Maha Al-Bassam1, Muzibunnisa A. Begam1, Saad Ghazal Aswad1 and Moamar Al-Jefout1,2,3,4
1The Department of Obstetrics and Gynecology, Tawam hospital, Al-Ain, UAE.
2The Department of Obstetrics and Gynecology, College of Medicine and Health Sciences (CM and HS), United Arab Emirates University, Al-Ain, UAE.
3 The Department of Obstetrics and Gynecology, Faculty of Medicine, Mutah University, Jordan.
4Sechenov First Moscow State Medical University, Russian Federation.
Corresponding Author E-mail: drmoamar@yahoo.co.uk
DOI : https://dx.doi.org/10.13005/bpj/1618
Abstract
Caesarean scar ectopic pregnancy (CSEP) is considered an extremely rare entity, but life threatening, however, with the increase in C/S rates it is becoming a more common finding. To explore the possibility of utilizing ultrasound guided transvaginal aspiration and injection of 50 mg methotrexate followed by mechanical disruption as a promising primary treatment approach for CSEP. In a series of 11 cases at Tawam Hospital (UAE) diagnosed with CSEP, an ultrasound guided transvaginal aspiration of the amniotic fluid and gestational tissue, followed by injection of 50 mg Methotrexate into the remaining chorionic sac and then disruption of the sac with the needle. B-hCG level were measured on: day of procedure, day 1 or 2 after the procedure and then weekly. Final resolution was considered when B-hCG returned to < 5mIU/mL. All cases presented and diagnosed in the 1st trimester, mean gestational age at diagnosis was 6.6 weeks (range 5-10). The presenting symptoms were mainly lower abdominal pain and vaginal bleeding in 8/11 patients (73%) while three women were asymptomatic (27%) and CSEP was diagnosed incidentally at viability scan. Nine out of eleven cases showed cardiac activity (82%). B-hCG day 1 or 2 post procedure dropped in all cases but by variable levels, ranging from 1.1% to 74.7%, while the drop during days 7-10, was more significant and reassuring; ranged from 45.7% to 92.5 %. No complications were reported with 100% success rate. Ultrasound guided transvaginal aspiration and injection of 50 mg Methotrexate followed by needle mechanical disruption is a promising primary treatment approach for CSEP. However, more studies with more numbers are needed to further support this treatment method.
Keywords
Caesarean Scar Ectopic Pregnancy (CSEP); Methotrexate Therapy; Ultrasound Guided Transvaginal Aspiration
Download this article as:| Copy the following to cite this article: Al-Bassam M, Begam M. A, Aswad S. G, Al-Jefout M, Ultrasound Guided Transvaginal Aspiration and Mechanical Destruction with Local Methotrexate Injection is a Promising Primary Treatment Approach for Caesarean Scar Ectopic Pregnancy (CSEP). Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Al-Bassam M, Begam M. A, Aswad S. G, Al-Jefout M, Ultrasound Guided Transvaginal Aspiration and Mechanical Destruction with Local Methotrexate Injection is a Promising Primary Treatment Approach for Caesarean Scar Ectopic Pregnancy (CSEP). Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2TM1Eu2 |
Introduction
Cesarean scar ectopic pregnancy (CSEP), described originally by Larsen et al. in 1978. As “Pregnancy in a uterine scar sacculus”.1 CSEP occurs when abnormal implantation of embryo within the myometrium and the fibrous tissue of the previous scar following caesarean section. It is a rare condition, the incidence has been reported as 1:1800 to 1:2216 of total pregnancies at a rate of 6.1% of all ectopic pregnancies in women with a history of a previous Caesarean section.2,3 However, the occurrence is thought to be rising globally with the advent of assisted reproductive technology and the increase in caesarean deliveries, as well as the improved diagnosis offered by the widespread use of transvaginal ultrasound.4,5 Presentation is usually in the first trimester, with vaginal bleeding and lower abdominal pain, however almost half of patients might be asymptomatic. Yu et al.6 reported in their series of 100 cases with scar ectopic pregnancies that 45 % patients were asymptomatic, 55 % had vaginal bleeding, and 7 % had pain in lower abdomen. Surprisingly, the number of caesarean deliveries seems not affecting the incidence of CSEP,7 it was reported that scar ectopic pregnancy occurred in 52 % of cases following prior one caesarean section, 36 % in prior two caesarean section and 12 % after three or more prior caesarean section.2
Early diagnosis is crucial as there are potential catastrophic complications such as massive haemorrhage, uterine rupture resulting in maternal morbidity and mortality. High index of suspicion from patient`s history, clinical presentation and imaging studies is vital for early diagnosis, prevention of these serious complications and to obviate the need for radical surgery thus, preserving the fertility.8
There is no consensus as to the optimal management of these rare ectopic pregnancies. Variety of treatment modalities have been utilized ranging from systemic or local medical management,4,5 minimally invasive surgical interventions such as transvaginal aspiration of sac content6 and uterine artery embolization,7 to more radical procedures.2,9 Both the medical and minimal surgical interventions focus on early interruption of the pregnancy for optimal outcome and preserving fertility.
