Chew June Li1, Aniza Aini Barkath1, Mohamad Zarrin Abdullah1, Nadiah Lingkan1, Nurul Hidayah Mohd Ismail1, Suria Hayati Md Pauzi2, Yusof Kamisah1 , Qodriyah HMS1, Kamsiah Jaarin3, Suhaila Mohamed4 and Norliana Masbah*1
, Qodriyah HMS1, Kamsiah Jaarin3, Suhaila Mohamed4 and Norliana Masbah*1
1Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Centre, Jalan Yaacob Latif, Bandar Tun Razak, 56000 Kuala Lumpur, Malaysia.
2Department of Pathology, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Centre, Jalan Yaacob Latif, Bandar Tun Razak, 56000 Kuala Lumpur, Malaysia.
3Faculty of Medicine, National Defence University of Malaysia (UPNM), Kem Sungai Besi, 57000 Kuala Lumpur, Malaysia.
4Institute of Bioscience, Faculty of Food Science and Technology, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia.
Corresponding Author E-mail: nmasbah@ukm.edu.my
DOI : https://dx.doi.org/10.13005/bpj/1649
Abstract
Prolonged consumption of heated palm oil causes detrimental effects on cardiovascular system, liver and kidneys. The detrimental effects of heated oils are associated with oxidative stress; hence the role of antioxidants in attenuating heated oil-induced effects has been widely studied. The aim of the present study was to determine the effects of polyphenol-rich Citrus leaf extract (CLE) on renal oxidative stress parameters, renal function and kidney histological changes in rats fed with heated palm oil. Fifty-six male Sprague-Dawley rats (n=56) were divided into seven groups. The control group was given normal rat chow, while other groups were fed with 15% weight/weight (w/w) palm oil-enriched diet of either fresh palm oil (FPO), five-time-heated palm oil (5HPO) or ten-time-heated palm oil (10HPO); with or without the addition of CLE (0.15%, w/w) supplementation. After 16 weeks, the rats were sacrificed and the kidneys were harvested for analysis. CLE supplementation improved heated palm oil-induced oxidative stress parameters in the kidneys, shown by reduced levels of renal thiobarbituric acid (TBARS) and renal nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in 5HPO and 10HPO groups. Renal haem oxygenase levels were restored with CLE supplementation in the heated oil groups. Decreased serum creatinine was observed in 5HPO group with CLE supplementation, but not in 10HPO group. Heated oil caused mild interstitial inflammation with vascular congestion in 5HPO and 5HPO+CLE groups, while 10HPO group had moderate inflammation and vascular congestion. CLE supplementation in 10HPO group was able to reduce these changes as only mild interstitial inflammation and congestion was observed. CLE has the potential to reduce renal oxidative stress parameters, improve renal function and reduce renal inflammation possibly mediated via its antioxidant properties.
Keywords
Citrus leaf; Heated Oil; Oxidative Stress; Polyphenols; Renal Function; Rutaceae
Download this article as:| Copy the following to cite this article: Li C. J, Barkath A. A, Abdullah M. Z, Lingkan N, Ismail N. H. M, Pauzi S. H. M, Kamisah Y, Qodriyah H. M. S, Jaarin K, Mohamed S, Masbah N. The Effects of Citrus Leaf Extract on Renal Oxidative Stress, Renal Function and Histological Changes in Rats Fed With Heated Palm Oil. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Li C. J, Barkath A. A, Abdullah M. Z, Lingkan N, Ismail N. H. M, Pauzi S. H. M, Kamisah Y, Qodriyah H. M. S, Jaarin K, Mohamed S, Masbah N. The Effects of Citrus Leaf Extract on Renal Oxidative Stress, Renal Function and Histological Changes in Rats Fed With Heated Palm Oil. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2S7jINn |
Introduction
Palm oil is commonly used as the major source of cooking oil in many households and food industries in this part of the world. In addition, the oils are commonly heated repeatedly as this practice helps to save cost.1 However, it is established that the components of the oils tend to degenerate after repeatedly heating, leading to increased viscosity and darkening in color due to increased fatty acid composition during hydrolysis.2
Heated palm oil undergoes hydrolysis, oxidation and polymerization which generates reactive oxygen species such as hydroperoxides and low molecular volatile compound2 leading to increased oxidative stress. Subsequently, during frying process, these reactive oxygen species will be absorbed into food and therefore poses health threats when consumed.3
