken (Latency) to enter dark compartment during exploration and retention phase. 1-B represents the total time spent in the dark compartment. 1-C Represents the number of transition during the experiment. (n = 6 per group). Significant values represented as p < 0.001(***), p< 0.01(**), p<0.05(*) versus AlCl3 group.
Assessment of Hippocampal Neuronal Cells
CA1 Region of the Hippocampus
CA1 sub-fields of the hippocampus with Haematoxylin and Eosin staining depicted in the figure 3A-B. Neuronal damage with swollen neuronal cell bodies because of phagocytosis process, hyper-dense neuronal soma cell, cell debris, and few pyknotic nuclei were observed in the AlCl3 group rats of hippocampal sections when compared to normal control group rats. Alternatively, artesunate + rivastigmine and artesunate + memantine combination treated group rats of hippocampal sections showed clear well shaped viable pyramidal neurons compared to AlCl3 group.
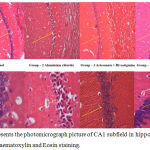 |
Figure 3: Represents the photomicrograph picture of CA1 subfield in hippocampal sections with Haematoxylin and Eosin staining.
|
3-A represents the all hippocampal sections in 10X magnification. 3-B represents all sections of rats in 40X magnification. Arrow mark represzents dense and dead neuronal cells in all groups.
CA3 Region of Hippocampus
CA3 sub-regions of the hippocampus with H and E staining were depicted in figure 4 A-B. Compared to CA1 and other regions of the hippocampus CA3 sub-region is a quite broad and has clear 4-5 layer of pyramidal cells and more sensitive to stress and injury. CA3 region sections of AlCl3 group rats showed less cell density, swollen irregular shaped hyper-dense cells with no well-defined boundary between cytoplasm and nucleus and more space between cells indicates that presence of dead cells, decreased in cell size and cerebral edema. While artesunate + rivastigmine and artesunate + memantine combination treatment group rats hippocampal sections showed an overall improvement in cell morphology.
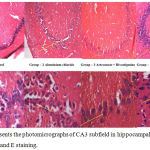 |
Figure 4: Represents the photomicrographs of CA3 subfield in hippocampal sections with H and E staining.
|
4 -A represents the all hippocampal sections in 10X magnification. 4 -B represents the all sections of rats in 40X magnification. Arrow mark represents dense and dead neuronal cells in all groups.
Discussion
Artesunate along with rivastigmine and memantine treated group of rats took more time to enter the dark compartment (Latency) after electric shock and also spent lesser time in the dark compartment and number of crossings were also less compare to AlCl3 group. This is due to the electric shock during learning phase after the exploration phase which the rats remembered after 24hrs. The AlCl3 treated group of rats took lesser time to enter the dark compartment and also spent more time in dark compartment in spite of electric shock and also a number of crossings were more in AlCl3 group when compared to artesunate. This is because they did not remember the electric shock during the learning phase. These results were also consistent with previous study which stated that memory consolidation and memory retrieval requires the coordinated sequential activity in a passive avoidance task.24
By taking all results together, combination therapy enhanced learning and memory This is evident from the significant increase of mean latency to enter into dark compartment, less time spent in the dark compartment where they experience foot shock, and less number of crossing between two compartments. This suggests that combination therapy protected the rats from AlCl3 induced neurotoxicity. Apart from this, these results also suggest that combination therapy provides a synergistic effect in retaining working memory. These results are also consistent with previous studies where an artesunate combination with erythropoietin improved hippocampal-dependent cognitive decline in cerebral malaria.12 Artesunate with rivastigmine and memantine combination demonstrated neuroprotection as evidenced by behavioural and histological findings. Artesunate and memantine have also been reversed the memory impairment; the possible mechanism may be due to synergistic NMDA receptor blockade by both memantine and artesunate.13
Histo-pathological Evaluation of hippocampal sections of AlCl3 treated group of rats confirms neurodegenerative changes in CA1 and CA3 sub-regions of hippocampus.22,23,24 Histopathological changes observed are massive depletion of cellular body, acidic cytoplasm, nuclear vacuolation and more inter cellular space in AlCl3 treared group of rats. Artesunate in combination with rivastigmine and memantine combination improved the viable cells, less inter-cell space, normal in structure and more in numbers in the CA1 subfield of hippocampus (figure 3 (A & B). Furthermore, neuronal cells are in a pyramidal, or spherical shape in the CA3 subfield suggest that treatment with artesunate with available drugs combated neurodegeneration and neuro-inflammation in CA3 subfield of hippocampus (Figure 4; A and B). Artesunate with rivastigmine and memantine combination therapy significantly improved the neurotoxicity by induced by AlCl3.