We present our experience of successful management of this rare form of ectopic pregnancies in our institution utilizing ultrasound guided trans-vaginal aspiration of the sac content with local injection of 50 mg Methotrexate.
Materials and Methods
This was a study of caesarean scar ectopic pregnancies diagnosed and treated at the Obstetrics and Gynaecology unit of Tawam hospital, United Arab Emirates for the period from June, 2012 to June, 2017. The study was approved by Institutional review board, Al Ain Medical District Human Research Ethics Committee (Protocol Number of AAMD IRR-CRD 316/14). All of the study subjects gave informed consent prior to treatment.
We utilized the following sonographic criteria for diagnosis of CSEP using TVS: 1- an empty uterine cavity and cervical canal, 2- a gestational sac located at the anterior wall of the isthmic portion, separated from endometrial cavity or fallopian tube in previous caesarean scar, 3- a gestational sac embedded within the myometrium and the fibrous tissue of caesarean section scar at the lower uterine segment with an absence of defect in the myometrium between the bladder and the sac and 4- a high-velocity low-impedance vascular flow surrounds the gestational sac.5,10 B-hCG was measured on presentation.
Aspiration of the sac content was performed under general anesthesia. This procedure was either done as a primary treatment or utilized after failed systemic methotrexate administration. We used 16-gauge oocyte-retrieval needles, (Cook Medical), which have double lumens, one for aspiration and the other one for injection. Under transvaginal ultrasound guidance, (Siemens Sonoline G60S), using the transvaginal probe, the needle was introduced through the nearest vaginal fornix into the chorionic sac cavity, the first step was aspiration of the amniotic fluid and gestational tissue, followed by injection of 50 mg Methotrexate into the remaining chorionic sac and then disruption of the sac with the needle. Average time of the procedure was 20 to 30 minutes. B-hCG level on day of the trans-vaginal aspiration was taken as day zero B-hCG, whether systemic methotrexate was given or not. Follow up was done with B-hCG level on day 1 or 2 after the procedure and then weekly. Final resolution was considered when B-hCG returned to < 5mIU/mL.
Results
Total of 11 cases were included in our study. The clinical details of those patients are presented in table 1. All women conceived spontaneously. Mean age of our study participants was 35 years (range, 29-44). Their obstetric profile was: mean gravidity of 6 (range, 1-12), and mean parity of 3 (range, 1-8). All patients with CSEP had previous Caesarean section, the mean number of previous Caesarean deliveries being 2.4 (range, 1-5).