Oxidative stress imposes multisystem detrimental effects on the body.4 It has shown to cause cardiac damage and remodeling,5 endothelial dysfunction6 and increased blood pressure leading to atherosclerosis.7 In addition, studies have shown that consumption of heated palm oil (HPO) results in increased inflammatory liver markers and liver damage in experimental rats.8-9 Chronic consumption of heated oils was also reported to cause increased oxidative stress measured as renal thiobarbituric acid reactive substances (TBARS),10-11 increased NADPH oxidase12 and reduced renal haem oxygenase activity,13 eventually leading to impairment in renal function14; which was manifested as increased serum creatinine as well as chronic inflammation of the renal parenchyma in rats.13,15
The citrus leaf extract (CLE) studied in this research was derived from citrus leaves of the Rutaceae plant family. A previous study using similar leaf extract has reported that CLE contains polyphenols such as diosmin, lutein, isoquercitrin, obacunone, hesperidin and didymin.16-17 It was originally developed and patented (patent number: US8425969B2) as an oil additive during frying process; targeted mainly to reduce oil absorption into food and at the same time provide anti-oxidative effects that render the frying oil to be more resistant against thermal oxidation.16 CLE, which was also known as ADD-X in previous studies, was reported to possess antihypertensive effects by reducing vascular damage in hypertensive rats fed with heated palm oil diet.17 An earlier study has also reiterated that CLE possessed antihypertensive properties as well as able to attenuate oxidative stress biomarkers when incorporated into the diet of ovariectomised rats.18 CLE supplementation has also been shown to display anti-inflammatory and cardio-protective properties by preventing heated oil-induced necrotic changes in cardiac tissues of experimental rats.19 Another study which reported the effects of CLE supplementation on aortic vascular reactivity in rats fed with heated palm oil has outlined that CLE supplementation managed to restore plasma nitric oxide levels, thereby reducing vasoconstriction-mediated vascular reactivity in the heated palm oil group.20 Although CLE supplementation has been shown to have multiple beneficial effects; particularly on blood pressure, inflammatory markers and oxidative stress parameters, its effects on the kidneys has not been explored. Therefore, this study was undertaken to evaluate the effects of CLE specifically on the kidneys, by measuring its effects on renal oxidative stress, renal function and kidney histological changes in rats fed with heated palm oil.
Materials and Method
Materials
CLE was obtained from University Putra Malaysia (UPM), Malaysia. Palm oil used for this study was purchased from a local manufacturer (Lam Soon Edible Oils) in Selangor, Malaysia. Creatinine (serum) Colorimetric assay kit was purchased from Cayman Chemical, Ann Arbor, MI, USA. Other chemicals were obtained from Sigma-Aldrich Chemical Co. (St. Loius, MO, USA).
Animals and Experimental Design
This study and animal handling was approved by Universiti Kebangsaan Malaysia Animal Ethics Committee. For this study, a total of fifty-six adult male Sprague-Dawley rats (weight between 180 to 200g) were obtained from the Laboratory Animal Research Unit, Universiti Kebangsaan Malaysia. All rats were housed in stainless steel cages in temperature maintained at 27ºC with 12-hours light-dark cycle. The animals had free access to water and food (rat chow) throughout the study. After one week of acclimatization, treatment was commenced according to the assigned groups. The animals were randomly divided equally into seven groups with eight rats in each group; namely control, fresh palm oil (FPO), fresh palm oil with CLE (FPO+CLE), five-times-heated palm oil (5HPO), five times-heated palm oil with CLE (5HPO+CLE), ten times-heated palm oil (10HPO) and ten times-heated palm oil with CLE (10HPO+CLE) groups. The control group were fed with normal rat chow without oil. The other groups were fed with diet consisting of rat chow fortified with 15% weight of total oil composition / total weight of basal diet (15% w/w). The total oil composition may be with or without CLE according to the assigned groups. After 16 weeks, the rats were sacrificed and subsequently the kidneys were weighed and harvested for histological analysis, renal thiobarbituric acid reactive substance (TBARS) measurement, serum creatinine, heme oxygenase and NADPH levels.
