Manuscript accepted on :13-Mar-2019
Published online on: 22-03-2019
Plagiarism Check: Yes
Reviewed by: Roopali Agarwal
Second Review by: Muhammad Alaa
Sajid Ali1, Sarfaraz Alam1, Sarfaraz Ahmad1, Maksood Ali1, Waquar Ahsan1, Masoom Raza Siddiqui2, Salahuddin Ansari3, Shamim4 and Mohammad Daud Ali*5
1College of Pharmacy, Jazan University, Jazan, KSA.
2Department of Chemistry, College of Science, King Saud University, Riyadh, Saudi Arabia, KSA.
3College of Pharmacy, Al-Dawadmi, Shaqra University, KSA.
4College of Pharmacy Teerthanker Mahaveer University Moradabad, Uttar Pradesh 244001, India.
5Department of Pharmacy, Mohammad Al-Mana college of Health Sciences, Abdulrazaq Bin Hammam Street, As Safa, Dammam, 34222, KSA.
Corresponding Author E-mail: dali.niper@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1611
Abstract
To evaluate the wound healing activity of ethanolic extract of Tamarix aphylla L. on animal model. Wound creation like circular excision and linear incision method were considered for this study. The various parameters were studied like DNA estimation, total protein estimation, estimation of Hexosamine and Uronic acid, estimation of lipid peroxides and antioxidant activity, Tensile Strength of tissues from incision wounds, Antioxidant activity, Antimicrobial activity, Period of epithelialization and finally TNF-a concentration in the wound tissue homogenate were estimated. The treatment groups with the extract showed significant antimicrobial activity with compare to the standard drug. Significantly, 93. 86% increase in the collagen content and significant 52% up regulation in tensile strength was observed in the treated group. 40% reduction was observed in epithelialization period of the treated wounds. The results of the current study confirm that the ethanolic extract of T. aphylla has potent wound healing capacity.
Keywords
Epithelialization Period; Tamarix Aphylla; Tensile Strength; TNF-Α; Wound Healing
Download this article as:| Copy the following to cite this article: Ali S, Alam S, Ahmad S, Ali M, Ahsan W, Siddiqui M. R, Ansari S, Shamim S, Ali M. D. Wound Healing Activity of Alcoholic Extract of Tamarix Aphylla L. on Animal Models. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Ali S, Alam S, Ahmad S, Ali M, Ahsan W, Siddiqui M. R, Ansari S, Shamim S, Ali M. D. Wound Healing Activity of Alcoholic Extract of Tamarix Aphylla L. on Animal Models. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2UR2stN |
Introduction
Wound healing is a series of conjugated process in which the damaged tissue at the site of injury is replaced by newly formed tissue. Healing is a five step process that includes haemostasis and blood clotting, fibroplasia and neovascularization, granulation tissue formation, re-epithelialization and finally the formation of new extracellular matrix and tissue remodeling.1,2 Several naturally occurring plant products are reported to up drive the process of healing,3,4 which are rich in active constituents such as triterpenes, tannins, flavonoids and alkaloids5 as well as other biomolecules.6 All these active phyto-principles are found to upregulate one or more steps of wound healing process.
In this study, we aimed to screen the alcoholic extract of Tamarix aphylla L. for possible wound healing activity on animal models. Tamarix aphylla, is commonly known as Athel or Tamarisk in English and Abal, Tarfaa, Ghaz or Athel in Arabic. It is mainly found as trees or tall shrubs that grow up to 12 m in height in alluvial or saline soil and has reddish brown or grey colored bark. Mostly, plants from this family are found to grow well in sub-tropical and temperate regions. These are the halophytes which can tolerate a variety of abiotic stresses such as draught, temperature, and saline impacts easily.7 Tamarix is a genus with over 55 species that are native to Middle East, Mongolia, North Africa, India, China and Europe with saline soils.8,9,10 In Saudi Arabia, the plant is reported to have natural vegetation properties in colder regions of Asir province such as Abha and Al-Baha.11 Although, the distribution is not restricted to the selective regions but the salty soils with low temperature range climate is suitable for its growth. This plant is widely available in Jazan province and commonly used for wound healing as a folklore medicine. The genera Tamarix is believed to possess a significant role in the phyto-remediation process and contributes to the reduction of pollutants from environment.12 Wound healing and anti-inflammatory action of Tamarix aphylla is also mentioned in Islamic literature as well as other sources from remote areas in Saudi Arabia. In Al-Qassim region of Saudi Arabia, dried powder of all parts of the plant was used to treat skin diseases of camel (allergic or mycotic dermatitis) by applying it on the affected skin for at least one week.13 Burnt smoke of the dried powdered leaves of the plant has the history to be used as wound healing agent as well as dental analgesic.14 Plant leaves when boiled in water and tied on the affected skin, works miraculously for wound healing, abscesses and rheumatism.15
Our current approach was to explore the wound healing capacity of this plant in a scientific manner by using modern techniques against the standard animal models. Many researchers previously reported various other activities of this plant but none of them explored the wound healing potential at molecular level and measure the role of TNF-α in wound healing. In view of these facts regarding medicinal values of the plant, we chose to examine the wound healing potential of an alcoholic extract of the leaves of Tamarix aphylla on rats.
