Anak Agung Wiradewi Lestari1 , I Dewa Made Sukrama2 and Dian Nurmansyah3
, I Dewa Made Sukrama2 and Dian Nurmansyah3
1Clinical Pathology Department, Faculty of Medicine, Udayana University, Sanglah General Hospital, Bali, Indonesia.
2Clinical Microbiology Department, Faculty of Medicine, Udayana University, Sanglah General Hospital, Bali, Indonesia.
3Master Degree of Biomedical Science, Udayana University, Bali, Indonesia.
Corresponding Author E-mail: wiradewilestari@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1643
Abstract
Lumbricus rubellus earthworm is known having antioxidant and antibacterial properties such as Polyphenolic, Glycoprotein G-90, and Lumbricin I. These substances also work as hepatoprotective agents in the parenchymal cell damage due to infection. This study aims to determine the antioxidant properties of earthworm extract (Lumbricus rubellus) in reducing the levels of ALT, AST, and a number of the bacterial colony in male Wistar rats infected by Salmonella thypimurium as a model of S.typhii infection. Posttest-only control group design method was carried out in 28 samples which divided into 4 treatment groups. The blood samples were taken for the ALT and AST measurement on day 18. The bacterial colony measurement was conducted by growing the bacteria in the feces with Total Plate Count (TPC) method. The ALT levels in T2 were significantly decreased (P<0.05; 25.9 ± 5.50 U/L), followed by T1 (P<0.05; 35.6 ± 1.46 U/L). The AST levels in the T2 and T1 groups were also significantly decreased (P<0.05; 81.4 ± 13.44 U/L and 107.8 ± 9.45 U/L respectively). The bacterial colony of S.thypimurium was also significantly decreased in the T2 and T1 with the mean of 6.89 ± 2.06 x 10-1 and 8.38 ± 2.15 x 10-1 respectively. The Kruskal-Wallis test found a significant difference between variable groups (P < 0.05), but the Mann-Whitney test showed no significant difference only between T1 and T2 group for the bacterial colonies (P = 0.180). The Lumbricus rubellus extract have a hepatoprotective and antibacterial properties by significantly reduce the levels of amino transaminase enzyme (ALT and AST) and bacterial colonies of S.thypimurium in male Wistar rats.
Keywords
Aminotransaminase; Bacterial Colonies; Earthworm Extract; Lumbricus Rubellus
Download this article as:| Copy the following to cite this article: Lestari A. A. W, Sukrama I. D. M, Nurmansyah D. The Earthworm (Lumbricus Rubellus) Extract Decreased Amino Transaminase Enzyme Level and Number of Bacterial Colony in Male Wistar Rats Infected With Salmonella Typhimurium. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Lestari A. A. W, Sukrama I. D. M, Nurmansyah D. The Earthworm (Lumbricus Rubellus) Extract Decreased Amino Transaminase Enzyme Level and Number of Bacterial Colony in Male Wistar Rats Infected With Salmonella Typhimurium. Biomed Pharmacol J 2019;12(1). Available from: http://biomedpharmajournal.org/?p=24865 |
Introduction
Typhoid fever is a foodborne disease caused by Salmonella enterica serovartyphi (S.typhi).1 The World Health Organization (WHO) in 2003 reported that the incidence of typhoid fever reached 17 million where 600,000 typhoid-related deaths occurred worldwide. Whereas in Indonesia, the incidence rate of typhoid fever is 900,000 cases annually with 20,000 deaths, 91% of cases reported at the age of 3-19 years.2,3
Besides to the increased of O and H antibodies in the diagnosis of typhoid fever, elevated levels of transaminase enzymes in S.typhi bacterial infections also occur such as AST (Aspartate Aminotransferase) and ALT (Alanine Transaminase).4 Both increases of these enzymes occur at the time of S. typhi adhesion on the small intestine then into the epithelial cells.S.typhi infection enters the bloodstream through the lymph vessels to the human organs, liver,and spleen particularly. S.typhi will stimulate the proliferation of inflammatory cells in theliver. The presence of liver parenchymal cells damage and membrane permeability results in the AST and ALT enzymes production, thus a good indicator for detecting liver damage is the measurement of AST and ALT enzymeslevelsince these enzymes will increase significantly formerly.4
Various types of drugs such as chloramphenicol, ampicillin, and cotrimoxazole are antibiotics that have been used for typhoid fever for decades until the emergence of resistance issues called multidrug-resistant (MDR).5 The cause of the emergence of resistance issues is the administration of the irrational drug; consumption behavior does not comply with the rules, and the intrinsic changes in the microbe itself.5 The antibiotic resistance is the underlying issues for researchers to find alternative medicine substances, such as earthworms (Lumbricus rubellus) from Indonesia.