Heated Oil and Animal Diet Preparation
For diet preparation, palm oil was used as either fresh palm oil (FPO), five-times heated palm oil (5HPO) or ten-times heated palm oil (10HPO) which was prepared according to the methods described earlier by Leong et al.7 2.5 litres of the oil were used to fry 1 kg of peeled sweet potato slices in a stainless-steel wok at 180ºC for 20 minutes. The hot oil was allowed to cool down for at least 5 hours before the frying process was repeated to produce 5HPO and 10HPO. The frying process was repeated with a fresh batch of sweet potatoes (without addition of fresh oil) for another four times to obtain five-times-heated palm oil (5HPO) and another nine times to obtained ten-times-heated palm oil (10HPO). For FPO+CLE, 5HPO+CLE and 10HPO+CLE groups, the respective oils used were then mixed with 10% diluted CLE, with ratio of CLE to oil equals to 1:10 before the diet preparation. Standard rat chow (obtained from Gold Coin, Selangor, Malaysia) were grounded and mixed with 15% w/w of the respective oils. The rats were fed with the respective diets for 16 weeks.
Measurement of Serum Creatinine
Serum creatinine was measured using a commercial kit available in the market (Cayman’s Creatinine (serum) Colorimetric Assay Kit. The developed color intensity was measured at 490 nm using ELISA microplate reader. The results for creatinine in serum were expressed as μmol/l.
Measurement of Renal Thiobarbituric Acid Reactive Substances (TBARS)
TBARS content in the kidney was determined by using previously described method by Ledwozyw et al. [21] with a few modifications. Firstly, kidney homogenate (0.5 mL) was added to 2.5 mL trichloroacetic acid (1.22mol/L) in 37% hydrochloric acid (HCl). Then, it was incubated for 15 min at room temperature. The mixture was mixed with 1.5 mL 0.67% of thiobarbituric acid in 0.05mol/L sodium hydroxide (NaOH) and incubated at 100 °C in a water bath for 30 min. The mixture was cooled down then added into 4 mL n-butanol. Subsequently, the mixture was vortexed vigorously and centrifuged at 3000 r/min for 10 minutes. The absorbance upper layer was read using a spectrophotometer at 532 nm against 1, 1, 3, 3-tetraethoxypropane standard curve. The results were expressed as nmol/g wet weight.
Measurement of Renal Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase
NADPH oxidase was measured based on its capacity to reduce ferricytochrome c in ferrocytochrome at pH of 7.8 [22]. Kidney homogenate (50 µg protein/experiment), cytochrome c (250 µg/l final concentration), and NADPH (100 µM) was incubated either in presence or absence of diphenyleneiodonium (DPI, 100 µM). The absorbance of reduction of cytochrome c was measured at 550 nm. The difference between the absorbance of samples between 0 to 120 min was measured in nmol/mg protein and the extinction coefficient 21 mmol/l/cm.
Measurement of Heme Oxygenase (HO-1) Activity
The method described by Vera et al.23 was used to determine heme oxygenase activity. Heme oxygenase activity was measured based on total bilirubin measurement. Initially, kidney homogenate in phosphate buffer (0.25mmol/L, pH 7.7) was centrifuged at 13 000 rpm at 4°C for a total of 15 minutes.5mg protein of sample was added into a reaction mixture containing 2mmol/L glucose-6-phosphate, 0.2 unit glucose-6-phosphate dehydrogenase, 0.8 mmol/L nicotinamide adenine dinucleotide phosphate, and 20 μmol/L hemin. The mixture was subsequently incubated in the dark for 1 hour at 37°C. 1mL of chloroform was added to extract formed bilirubin. The samples were then centrifuged at 7000r/min for 10 minutes. The optical density of the chloroform later was read at 466-530nm, Finally, the heme oxygenase activity was calculated based on extinction coefficient of 40 mM-1cm-1.
Kidney Histology
Kidney samples were initially fixed in phosphate formalin buffer solution (10%) before being blocked in paraffin, sectioned at 5 μmol/L and stained with haematoxylin-eosin. Kidney histology was assessed by light microscopic examination and slides were read under 200x and 400x magnifications. All the slides were examined by an experienced pathologist in a blinded fashion and graded for the presence of inflammation (mononuclear cell infiltration), red blood cell (RBC) congestion and necrosis. The extent of the above mentioned histological changes in the kidney was graded using the following pre-defined scale:
Normal : 0 % of area is affected.
Mild changes : <25% of the area is affected.
Moderate changes : 25-50% of the is affected.
Severe changes : >50% of the area is affected.
Statistical Analysis
All results for this study were expressed as mean ± SEM (standard error of mean). Shapiro-Wilk test was applied to check for normality of data. Normally distributed data were analysed using ANOVA and followed by Tukey’s Honestly Significant Differences (HSD) post-hoc test. A value of p<0.05 was considered as significant. All statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS) version 23 software (SPSS Inc, Chicago, IL, USA).