Table 1: Clinical data of patients.
| No. | Age | Gravida
Para (GP) |
Uterine surgery | GA
(Weeks) |
Presenting symptoms | Initial
B-hCG (mIU/L) |
Fetal Cardiac Activity
(Yes/No) |
| 1 | 37 | G5P4 | 4 C-Sections | 7 | Asymptomatic | 17180 | Yes |
| 2 | 30 | G4P2 | 2 C-sections Uterine septum removal
Hysteroscopy polypectomy |
6 | Lower abdominal pain Mild vaginal bleeding | 6435 | Yes |
| 3 | 29 | G4P2 | 2 C-sections | 6 | Lower abdominal pain Mild vaginal bleeding | 6763 | Yes |
| 4 | 40 | G7P6 | 2 C-Sections | 5 | Asymptomatic | 12191 | No |
| 5 | 34 | G12P2 | 1 C-section
Abdominal cervical Cerclage Dilatation and curettage |
8 | Lower abdominal pain, Mild vaginal bleeding | 4445 | Yes |
| 6 | 37 | G7P5 | 5 C-Sections | 10 | Lower abdominal pain, Mild vaginal bleeding | 142940 | Yes |
| 7 | 31 | G4P3 | 3 C-Sections | 5 | Lower abdominal pain, Mild vaginal bleeding | 8005 | No |
| 8 | 33 | G8P3 | 3 C-Sections | 7 | Mild vaginal bleeding | 19850 | Yes |
| 9 | 44 | G12P8 | 2 C-Sections | 6 | Lower abdominal pain | 53631 | Yes |
| 10 | 33 | G3P1 | 1 C-Section | 7 | Lower abdominal pain | 40198 | Yes |
| 11 | 39 | G4P3 | 2 C-Sections | 6 | Asymptomatic | 24012 | Yes |
All presented and diagnosed in the first trimester, mean gestational age at diagnosis was 6.6 weeks (range 5-10 weeks). An example is presented in figure 1(A, B &C). The presenting symptoms were mainly lower abdominal pain and vaginal bleeding in 8/11 patients (73%) while three women were asymptomatic (27%) and CSEP was diagnosed incidentally at viability scan. Nine out of eleven cases showed cardiac activity (82%). B-hCG was measured on presentation as the initial B-hCG, it ranged from 4445 to142940 mIU/L, it did not add value to the diagnosis, but was in follow up of treatment success.
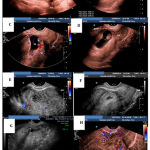 |
Figure 1: TV scan of case (no 6). Pre-treatment (A, B, C &D) & Post-treatment (E-day 1, F &G- day 10 & H-4).
|
Systemic methotrexate was initially tried in 4 cases as single or multiple doses regimens (cases 1-4) according to our departmental medical protocol of management of ectopic pregnancies. However, it failed to resolve the issue and there was no biochemical resolution as B-hCG levels increased by 25% to 118% of the initial values, nor sonographic resolution of CSEP. Hence, they were considered as failed medical treatment that warranted further intervention. US guided trans-vaginal aspiration and disruption was utilized as a secondary approach in those 3 cases while it was the primary treatment modality in the remaining cases.
Case number 2 had ultrasound guided local methotrexate injection alone, after failed systemic Methotrexate treatment, without aspiration or disruption of the gestational sac as the initial local treatment. Secondary intervention with ultrasound guided disruption and aspiration of the sac was required as there was no sign of resolution of the pregnancy. The mechanical disruption eventually helped in the resolution.
B-hCG day 1 or 2 post US guided trans-vaginal aspiration of the sac dropped in all cases but by variable levels, ranging from 1.1% to 74.7%, while the drop during days 7-10, was more significant and reassuring; ranged from 45.7% to 92.5 %.( Table 2). The mean time for final resolution of B-hCG was 5.1 weeks (range 3-8 weeks), and was not well correlated to the initial level of B-hCG (Table 2).
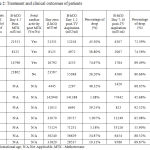 |
Table 2: Treatment and clinical outcomes of patients.
|
Ultrasound examination few days post aspiration showed collapsed sacs in all cases, an example is demonstrated in Figure 1(D, E & F), follow up scans continued to show echogenic area at the site of the scar ectopic pregnancy up to 6 months after the procedure. Cytology examination of the aspirated tissue showed embryonic tissue in one case while in the rest was trophoblast and chorionic villi or chorionic villi only.