Several studies have reported thatthe bioactive compounds and antimicrobial properties were found in earthworms to inhibit pathogenic bacteria. These active substances include G-90 glycoprotein and fetidin from Eisenia feotida,6 lysozyme from E. fetida Andrei,7 as well as histidinefromDendrobaena venetaand Nereis diversicolor earthworms.8,9 In addition to the inhibition of pathogenic bacteria, earthworm flour (L. rubellus) has a fairly high protein content of 63.06% of its dry matter.10
The effects of Lumbricus rubellus earthworms against pathogenic bacteria have also been widely performed in vitro. A study conducted by Purwaningroom showed that Lumbricus rubellus earthworms were better at generating inhibition zones against S.typhi bacteria growth compared to Pheretima aspergillum.11 While study conducted by Ratriyani(found that the Lumbricus rubellus earthworms were proven to reduce the number of S.typhi bacterial colonies in vitro.12
Salmonella typhimurium bacteria used as a model of typhoid fever by using rats in this study due to Salmonella typhimurium infection may represent the pathological state of typhoid fever in humans caused by Salmonella typhi according to Rosenberger et al.The bacteria will enter through the gastrointestinal tract and migrate to lymph nodes followed by spleen and liver, similar to the pathogenesis of typhoid fever occurring in humans.13 Thus, the use of Salmonella typhimurium bacteria can be used as a model of typhoid fever in rats.
Based on the above explanation, the researchers wanted to determine the effect of earthworm (Lumbricus rubellus) extract in decreasing ALT, AST, and S.typhimuriumbacterial colonies in vivo.
Material and Methods
This study was an experimental study using posttest-only control group design since December 2016 – January 2017. The samples were male Wistar rats strain, 2 months old, weighing 200-250 grams, infected bacteria S.typhimurium, and meet the inclusion and exclusion criteria. The sample size was 28 male Wistar rats divided into 4 groups (C+, C-, T1, and T2). This study had received a recommendation from the Ethics Committee on animal research, Faculty of Medicine, Udayana University.
Animal Preparation
All 28 male Wistar male ratswere adapted for 1 week then divided into 4 groups, each group contained 7 rats. The groups consisted withnegative control group (only gave placebo), positive control (infected by S. typhimuriumbacteria), treatment group 1 (infected by S. typhimuriumon day 1 and giving L. rubellus earthworm extract on the following days until day 18) and treatment group 2 (giving L. rubellus earthworm extract in the first week then infected by S. typhimuriumon day 8, followed by extract until day 18).The number of mice infected by S. typhi was 105 and dosage of earthworm extract was 100 mg/kg based on previous study. All rats were fed and given drink in ad libitum way. On the 18th day, blood samples were taken for further measurement of the ALT and AST enzyme levels as well as abacterial colony from feces.
Crude Extract Preparation of Lumbricus rubellus earthworm
The collected Lumbricus rubellus earthworms were washed with running water to remove the mucus on its surface.After the earthworms were cleaned then it dried at 40°C constant temperature in the oven for 24 hours. The dried earthwormswere cut into small pieces then put into a glass tube of 80% ethanol solvent for evaporation process in obtaining thecrude extract. This process has been conducted for 2 days.
Salmonella thypimurium suspension preparation
S.thypimurium ATCC 14028 bacterial isolates were obtained from the Microbiology Department, Faculty of Medicine, Udayana University. Bacteria were cultured in Salmonella Shigella Agar, then incubated for 18-24 hours at 37°C. The S.thypimurium suspension preparation used cop count method. About 3-5 colonies were inserted into a tube containing 10 ml of 0.1% peptone;thenthe S.typhimuriumbacteria suspension was diluted in multiple series of 106.In addition, 0.1 ml of 0.1% peptone was planted to control contamination. The colony counting was performed if the number of colonies growing between 30-300 colonies. To determine the culture contamination, gram staining was performed and observed under a microscope for each dilution. The results of the concentration obtained will be made bacteria suspension containing 105 cells/ml.
Assessment of AST and ALT levels
The AST and ALT enzyme measurement methods were using IFCC method (International Federation of Clinical Chemistry and Laboratory Medicine). The measurement steps as follows: 1) Prepared test tubes and other; 2) Four parts of reagent A were mixed with one part of reagent B then homogenized (monoreagen); 3) Approximately 1 mL of monoreagen was taken and inserted into the test tube, then incubated in the 37oC temperature at least 1 hour; 4) Added serum/sample as much as 0.1 ml; 5) Incubated in water bath at 37°C for 1 minute; 6) the sample absorbance in a spectrophotometer with a wavelength of 340 nm was read.14
Assessment of Bacterial Colony in Feces
The measurement of bacterial colonies in the feces were conducted by a modified Total Plate Count (TPC) method with the following steps: 1) The intestine specimens or rats were taken with aseptic techniques and then feces collected in sterile containers; 2) The transport medium (TSB) was used to carry samples to the laboratory; 3) 1 gram sample was put into a 10 mL sterile NaCl; 4) Dilution was carried out from 101 – 106 to then transferred into a medium plate to Salmonella Shigella Agar (SS Agar); 5) Media plate Salmonella Shigella Agar (SS Agar) then incubated at 37oC for 1×24 hours; 6) the colony measurement was performed with a colony counter on a plate that was eligible to be calculated with colony counts of 30-300 colonies; 7) the average number calculation of bacterial colonies grown expressed by cfu/g sample unit; 8) the gram staining was carried out to ensure that the growing bacterial colony was S.typhimuriumwith red color and rod-shaped; and 9) The calculation of cfu / gram of sample was carried out by using the formula: (CFU number x dilution x 10) / sample weight (gram).15,16
Statistical Analysis
Data on AST and ALT levels as well as abacterial colony were described, followed by normality test using Saphiro Wilk, homogeneity test using Levene’s test, and comparability test using Kruskal-Wallis due to the data was not normally distributed and not homogeneous of data variants. In addition, Mann-Whitney test was also conducted to know the difference between each variable.