Materials and Methods
Animals
Male albino rats weighing 140–210 g body weight were selected for study. Animals were kept in clean, sterile, polyvinyl cages and fed with commercial rats feed. Food and water were provided frequently to the animals ad libitum. All experimental procedures were carried as per institutional guidelines on ethics for animal handling.
Chemicals
L-hydroxyproline, pepsin, glucuronic acid, calf thymus DNA, chloramine-T and bovine serum albumin were purchased from Sigma Aldrich, Saudi Arabia. p-dimethyl aminobenzaldehyde and Folin’s Phenol reagent were purchased from Loba Chemie, Mumbai, India. Methyl cellulose was also obtained from Sigma Aldrich, Saudi Arabia. Anti-TNF-α mAb was purified using a protein G column kit (Kirkegaard & Perry Lab., Gaithersburg, MD) from the culture supernatants of hybridoma cells (clone MP6-XT2.2-11).
Preparation of alcoholic extract of Tamarix aphylla
The leaves of T. aphylla were collected from Jazan Region, Saudi Arabia. Leaves were cut into very small pieces, weighed, dried, powdered and homogeneously mixed in 10–20 volumes (by weight) of 70% ethanol and filtered to yield a viscous supernatant. This viscous supernatant liquid used as the crude alcoholic extract. Lyophilization of an aliquot of the extract was carried out and weighed.
Animal grouping, drug administration and Wound creation
Hairs present at the back side of the rats were removed and a 4 cm2 full thickness open excision wound was created by removing a patch of skin under anesthesia. Animals were segregated in two groups of six rats each. Control group (group 1) received 200 µL of unbuffered physiological saline, once a day, for a period of 15 days. The experimental group (group 2) received 200 µL of the alcoholic extract of T. aphylla, applied topically once a day for a period of 15 days. The granulation tissues formed were removed on 3, 6, 9 and 15 days post-wounding and used for analyses.
Incision wounds: Rats were distributed in two groups of six animals each. Rat skin was shaved and a six cm linear incision was made with a sterile sharp blade. Surgical sutures were used to close the incision maintaining a distance of approximately 1 cm between two. Extract was applied topically for the excision group animals. Sutures were removed on day 7 post-wounding. Dumbbell shaped tissue pieces were removed from the wound site on day 10 post wounding and used for tensile strength measurements.
Biochemical parameters
Nasir et al. (2017)16 method was utilized to extract Nucleic acids. Homogenization of 100 mg of granulation tissue was carried out in 5 mL of ice cold distilled water. To this, was added 5 mL of 10% trichloroacetic acid (TCA), and kept in an ice bath for 0.5 h in order to precipitate proteins and nucleic acids. Contents were then centrifuged and the pellets were first washed with 1 mL of 10% TCA followed by 3 mL of absolute alcohol to remove lipid content. To separate nucleic acids, the lipid free sediment was resuspended in 5 mL of 5% TCA and heated at 90° C for 15 min. The contents were centrifuged and the supernatant was used for the estimation of DNA by the method reported previously.17 Estimation of total protein was performed according to Lowry et al., (1951) method []. Pellets were resuspended in 0.1M Tris-HCl (pH 7.4) and the total collagen content in granulation tissues was estimated based on the hydroxyproline index by the method of Wold et al., (1999).18 Levels of Hexosamine and Uronic acid were estimated by following the methods of Saeed et al., 2016 and Mojica et al., 2010 respectively.19,20
Estimation of lipid peroxides and antioxidant activity
The thiobarbituric acid reaction method was used to determine Lipid peroxide levels (as nanomoles of malondialdehyde) in granulation tissues as described by Santos et al., (1980).21 To test the antioxidant potential of the plant, radical analysis was carried out by ESR measurements at room temperature in X-band using a Varian type ESR spectrophotometer Model E112 with 100 KHz field modulation. DPPH free radicals were measured according to Santiago et al., (1992) method.22 To 10 µL of the T. aphylla extract, was added 500 µL of 50 µM DPPH in methanol, mixed well and immediately transferred to the ESR spectrophotometer cell and analyzed exactly after 60 s. Blank was performed using 10 µL of methanol.