On follow up; all those patients continued to have on and off mild vaginal bleeding for 2-6 weeks post aspiration. None of the women had any complications or side-effects related to local methotrexate treatment or the procedure and none needed further surgical intervention. The uterus was preserved in all (100%) patients. Normal menstruation resumed in all, four got spontaneous pregnancies after the successful treatment of CSEP 8 moths to 3 years, and all had Elective Caesarean sections without complications.
Discussion
Our study clearly showed that ultrasound guided transvaginal aspiration and injection of 50 mg Methotrexate followed by mechanical disruption is a promising primary treatment approach for CSEP. Once considered an extremely rare entity, low implantation ectopic pregnancies are becoming a common finding.9 Many theories have been proposed to explain the occurrence of this phenomenon, the most plausible one suggested that the blastocyst enters and gets implanted into the myometrium through a microscopic dehiscent tract that might be created as a result of trauma during previous uterine surgery.9 While some authors argue the low prevalence of recurrent CSEP indicates that the implantation into the scar is likely to be a chance event, rather than the result of particular affinity of a pregnancy for implanting into the scar.11
Early intervention is recommended especially in cases of CSEP with viable pregnancy to avoid serious complications such as hemorrhage which may need hysterectomy and endanger the woman`s life. The challenge in early diagnosis and detection is further complicated by a challenge in treatment. Due to the relative rarity of the condition, there is no optimal line of management.12
The initial 4 cases in our series were given systemic methotrexate as the primary treatment, and the regimen of Methotrexate therapy was given as per our departmental porotocol for management of ectopic pregnancies. The failure of Medical treatment with Systemic Methotrexate in those cases can be explained by the limited absorption of systemic methotrexate by the conceptus, due to poor vascularization of the fibrous Caesarean scar.13,14 This is especially noted when there was fetal cardiac activity, as our previous experience with systemic methotrexate has proven useful for CSEP without fetal viability (Unpublished data). During the trial period, the B-hCG levels rose up for these 4 cases, further complicating the management while potentially increasing the risks to the women from CSEP (Table 1&2).
As our learning curve improved with the management of these rare pregnancies, we started to utilize local treatment as our primary treatment modality. Ultrasound guided aspiration of the gestational sac content has been used as a minimally invasive approach.8 However, the mechanical disruption of the GS with the needle rather than aspiration of the sac contents is likely the key component in the resolution of the pregnancy, as it was not possible to aspirate the whole embryo in all our cases. This was proven by the cytology results which showed embryonic tissue or trophoblast and chorionic villi in the aspirated specimen.
The importance of the mechanical disruption was further shown in Case number 2 who had ultrasound guided local methotrexate injection alone without aspiration as the primary procedure. Local methotrexate probably plays a role in the process of final resorption of the remaining gestational tissue after aspiration.
The percentage of initial drop in B-hCG in day 1 or 2 following the procedure was variable but reassuring. Follow up of the patient is essential even after the initial drop. The patients need to be educated about it and can be organized as outpatient weekly BhCG levels.
Women may continue to have intermittent mild vaginal bleeding following the procedure resulting from the resorption of the remaining gestational tissue, which may increase their anxiety. This aspect needs to be explained to the patient clearly.
Follow up with ultrasound might not be indicated after progressive reassuring drop in B-hCG, as the residual sac structure could continue to be detected on ultrasound as echogenic area from 2 moths up to 1 year before complete regression.8,15 This might lead to unnecessary interventions such as hysteroscopy or dilatation and curettage.
Although, the small numbers reported by us may be a weakness to draw final conclusions about the validity and applicability of this procedure into wider clinical practice but we were encouraged by the 100% resolution in our pilot 10 cases and the fact that no complications occurred.