Results
Serum Creatinine
Fig. 1 shows the serum creatinine level of all experimental groups at the end of the study (week 16). There was no significant difference in the levels of serum creatinine of FPO and FPO + CLE groups when compared to control. Serum creatinine was significantly increased in 5HPO and 10HPO groups as compared to control and FPO groups. Serum creatinine level in 5HPO + CLE group was significantly lower compared to 5HPO (p < 0.05). However, serum creatinine level in 10HPO + CLE group was not significantly different compared with 10HPO group.
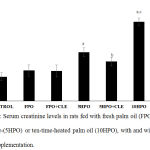 |
Figure 1: Serum creatinine levels in rats fed with fresh palm oil (FPO), five-time-(5HPO) or ten-time-heated palm oil (10HPO), with and without CLE supplementation.
|
Data are means ± SEM (n=8). asignificant difference vs control and FPO (p<0.05); bsignificant difference vs 5HPO (p<0.05); csignificant difference vs 5HPO (p<0.05).
Renal Thiobarbituric Acid Reactive Substances (TBARS)
Figure 2 shows renal TBARS level for all experimental groups. There was no significant difference between renal TBARS in FPO and FPO+CLE compared to control group. There was a significant increase in renal TBARS in 5HPO and 10HPO groups compared to control (p < 0.05). Renal TBARS level is significantly reduced in 5HPO+CLE and 10HPO+CLE compared to 5HPO and 10HPO groups respectively.
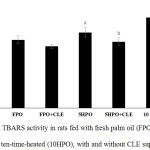 |
Figure 2: Renal TBARS activity in rats fed with fresh palm oil (FPO), five-time-heated (5HPO) or ten-time-heated (10HPO), with and without CLE supplementation.
|
Data are means ± SEM (n=8). a significant difference vs control and FPO (p<0.05); b significant difference vs 5HPO (p<0.05); c significant difference vs 10HPO (p<0.05).
Renal Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase
Figure 3 shows renal NADPH oxidase activity of all experimental groups at the end of the study. There was no significant difference in renal NADPH oxidase activity in FPO and FPO +CLE compared to control. NADPH oxidase activity in 5HPO and 10HPO groups were significantly increased compared to control (p < 0.05). NADPH oxidase activities in 5HPO + CLE and 10HPO + CLE were significantly reduced compared to 5HPO and 10HPO groups respectively (p< 0.05).
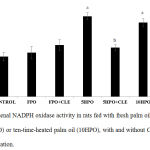 |
Figure 3: Renal NADPH oxidase activity in rats fed with fresh palm oil (FPO), five-time-(5HPO) or ten-time-heated palm oil (10HPO), with and without CLE supplementation.
|
Data are means ± SEM (n=8). asignificant difference vs control and FPO (p<0.05); bsignificant difference vs 5HPO (p<0.05); csignificant difference vs 10HPO (p<0.05).
Haem Oxygenase (HO) Activity
Figure 4 shows HO levels for rats fed with heated palm oil with or without CLE supplementation. FPO and FPO+CLE groups were comparable to the control group. 5HPO and 10HPO groups showed significant decrease in HO activity compared to control and FPO groups (p < 0.05). With CLE supplementation, HO activity were significantly restored in both 5HPO and 10HPO groups (p < 0.05).
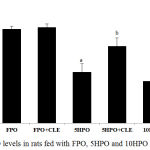 |
Figure 4: Shows HO levels in rats fed with FPO, 5HPO and 10HPO with or without CLE.
|
Data are means ± SEM (n = 8). asignificant difference vs control and FPO (p < 0.05); bsignificant difference vs 5HPO (p < 0.05); csignificant difference vs 10HPO (p < 0.05).
Kidney Histology
Figure 5 shows photomicrographs (A – C) of the control, FPO and FPO+CLE groups. There was no congestion, inflammation or necrosis noted in the tubules, glomerulus and interstitium of control, FPO and FPO+CLE groups. Figure 6 shows photomicrograph (D – G), whereby there were mild congestion in the glomerulus and mild inflammation in the interstitium of the 5HPO and 5HPO+CLE groups. Figure 7 shows photomicrographs (H – K), which shows moderate congestion and inflammation in 10HPO group. In contrast, the 10HPO + CLE group showed mild congestion and inflammation in the interstitium and glomerular areas. No necrosis was observed in any of the experimental groups.