Biophysical parameters
To determine the rate of contraction of wounds, wound surface was traced on a transparent graph paper and the surface area was measured by planimetry. The period of epithelialization was considered as the total number of days needed for eschar shedding without any remains of raw wound. Muehlberger (2005)23 method was utilized for the measurement of tensile strength of wound tissues.
Measurement of cytokine concentrations
To neutralize TNF-α biological activity, mice were injected intraperitoneally with mAb against cytokine at 400 μg one day prior to and three days after the wound creation. Rat IgG was used as the control Ab. An amount of 25 μg/kg TNF-α, purified from a human B-cell lymphoblastoid cell line (BALL-1) was injected into the peritoneal cavity once daily beginning on the day of wound creation.24 PBS was used as the control vehicle. Wound tissues were homogenized in PBS and the TNF-α concentration in the supernatant was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Endogen, Cambridge, MA). Results were expressed as the values per one wound with a detection limit 5.1 pg/mL.
Antibacterial activity
The antibacterial activity of the alcoholic extract was measured by reported method.25,26 1 mm2 wells were made on nutrient agar plates, using a cork borer, and 20 µL of the extract was applied to the wells. Microorganisms to be tested were streaked on the agar plates and grown overnight at 37°C. The presence of clear zones of inhibition was taken to represent antibacterial activity. Equal amount of alcohol (vehicle) were inoculated into another set of similar plates, to serve as controls.
Results
Estimated results of the total protein, DNA and collagen content in the granulation tissues of control and treated wounds are summarized in Figure 1.
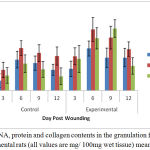 |
Figure 1: DNA, protein and collagen contents in the granulation from control and experimental rats (all values are mg/ 100mg wet tissue) mean ± SD, n = 6.
|
Significantly, 93. 86% increase in the collagen content was observed in the treated group at the day 6 of wound creation. Similar pattern was noticed in case of DNA content, with a parallel increase in total protein content. The findings of Hexamine and uronic acid levels in the granulation tissues of control and experimental wounds are shown in Figure 2.
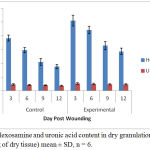 |
Figure 2: Hexosamine and uronic acid content in dry granulation tissue (µg/100mg of dry tissue) mean ± SD, n = 6.
|
The ground substance level was up regulated upto day 12 post-wounding in the treated group, and fell back to normal afterwards. Figure 3 depicts results of the rates of contraction of control and experimental wounds.
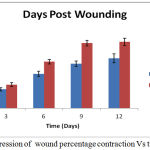 |
Figure 3: Progression of wound percentage contraction Vs time.
|
The contraction of wound for treated group was found to be much faster. Interestingly, a sharp (40%) reduction was observed in epithelialization period of the treated wounds Figure 4. The tensile strengths of tissues for the control and experimental incision wounds are compared in table 1.
Table 1: Tensile Strength of tissues from incision wounds of the control and treated rats (measured as kg/cm2), Mean ± SD, n=6).
| Control | Treated | |
| Tensile strength (Kg/cm2) | 8.35 ± 1.66 | 12.71 ± 1.35 |
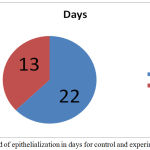 |
Figure 4: Period of epithelialization in days for control and experimental wound.
|
A significant 52% up regulation in tensile strength was observed in the treated group. Table 2 reveals the antioxidant activity of Tamarix aphylla plant extract, estimated by ESR measurement technique.
Table 2: Antioxidant activity of T. phyllus methanolic extract quantified through ESR measurements of DPPH radical spin reduction
| Control | Treated | |
| DPPH radicals (× 1017 spins/mL) | 0.301 ± 0.0193 | 0.163 ± 0.0121 |
Up to 41% reduction of DPPH spins from the control value was observed in the treated group. The TNF-a concentration in the homogenate supernatants of the collected wounds at various time intervals estimated as shown in Figure 5.