The probable mechanisms of improved memory may be due to an increase in the acetylcholine or blocking the excitatory glutamatergic neurons to reduce neuro-inflammation. Rivastigmine is well known for increasing brain acetylcholine level by inhibiting the acetyl-cholinesterase enzyme and memantine a known NMDA receptor blocker when with artesunate significantly reversed and protected the neuroinflammation. Artesunate combination with memantine showed a significant effect on learning and memory deficit induced by AlCl3. Wheather both the drugs block NMDA receptors and could decrease the glutamate inputs to neurons as the underlying mechanism needs further evaluation.
Conclusion
Treatment with Artesunate and its combination with rivastigmine and memantine exerted neuroprotective action against AlCl3 induced learning and memory deficit. A well-planned clinical study involving the above combination of drug in Alzheimer’s patients is worthwhile in order to establish the useful ness of this combination in clinical practice.
Acknowledgements
The authors express their gratitude to HOD, Department of Pharmacology, KMC, Manipal, Department of Anatomy, MMMC, Manipal and Manipal Academy of Higher education for their support and help.
Conflict of Interest
There is no conflict of interest.
Ethics of Human and Animal Experimentation
Experiments were performed as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
References
- Prema A, Thenmozhi AJ, Manivasagam T, Essa MM, Akbar MD, Akbar M. Fenugreek Seed Powder Nullified Aluminium Chloride Induced Memory Loss, Biochemical Changes, Aβ Burden, and Apoptosis via Regulation Akt/GSK3β Signaling Pathway. PLoS ONE., 2016 Nov 28 ;11(11): e0165955.
- Bhattacharya B, Mangilal T, Nagakishore R. Alzheimer’s disease-pathophysiology, diagnosis and modern approach to treatment. World J Pharm Pharm Sci., 2014;3(10):1452-60.
- Reddy SK, Sudheer A, Arunamma M, Sree PL, Jyothirmayi E. Protective effect of Picrorhiza kurroa on Alzheimer’s disease induced by aluminium chloride in rats. Int J Basic Clin Pharmacol., 2017;6:602-7.
- Dhivya Bharathi Mathiyazahan, Arokiasamy Justin Thenmozhi, Thamilarasan Manivasagam. Protective effect of black tea extracts against aluminium chloride-induced Alzheimer’s disease in rats: A behavioural, biochemical and molecular approach. Journal of Functional Foods., 16 (2015); 423 – 35.
- Kafeel A. Khan, Nitesh Kumar, Pawan G. Nayak, Madhavan Nampoothiri, Rekha R. Shenoy, Nandakumar Krishnadas, C. Mallikarjuna Rao and Jayesh Mudgal. Impact of caffeic acid on aluminium chloride-induced dementia in rats. Royal Pharmaceutical Society, Journal of Pharmacy and Pharmacology., 2013;2:1-6.
- Justin Thenmozhi A, William Raja TR, Janakiraman U, Manivasagam T. Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem Res., 2015 Apr; 40(4):767-76.
- Dunjing Wang, Jun Shi, Shuiqing Lv, Weiwei Xu, Jizhen Li, Wei Ge, Chenghua Xiao, Deqin Geng, and Yonghai Liu. Artesunate Attenuates Lipopolysaccharide-Stimulated Proinflammatory Responses by Suppressing TLR4, MyD88 Expression and NF-κB Activation in Microglial Cells. Inflammation., 2015 May; DOI: 10.1007/s10753-015-0172-7.
- Cheng C, Ho WE, Goh FY, Guan SP, Kong L. Anti-Malarial Drug Artesunate Attenuates Experimental Allergic Asthma via Inhibition of the Phosphoinositide 3-Kinase/Akt Pathway. PLoS ONE., 6(6): e20932.
- Shilun Zuo, Hongfei Ge, Qiang Li, Xuan Zhang, Rong Hu, Shengli Hu, Xin Liu, John H. Zhang, Yujie Chen, Hua Feng. Artesunate Protected Blood-Brain Barrier via Sphingosine 1 Phosphate Receptor 1/Phosphatidylinositol 3 Kinase Pathway After Subarachnoid Hemorrhage in Rats. Mol Neurobiol., 28 Jan 2016; 54(2):1213-1228.