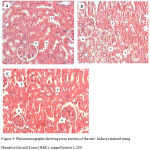 |
Figure 5: Photomicrographs showing cross section of the rats’ kidneys stained using Hematoxylin and Eosin (H&E), magnification x 200.
|
All three groups namely A) Control, B) Fresh Palm Oil (FPO) and C) FPO+CLE groups showed no congestion and inflammation. G: Glomerulus ; T: Tubule.
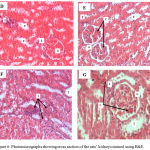 |
Figure 6: Photomicrographs showing cross section of the rats’ kidneys stained using H&E.
|
D) 5 times heated palm oil (5HPO) group showed mild inflammation in the interstitium (X200). E) 5HPO group showed mild congestion at the glomerulus (X200). F) 5HPO + CLE group showed mild inflammation at the interstitium (X200). G) 5HPO + CLE group showed mild inflammation at the interstitium and mild congestion at the glomerulus (x400). C: Congestion; G: Glomerulus I: inflammation; T: Tubule.
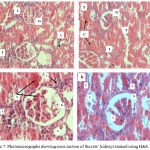 |
Figure 7: Photomicrographs showing cross section of the rats’ kidneys stained using H&E.
|
H) 10HPO group showed moderate congestion in the glomerulus and tubular sections and moderate inflammation at the interstitium. I) 10HPO + CLE group showed mild inflammation at the interstitium and mild interstitial congestion. J) 10HPO group showed moderate congestion in the glomerulus and moderate inflammation in the interstitium. K) 10HPO+ CLE showed mild inflammation at the interstitium and mild congestion in the glomerulus. C: Congestion; G: Glomerulus; I: inflammation; T: Tubule.
Discussion
Long-term consumption of repeatedly heated palm oil is known to cause detrimental health effects, including to the kidneys. In this study, intake of 5HPO and 10HPO diet for 16 weeks was observed to significantly increase serum creatinine and oxidative stress parameters such as renal TBARS and NAPDH oxidase, as well as reduced HO activity in the experimental groups. A significant increase in serum creatinine levels in 5HPO and 10HPO groups was observed when compared to control group. This finding is in accordance with other studies which reported a significant increase in serum creatinine concentration in thermoxidised palm oil group and repeatedly heated cooking oil groups respectively.14,24 One of the mechanisms proposed for causing deterioration of renal function is due to oxidative stress, whereby excessive reactive oxygen species (ROS) such as hydroperoxides produced as a result of repeated heating of oil,2 led to cellular damage by reacting with various biomolecules such as proteins, nucleic acids or lipids.25 However, our research finding was in contrary with the findings of Jaarin et al.,15 which reported that there was no significant difference in serum creatinine among the groups at the end of study period. The reason for the discrepancy in this finding was unclear, although it could possibly be attributable to the shorter frying duration in the previously mentioned study. In our study, supplementation of CLE had significantly reduced the serum creatinine level in 5HPO but not in 10HPO group. This finding shows that CLE supplementation, through its ROS-scavenging activity was able to reduce the extent of renal damage only in the 5HPO group due to less oxidative damage induced by less number of oil heating in this group. Therefore, the antioxidant properties of CLE might no longer be effective in 10HPO group when the oxidative insult is greater,17 as caused by the increased number of oil heating.