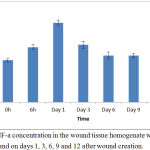 |
Figure 5: TNF-a concentration in the wound tissue homogenate was measured at 0 and 6 h and on days 1, 3, 6, 9 and 12 after wound creation.
|
TNF-a was detected just after wound creation, and rapidly increased in concentration during the first several hours. The peak concentration level was reached on day 1, and levels declined thereafter to the basal level. TNF-a production was detected in the homogenate of wound tissues with a peak level on day 3 after wound creation; wound healing was significantly delayed on day 3, but not on day 6, in mice treated with anti-TNF-a mAb compared with mice that received control IgG; and TNF-a accelerated wound repair on day 3, but not on day 6. Table 3 summarizes the extent of antibacterial activity of Tamarix aphylla. The drug was found to be potentially active against S. aureus and P. aeruginosa.
Table 3: Antimicrobial activity of control and treated groups against S. aureus and P. aeruginosa
| Organism | Zone of inhibition (mm) | |
| Control | Treated | |
| S. aureus | 5.36 ± 0.0136 | 22.29 ± 0.0438 |
| P. aeruginosa | 10.03 ± 0.0235 | |
Discussion
This work is aimed to assess the beneficial effects of plant, Tamarix aphylla, on rat dermal wound healing process using standard animal models. Plant metabolites are reported to be promising agents for wound healing and are preferable because of their availability, non-toxic nature, easy administration, lesser side effects and effectiveness as crude preparations. Previously, many plants and their products are reported to have significant wound healing activities such as C. asiatica, a sweet, acrid, tropical weed/green, was found to be effective in wound healing.6 Likewise, the positive influence of atropical cactus Aloe vera on wound healing was also documented.4 These findings encouraged us to further examine other plants which had other reported medicinal values, for wound healing. Therefore, systematic in vivo studies on Tamarix aphylla for wound healing were conducted. Cellular hyperplasia indicated that there was an increase in DNA content in the treated wounds. Simultaneously, there was also an increase in the total protein content representing active synthesis and deposition of matrix proteins in the granulation tissues. Collagen constitutes an essential component of the extracellular matrix and the healing process depends, to a large extent, on the regulated biosynthesis and deposition of new collagens and their subsequent maturation. The type III collagen levels are particularly increased during early stages of wound healing.6 Assessment of collagen content in granulation tissues indicated that there is an enhanced production of new collagens in treated wounds post treatment with Tamarix aphylla. Hexosamine and uronic acid, commonly known as matrix molecules, are involved in the synthesis of new extracellular matrices. During early stages of wound healing, an increase in the levels of these components was reported, following which normal levels were restored (Dunphy and Udupa, 1955). Similar results were obtained in case of Tamarix aphylla treated wounds, where the levels of hexosamine and uronic acid increased significantly. Tissue damage can be aggravated by free radicals and oxidative reaction products which arise through lipid peroxidation. These play a major role in various tissue disorders27 and are predominantly noticed during fibrosis as well as during wound healing.28 In view to these contexts, several plant products having antioxidant action such as curcumin, vitamin E, etc. have been testified to reduce oxidative damage to tissues.29 In fact, the use of antioxidants became very popular in recent years for effective strategy for therapeutic approaches to such type of disorders. Numerous medicinal plants have been reported to possess antioxidant properties.30 Our studies on the lipid peroxide status and DPPH radical reducing properties were carried out through ESR technique. It was observed that Tamarix aphylla extract has significant antioxidant activity, which would help inhibit oxidative damage and stimulate the healing process. Wound contraction rate appeared to be faster in treated groups due to the larger availability at the wound site when applied topically. The period of epithelialization was also found to be lesser for the treated wounds. All these results additionally supported the effectiveness of Tamarix aphylla in wound healing process. Newly synthesized collagens deposited at the wound site increases the collagen amount per unit area as well as the tissue tensile strength.31 Tamarix aphylla treated incision wound tissues exhibited higher tensile strength, indicating larger collagen content at the wound site. Post-operative wound infection is frequently encountered in several surgical procedures owing to the exposure of wound to microorganisms. Several plant products are known to possess intrinsic antibacterial activity, improving their medicinal values. The results indicate that TNF-a is closely involved in the very early process of wound healing in skin. TNF-a was produced in the wounded tissues and reached a peak level on day 1, after which it returned to the basal level. However, this increase in TNF-a level was not statistically significant even at the time point of maximal production. This may be a result of the high TNF-a level detected just after wounding (0 h), which this constitutive expression of TNF-a is thought to be involved in the regulation of homeostasis in skin tissue.32 Thus, TNF-a levels measured in the wound tissues were likely higher than the normal basal levels, which suggests that this cytokine may be involved in the process of wound healing.