- Yuan-Bo Wua, Li Zhanga, Wen-ting Lib, Yi Yanga, Jiang-ming Zhaoa. Artesunate restores spatial learning of rats with hepatic encephalopathy by inhibiting ammonia-induced oxidative damage in neurons and dysfunction of glutamate signaling in astroglial cells. Biomed & Pharmacother., 2016 Dec; 84:972-978.
- Shao M, Shen Y, Sun H, Meng D, Huo W, and Qi Xu. Protectiveness of Artesunate Given Prior Ischemic Cerebral Infarction is mediated by Increased Autophagy. Front. Neurol., 9:634. doi: 10.3389/fneur.2018.00634.
- Yunting Dua, Guang Chenb, Xuexing Zhanga, Chunyun Yua, Yaming Caoa, Liwang Cuic. Artesunate and erythropoietin synergistically improve the outcome of experimental cerebral malaria. International Immunopharmacology., 48 (2017); 219 –230.
- Sanjana K, Shyamjith M, Deepa B, Rao S, Pai P. Effect of artesunate on maximal electroshock and pentylenetetrazole-induced seizures in albino mice. Int. J. Green Pharm., 2012;6(1):63.
- Peter Georgiev Yanev, Darinka Slavcheva Dimitrova, Damianka Peteva Getova-Spassova. Effects of rivastigmine and memantine alone and in combination on learning and memory in rats with scopolamine-induced amnesia. Open Med., 2015; 10: 338-345.
- Jan Bures, Olga Buresova, Joseph P. Huston. Techniques and basic experiments for the study of brain and behaviour. Newyork: Elsevier Science Publishers, Amsterdam; 1983. p. 148- 52.
- Rai KS, Murthy KD, Karanth KS, Rao MS. Clitoria ternatea (Linn) root extract treatment during growth spurt period enhances learning and memory in rats. Indian J Physiol Pharmacol., 2001;45(3):305-313.
- Bharath G, Shalini Adiga, Shiva Kumar Reddy, Amruta Tripathy. Comparison of effects of carvedilol and propranolol on learning and memory in rats. International Journal of Advanced Research., 2015; 3 (10) :1164 – 1168.
- Vijayalakshmi, Adiga S, Bhat P, Chaturvedi A, Bairy KL, Kamath S. Evaluation of the effect of Ferula asafoetida Linn. gum extract on learning and memory in Wistar rats. Indian J Pharmacol., 2012; 44(1):82-87.
- Chetty S, Adiga S, Reddy S. Evaluation of the effect of costus igneous on learning and memory in normal and diabetic rats using passive avoidance task. International Journal of Pharmacy and Pharmaceutical Sciences., 2014 Jan 1; 6:835-838.
- AVR Mohanbabu, Meena Kumari K K, B R Chandrashekar, H D Pradeepa, Rockson C, P B Nandit. Evaluation of potential antiamnesic activities of aqueous extract of Vitex trifolia leaves against scopolamine-induced amnesia and in normal rats. J Basic clin Physiol Pharmacol., 2015; 26(2): 201–209.
- Sivakumar G, Vidyadhara DJ, Reddy S, Rajesh T, Babu Ramesh MG, Rao KGM, Rai KS. Prophylactic combined supplementation of choline and docosahexaenoic acid attenuates vascular cognitive impairment and preserves hippocampal cell viability in a rat model of chronic cerebral hypoperfusion ischemic brain injury. Int J Basic Clin Pharmacol., 2015; 4:522-30.
- Maccioni RB, Munoz JP, Barbeito L. The molecular basis of Alzheimer’s disease and other neurodegenerative disorders. Arch Med Res., 2001; 32:367-381.
- Ouafa Rebai and Nour Eddine Djebli. Chronic Exposure to Aluminium Chloride in Mice: Exploratory Behaviors and Spatial Learning. Advan. Biol. Res., 2 (1-2): 26-33, 2008.
- Esam S. Kamela and Nashwa Mostafab. Effect of aluminium chloride on the hippocampus of adult rats and the possible protective role of Nigella sativa: a histological and immunohistochemical study. The Egyptian Journal of Histology., 2013, 36:505-513.