Renal TBARS levels were significantly increased in the 5HPO and 10HPO groups compared to control. This finding is in line with previous studies indicating that repeated heating increases oil oxidation, which subsequently increases lipid peroxidation.10,11,18,26 CLE supplementation was able to significantly reduce the renal TBARS in 5HPO and 10HPO groups. This finding was in agreement with previous studies which reported that citrus leaf extract supplementation reduced plasma TBARS in heated oil groups.10,18 Similarly, as highlighted by a previous report, oxidative stress has the capability to promote renal injury by inducing cytotoxicity, as shown by accumulation of renal malonyldialdehyde levels which represents renal TBARS levels in our present study.25
Chronic consumption of 5HPO and 10HPO were shown to increase NADPH oxidase levels in our present study. Similarly, a study by Perez-Herrera et al.12 has demonstrated that consumption of repeatedly heated oil increased NADPH oxidase gene expression. Jaarin et al. also showed the association between oxidative stress and cardiac NADPH oxidase activity in hypertensive rats.27 As reported previously, the reduction in NADPH oxidase activity comes hand in hand with decreased ROS production,28-29 therefore as proven in our study, CLE supplementation was able to attenuate the ROS-associated increase in NADPH levels in the heated oil groups. In favour of this, several studies have also showed the efficacy of polyphenols and dietary flavonoids in reducing cellular NADPH oxidase activity,30-31 which may be attributed to reduced ROS production that subsequently attenuated high levels of NADPH oxidase activity.28-29
There is an inverse relationship between heme oxygenase-1 (HO-1) levels and oxidative stress. Higher levels of oxidative stress induced by chronic consumption of repeatedly heated palm oil have been shown to reduce renal heme oxygenase,13,32-33 which is supposed to have anti-oxidative effects. In contrast, when the activity of the enzyme is increased, bilirubin formation will rise, which subsequently will function as an antioxidant with anti-inflammatory properties.34 In this present study, the heated oil groups (5HPO and 10HPO groups) had reduced levels of heme oxygenase activity, indicating that oxidative stress were markedly increased due to repeated heating in these experimental groups. However, with CLE supplementation, heme oxygenase activity was restored in 5HPO and 10HPO groups which are in accordance with the study reported by Siti et al.17
Histological analysis showed that there was evidence of inflammation in the heated oil groups after 16 weeks of treatment. Mild interstitial inflammation and mild congestion were observed at the glomeruli area of the rats’ kidneys for both 5HPO groups, with and without CLE supplementation. This further reiterates the fact that oxidative process which occurs as the oil is repeatedly heated is damaging to the renal cells25,35 as the findings in our study has also highlighted that there was a similar increasing trend of serum creatinine levels for both heated oil groups. However, 10HPO group showed more severe histological changes, as moderate inflammation and congestion were observed surrounding the glomeruli and interstitium. This was in accordance with Jaarin et.al15 which highlighted that the degree of congestion and inflammation in the kidneys was more severe in 10HPO compared to 5HPO experimental groups. However, in our study, the addition of CLE into 10HPO group was able to reduce the severity of congestion and interstitial inflammation observed, possibly due to its antioxidant properties. This finding is in accordance with the findings highlighted by Jones and Shoskes [36], which reported that the addition of polyphenols such as curcumin and quercetin were able to ameliorate ischaemic perfusion injury that is associated with irreversible lipid peroxidation in animal model. Other previous studies have also shown a strong correlation between nephroprotective effects of flavonoid-rich plant extracts against nephrotoxicity induced in experimental rat model, possibly mediated via their high antioxidant content and free-radical scavenging properties.37-38 Although the method of inducing nephrotoxicity in the experimental animals might differ with our study (drug-induced as opposed to nephrotoxicity induced via heated oil diet supplementation), it is possible that similar nephroprotective mechanisms offered by the polyphenol contents in the CLE has managed to restore the oxidative stress parameters in this study and reversed the histological changes in the heated palm oil groups.
Conclusion
Based on the findings of this study, CLE supplementation after 16 weeks of treatment was capable to improve renal oxidative stress parameters, reduce renal injury and preserve renal function in heated oil group. These findings suggest that CLE has renoprotective effects against heated oil-induced oxidation and renal injury, possibly mediated by its antioxidant properties that is rich in polyphenols.
Conflict of Interest
The authors declare that there is no conflict of interest in this study.
Acknowledgements
This research was funded by grant from Universiti Kebangsaan Malaysia Medical Centre (grant number FF-2017-077). The researchers would like to acknowledge Mr. Fadhlullah Zuhair Japar Sidik, Mrs. Juliana Abd Hamid and Ms Nur Hani Suhaimi for providing technical assistance for the research.
References
- Azman A., Shahrul S. M., Chan S. X., Noorhazliza A. P., Khairunnisak M., Azlina M. N., Qodriyah H. M., Kamisah Y., Jaarin K. Level of knowledge attitude and practice of night market food outlet operators in Kuala Lumpur regarding the usage of repeatedly heated cooking oil. Medical Journal of Malaysia. 2012;67(1):91-101.
- Choe E., Min D. B. Chemistry of deep‐fat frying oils. Journal of Food Science. 2007;72(5):R77-86.
- Choe E., Min D. B. Chemistry and reactions of reactive oxygen species in foods. Critical Reviews in Food Science and Nutrition. 2006;46(1):1-22..