Our studies indicated that Tamarix aphylla is a potent inhibitor of growth of microorganisms such as S.aureus and P. aeruginosa. These data are in accordance with previously reported study on the antifungal33 and antibacterial34 activities of other medicinal plants, without cellular toxicity. The outcome of the present study strongly indicated that Tamarix aphylla is a potential candidate for wound healing owing to its positive influences on various phases of the process. Antioxidant and antimicrobial properties additionally played an important role in their wound healing capacity.
Conclusion
It was concluded that Tamarix aphylla may be the potential wound healing agent and it may be free from other problem which was associated with synthetic drug.
Acknowledgements
Authors are thankful to Jazan University, KSA and Teerthanker Mahaveer University, India for providing different types of facilities to complete this project.
Conflict of interest
There is no conflict of interest.
References
- Atala A., Irvine D., Moses M., Shaunak S. Wound Healing Versus Regeneration: Role of the Tissue Environment in Regenerative Medicine. MRS Bulletin. 2010;35(8):597-606.
CrossRef - Martinotti S., Propolis R. E . A new frontier for wound healing? Burns & Trauma. 2015;3:9.
CrossRef - Pereira R. F and Bártolo P. J. Traditional Therapies for Skin Wound Healing. Advances in Wound Care. 2016;5(5):208–229.
- Suguna L., Chandrakasan G., Joseph K. T. Influence of honey on biochemical and biophysical parameters of wounds in rats. J Clin Biochem Nutr. 1193;14:91–99.
CrossRef - Sarma S. P., Aithal K. S., Srinivasan K. K et al. Anti- inflammatory andwound healing activities of the crude alcoholic extracts and flavonoids of Vitex leucoxylon. Fitoterapia. 1990;61:263–265.
- Chithra P., Suguna L., Chandrakasan G. Influence of arginine on wound healing in rats. J Clin Biochem Nutr. 1995;18:111–117.
CrossRef - Saïdana D., Mahjoub M. A., Boussaada O., Chriaa J., Chéraif I., Daami M., Mighri Z., Helal A. N. Chemical composition and antimicrobial activity of vol atile compounds of Tamarix boveana (Tamaricaceae). Microbiol. Res. 2008;163:445–455.
CrossRef - Heywood V. H., Brummitt R. K., Culham A., Seberg O. Flowering plant families of the world.Royal Botanic Gardens. 2007.
- Baum B. R. The genus Tamarix. Israel Academy of Sciences and Humanities, Jerusalem. 1978.
- DeLoach C. J., Lewis P. A., Herr J. C., Carruthers R. I., Tracy J. L., Johnson J. Host specificity of the leaf beetle, Diorhabda elongata deserticola (Coleoptera: Chrysomelidae) from Asia, a biological control agent for saltcedars (Tamarix: Tamaricaceae) in the Western United States. Biol Control. 2003;27:117–147.
CrossRef - Alrumman S. A. Phytochemical and Antimicrobial properties of Tamarix aphylla L. leaves growing Naturally in the abha Region, Saudi Arabia. Arab J Sci Eng. 2016;41:2123-2129.
CrossRef - Marlin D., Newete S. W., Mayonde S. G., Smit E. R., Byrne M. J. Invasive Tamarix(Tamaricaceae) in South Africa: current research and the potential for biological control. Biol Invasions. 2017;19: 2971–2992
CrossRef - Abbas B., Al-Qarawi A. A., Al-Hawas A. The ethanoveterinary knowledge and practice of traditional healers in Qassim Region, Saudi Arabia. J Arid Environ. 2002;50:367-379.
CrossRef - Kamal M., Wazir S. M., Hassan M., Subhan M., Khan S. U., Muhammad M., Taj S. Ethanobotanically important plants of District Bnnu, Pakistan. Pak J Plant Sci. 2009;15:87-93.