In conclusion, ultrasound Guided Transvaginal aspiration and injection of 50 mg Methotrexate followed by mechanical disruption is a promising primary treatment approach for Caesarean scar ectopic pregnancy (CSEP). This should especially be considered as primary treatment in pregnancies with viable fetus and/or high B-hCG levels; it is minimally invasive, with no systemic side effects with the exception of minimal risk of very mild bleeding. It can obviate the need for major surgery and preserve fertility. Moreover, compared to systemic treatment, this approach will avoid long treatment duration, thereby potentially reducing the risk of morbidities associated with this rare, yet increasing in incidence condition.
Acknowledgements
The author(s) received no specific funding for this work.
Conflict of Interest
There is no conflict of interest.
References
- Larsen J., Solomon M. Pregnancy in a Uterine Scar Sacculus-an Unusual Cause of Postabortal Haeinorrhage. S Afr med J. 1978;53:142.
- Rotas M. A., Haberman S., Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstetrics & Gynecology. 2006;107(6):1373-81.
CrossRef - Jurkovic D., Hillaby K., Woelfer B., Lawrence A., Salim R., Elson C. First‐trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound in Obstetrics and Gynecology. The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2003;21(3):220-7.
CrossRef - Ash A., Smith A., Maxwell D. Caesarean scar pregnancy. BJOG: An International Journal of Obstetrics & Gynaecology. 2007;114(3):253-63.
CrossRef - Rosen T. Placenta accreta and cesarean scar pregnancy: overlooked costs of the rising cesarean section rate. Clinics in perinatology. 2008;35(3):519-29.
CrossRef - Yu X., Zhang N., Zuo W. Cesarean scar pregnancy: an analysis of 100 cases. Zhonghua yi xue za zhi. 2011;91(45):3186-9.
- Shen L., Tan A., Zhu H., Guo C., Liu D., Huang W. Bilateral uterine artery chemoembolization with methotrexate for cesarean scar pregnancy. American journal of obstetrics and gynecology. 2012;207(5):386.e1-. e6.
- Hwu Y. M., Hsu C. Y., Yang H. Y. Conservative treatment of caesarean scar pregnancy with transvaginal needle aspiration of the embryo. BJOG: An International Journal of Obstetrics & Gynaecology. 2005;112(6):841-2.
CrossRef - Wu R., Klein M. A., Mahboob S., Gupta M., Katz D. S. Magnetic resonance imaging as an adjunct to ultrasound in evaluating cesarean scar ectopic pregnancy. Journal of clinical imaging science. 2013;3.
- Pascual M. A., Hereter L., Graupera B., Tresserra F., Fernandez-Cid M., Simon M. Three-dimensional power Doppler ultrasound diagnosis and conservative treatment of ectopic pregnancy in a cesarean section scar. Fertility and sterility. 2007;88(3):706. e5-. e7.
- Nagi J. B., Ofili‐Yebovi D., Sawyer E., Aplin J., Jurkovic D. Successful treatment of a recurrent Cesarean scar ectopic pregnancy by surgical repair of the uterine defect. Ultrasound in Obstetrics and Gynecology. 2006;28(6):855-6.
CrossRef - Maymon R. Ectopic pregnancies in a Cesarean scar: review of the medical approach to an iatrogenic complication. Human Reproduction Update 2004. 2004;10:869-70.
- Fylstra D. L. Ectopic pregnancy within a cesarean scar: a review. Obstetrical & gynecological. survey. 2002;57(8):537-43.
CrossRef - Stevens E. E., Ogburn P. Cesarean scar ectopic pregnancy: a case report of failed combination local and systemic methotrexate management requiring surgical intervention. The Journal of reproductive medicine. 2011;56(7-8):356-8.
- Seow K. M., Huang L. W., Lin Y. H., Lin M. Y. S., Tsai Y. L., Hwang J. L. Cesarean scar pregnancy: issues in management. Ultrasound in Obstetrics and Gynecology. 2004;23(3):247-53.