- Ku S. K., Muhamad M. R., Fatin S. S., Saffana M., Taty K. A., Das S., Kamsiah J. The harmful effects of consumption of repeatedly heated edible oils: a short review. La Clinica terapeutica. 2014;165(4):217-21..
- Leong X. F., Aishah A., Aini U. N., Das S., Jaarin K. Heated palm oil causes rise in blood pressure and cardiac changes in heart muscle in experimental rats. Archives of Medical Research. 2008;39(6):567-72.
CrossRef - Ng C. Y., Kamisah Y., Faizah O., Jubri Z., Qodriyah H. M., Jaarin K. Involvement of inflammation and adverse vascular remodelling in the blood pressure raising effect of repeatedly heated palm oil in rats. International journal of vascular medicine. 2012;2012.
- Leong X. F., Najib M. N., Das S., Mustafa M. R., Jaarin K. Intake of repeatedly heated palm oil causes elevation in blood pressure with impaired vasorelaxation in rats. The Tohoku journal of experimental medicine. 2009;219(1):71-8.
CrossRef - Jaarin K., Hwa T. C., Umar N. A., Siti M. A., Das S. Enzymatic and microstructural changes in the liver of experimental rats fed with fatty diet and fresh or heated soy oil concurrently. La Clinica terapeutica. 2010;161(5):429-33.
- Chen Y. J., Liu Y. J., Yang H. J., Yuan Y., Liu F. J., Tian L. X., Liang G. Y., Yuan R. M. Effect of dietary oxidized fish oil on growth performance, body composition antioxidant defence mechanism and liver histology of juvenile largemouth bass Micropterus salmoides. Aquaculture Nutrition. 2012;18(3):321-31.
CrossRef - Jaarin K., Kamisah Y. Repeatedly heated vegetable oils and lipid peroxidation. Lipid Peroxidation InTech.
- Xin-Fang L., Jumat S., Rais M. M., Kamsiah J. Effect of Repeatedly Heated Palm Olein on Blood Pressure—Regulating Enzymes Activity and Lipid Peroxidation in Rats. The Malaysian journal of medical sciences. 2012;19(1):20.
- Perez-Herrera A., Rangel-Zuñiga O. A., Delgado-Lista J., Marin C., Perez-Martinez P., Tasset I., Tunez I., Quintana-Navarro G. M., Lopez-Segura F., De Castro M. D., Lopez-Miranda J. The antioxidants in oils heated at frying temperature, whether natural or added, could protect against postprandial oxidative stress in obese people. Food chemistry. 2013;138(4):2250-9.
CrossRef - Kamisah Y., Ang S. M., Othman F., Nurul-Iman B. S., Qodriyah H. M. Renoprotective effect of virgin coconut oil in heated palm oil diet-induced hypertensive rats. Applied Physiology, Nutrition and Metabolism. 2016;41(10):1033-8.
CrossRef - Ani E. J., Nna V. U., Owu D. U., Osim E. E. Effect of chronic consumption of two forms of palm oil diet on serum electrolytes, creatinine and urea in rabbits. Journal of Applied Pharmaceutical Science. 2015;5(6):115-9.
CrossRef - Jaarin K., Yeen C. P., Masbah N. Consumption of heated palm oil and its effect on kidney in rats. Pakistan Journal of Nutrition. 2016;15(2):148.
CrossRef - Mohamed S., Nor F. M. inventors; Universiti Putra Malaysia (UPM), assignee. Cooking oil composition with additive to reduce oil absorption. United States patent US 8425969B2. 2013.
- Siti H. N., Kamisah Y., Iliyani M. I., Mohamed S., Jaarin K. Citrus leaf extract reduces blood pressure and vascular damage in repeatedly heated palm oil diet-Induced hypertensive rats. Biomedicine & Pharmacotherapy. 2017;87:451-60.
CrossRef - Sukalingam K., Jaarin K., Saad Q. H., Mohamed S., Othman F. Consumption of ADD-X and repeatedly heated palm oil on the blood pressure and oxidative stress markers in ovarectmized rats. International Journal of Pharmacology. 2016;12(5):514-22.
CrossRef - Sukalingam K., Jaarin K., Saad Q. H., Mohamed S., Othman F. Effect of rutacea plant extract (ADD X) on inflammatory biomarkers, cardiac LDL, troponin T and histological changes in ovariectomized rats fed with heated palm oil. International Journal of Toxicological and Pharmacological Research. 2016;8(4):223-31.