- Marwat S. K., Rehman F. U., Khan M. A., Ahmad M. A. Q., Zafar M., Ghulam S. Medicinal folk recipes used as traditional phytotherapies in district Dera Ismail Khan, KPK, Pakistan. J Bot. 2011;43(3):1453-1462.
- Ali N., de Rampazzo P. R. C., Costa T. D. A and Krieger A. M. “Current Nucleic Acid Extraction Methods and Their Implications to Point-of-Care Diagnostics,”Bio Med Research International. 2017;1-13.
CrossRef - De Mey M., De Maeseneire S., Van Nieuland K., Lequeux G., Soetaert W., Vandamme. Evaluation of different methods for nucleic acids quantification. Commun Agric Appl Biol Sci. 2005;70(2):97-100.
- Wold J. P., Lundby F., Egelandsdal B. Quantification of connective tissue (hydroxyproline) in ground beef by autofluorescence spectroscopy. J Food Sci. 1999;64:377–383.
CrossRef - Mojica K. D., Cooney M. J. The uronic acids assay: a methodfor the determination of chemical activity on biofilm EPS. Biofouling. 2010;26(3):301-312.
CrossRef - Saeed M. T., Ahmad J., Kanwal S., Holowatyj A. N., Sheikh I. A.,Paracha R. Z., Shafi A., Siddiqa A., Bibi Z., Khan M., Ali A. Formal modeling and analysis of the hexosamine biosynthetic pathway: role of O-linked N-acetylglucosamine transferase in oncogenesis and cancer progression. PeerJ. 2016;4:e2348.
CrossRef - Santos M. T., Valles J., Aznar J. A., Vilches J. Determination of plasma malondialdehyde-like material and its clinical application in stroke patients. J Clin Pathol. 1980;33:973–976.
CrossRef - Santiago L. A., Midori H., Akitane M. Japanese soybean paste miso scavenges free radicals and inhibits lipid peroxidation. J Nutr Sci Vitaminol. 1992;38:297–304.
CrossRef - Muehlberger T., Moresi J. M., Schwarze H., Hristopoulos G., Laenger F., Wong L. The effect of topical tretinoin on tissue strength and skin components in a murine incisional wound model. Am. Acad. Dermatol. 2005;52:583-588.
CrossRef - Fukuda S., Ando S., Sanou O., et al. Simultaneous production of natural human tumor necrosis factor-a, -b and interferon-a from BALL-1 cells stimulated by HVJ. Lymphokine Res. 1998;7:175–185.
- Eldesouky Z., Amani S. A., Mounerah R. A., Reham M. E. Antimicrobial activities of Saudi Arabian desert plants. Phytopharmacology. 2012;2(1):06-113
- Hamid I.,Muhammad I., Abbas N., Muhammad & Ijaz A., Ali R., Amin B., Syed & Bibi S.,Musaratv U. In vitro Antimicrobial Study of Tamarix aphylla in View of Phytochemical Constituents. Pharmacologia. 2015;6:333-336.
CrossRef - Nohl H., Esterbauer H., Rice-Evans C. In Free Radicals in the Environment,Medicine and Toxicology. Richelieu Press: London.
- Sonel O., Cetinkale O., Ozbay G., Ahcloglu F., Bulan R. Oxygen free radicals impair wound healing in ischemic rat skin. Ann Plast Surg. 1997;3:516–523.
CrossRef - Sieradzki E., Olejarz E., Strauss K., Marzec A., Mieszkowska M., Kauzringny J. The effect of selenium and vitamin E on the healing process of experimental corneal lesions in the eye of the rabbit. Klin Oczna. 1998;100:85–88.
- Kumar K. C., Muller K. Medicinal plants from Nepal. Evaluation as inhibitors of lipid peroxidation in biological membranes. J Ethnopharmacol. 1999;64(2):135–139.
CrossRef - Xue M., Jackson C. J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Advances in Wound Care. 2015;4(3):119-136.
CrossRef - Mckay I. A., Leigh I. M. Epidermal cytokines and their roles cutaneous wound healing. Br. J. Dermal. 1991;124:513–518.
CrossRef - Dutta B. K., Rahman I. Das Antifungal activity of Indian medicinal plant extracts. Mycoses. 1998;41:535–536.
CrossRef - Ahmad I., Mehmood Z., Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol. 1998;62:183–193.
CrossRef