- Nordin S. H., Kamisah Y., Mohamed S and Jaarin K. Effects of citrus leaf extract on aortic vascular reactivity in repeatedly heated vegetable oil-induced hypertensive rats. Applied Physiology, Nutrition and Metabolism. 2018 (in press). doi: 10.1139/apnm-2018-0175.
CrossRef - Ledwożyw A., Michalak J., Stȩpień A., Ka̧dziołka A. The relationship between plasma Triglycerides, cholesterol total lipids and lipid peroxidation products during human atherosclerosis. Clinica Chimica Acta. 1986;155(3):275-83.
CrossRef - Carter W. O., Narayanan P. K., Robinson J. P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. Journal of leukocyte biology. 1994;55(2):253-8.
CrossRef - Vera T., Kelsen S., Yanes L. L., Reckelhoff J. F., Stec D. E. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin II hypertensive mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2007;292(4):R1472-8.
- Venkata R. P., Subramanyam R. Evaluation of the deleterious health effects of consumption of repeatedly heated vegetable oil. Toxicology reports. 2016;3:636-43.
CrossRef - Cachofeiro V., Goicochea M., de Vinuesa S. G., Oubina P., Lahera V.,Luno J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney International. 2008;111:54-59.
CrossRef - Adam S. K., Das S., Soelaiman I. N., Umar N. A., Jaarin K. Consumption of repeatedly heated soy oil increases the serum parameters related to atherosclerosis in ovariectomized rats. The Tohoku journal of experimental medicine. 2008;215(3):219-26.
CrossRef - Jaarin K., Foong W. D., Yeoh M. H., Kamarul Z. Y., Qodriyah H. M., Azman A., Zuhair J. S., Juliana A. H., Kamisah Y. Mechanisms of the antihypertensive effects of Nigella sativa oil in L-NAME-induced hypertensive rats. Clinics. 2015;70(11):751-7.
CrossRef - Taylor N. E., Glocka P., Liang M., Cowley Jr A.W. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47(4):692-8.
CrossRef - Mazor R., Itzhaki O., Sela S., Yagil Y.,Cohen-Mazor M., Yagil C & Kristal B. A Possible Priming Agent for the Polymorphonuclear Leukocyte-Reduced Nicotinamide Adenine Dinucleotide Phosphate Oxidase in Hypertension. Hypertension. 2010; 55(2):353-362.
- Steffen Y., Gruber C., Schewe T., Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Archives of biochemistry and biophysics. 2008;469(2):209-19.
CrossRef - Calabriso N., Massaro M., Scoditti E., D’Amore S., Gnoni A., Pellegrino M., Storelli C., De Caterina R., Palasciano G., Carluccio M. A. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. The Journal of nutritional biochemistry. 2016 ;28:19-29.
CrossRef - Leong X. F., Mustafa M. R., Das S., Jaarin K. Association of elevated blood pressure and impaired vasorelaxation in experimental Sprague-Dawley rats fed with heated vegetable oil. Lipids in Health and Disease. 2010;9(1):66.
CrossRef - Kamisah Y., Lim J. J., Lim C. L., Asmadi A. Y. Inhibitory effects of palm tocotrienol-rich fraction supplementation on bilirubin-metabolizing enzymes in hyperbilirubinemic adult rats. PloS one. 2014;9(2):e89248.
- Xin-Fang L., Jumat S., Rais M. M., Kamsiah J. Effect of Repeatedly Heated Palm Olein on Blood Pressure—Regulating Enzymes Activity and Lipid Peroxidation in Rats. The Malaysian journal of medical sciences: MJMS. 2012;19(1):20.
- Totani N., Ojiri Y. Thermal deterioration of oil and frying foodstuffs. Journal of oleo science. 2007;56(10):543-51.
CrossRef - Jones E. A., Shoskes D. A. The effect of mycophenolate mofetil and polyphenolic bioflavonoids on renal ischemia reperfusion injury and repair. The Journal of urology. 2000;163(3):999-1004.
CrossRef - Adeneye A. A and Benebo A. S. Protective effect of the aqueous leaf and seed extract of Phyllanthus amarus on gentamicin and acetaminophen-induced nephrotoxic rats. Journal of Ethnopharmacology. 2008;118:318-323.
CrossRef - Annie S., Rajagopal P. L and Malini S. Effect of Cassia auriculata Linn. Root extract on cisplatin and gentamicin-in.