Results
AST and ALT levels
Table 1 showed the upper, lower, and mean values of aspartate transaminase (AST) serum levels.The results showed that the highest mean value of AST levels was in the positive control group (C +) of 184.7± 42.95 U/L. While the lowest mean value of AST levels was found in the treatment group 2 (T2), 81.4 ± 13.44 U/L. Based on the highest and lowest AST values suggested that the lowest values were in the T2 group (92.4 U/L and 66.2 U/L).The AST levels were known having a P value of 0.001 (<0.05), so it can be concludedthat there was a significant difference in AST levels between treatment groups (Table 1).
Table 1: The results of AST level measurement in rats.
| Group | Upper Values | Lower Values | Mean | P-Values |
| Control (+) | 274.4 U/L | 150.4 U/L | 184.7 ± 42.95 U/L | 0.001 |
| Control (-) | 133.2 U/L | 124.2 U/L | 128.1 ± 3.89 U/L | |
| T1 | 117.9 U/L | 96.3 U/L | 107.8 ± 9.45 U/L | |
| T2 | 92.4 U/L | 66.2 U/L | 81.4 ± 13.44 U/L |
Table 2 showed the highest, lowest, and mean value of alanine transaminase (ALT) serum levels by using IFCC method without peroxide. The highest mean value of ALT levels was found in the positive control group (C +) which accounted for 58.6 ± 21.92 U/L. Based on the highest and lowest ALT values suggested that the lowestvalues were in the T2 group (33,6 U/L and 20 U/L). Besides, the ALT levels were known having a P value of 0.001 (<0.05), so.
Table 2: The results of ALT level measurement with IFCC method.
| Group | Upper Values | Lower Values | Mean | P-Values |
| Control (+) | 106.1 U/L | 41.9 U/L | 58.6 ± 21.92 U/L | 0.001 |
| Control (-) | 42.2 U/L | 38.4 U/L | 39.8 ± 1.46 U/L | |
| T1 | 37.7 U/L | 34.2 U/L | 35.6 ± 1.46 U/L | |
| T2 | 33.6 U/L | 20 U/L | 25.9 ± 5.50 U/L |
Bacterial Colonies
The results of bacterial colonies were performed by TPC and Harigan methods that demonstrated in Figure 1. S. typhimurium bacteria that grow on SS agar media have a clear and transparent colony. Some have a black dot on the colony due to H2S produced by S.typhimurium. Gram staining was carried out to see the properties and morphology of bacteria in which indicate for gram negative due to rods shape and red appearance from Safranin Red stain (Figure 2). In the negative control group, there were no colonies eligible for 30-300 counting colony so that all results in the group could not be calculated. The results of this study also showed that the mean colony growth of S. typhimurium bacteria was the lowest in the treatment group 2 (P2) about 6.89 ± 2.06 x 10-1. Besides, the colony countswere known having a P value of 0.001 (<0.05), so it can be concluded there was a significant difference in the bacterial colony counts between treatment groups (Table 3).
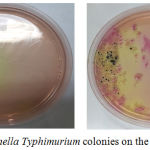 |
Figure 1: Salmonella typhimurium colonies on the SS agar medium. |
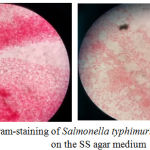 |
Figure 2: Gram-staining of Salmonella typhimurium colonies on the SS agar medium. |
Table 3: Colonies count results of S.typhimurium with TPC method (Total Plate Count) .
| Group | Σ The Highest Colonies | Σ The Lowest Colonies | Σ Mean | P-Values |
| Control (+) | 29.92 x 10-1 | 14.15 x 10-1 | 21.28± 5.64 x 10-1 | |
| Control (-) | 0 | 0 | 0 | 0.001 |
| T1 | 11.74 x 10-1 | 6.03 x10-1 | 8.38 ± 2.15 x 10-1 | |
| T2 | 9.69 x10-1 | 4.34 x10-1 | 6.89 ± 2.06 x 10-1 |
Multivariate Analysis
Mann-Whitney test was carried out to determine whether there were differences between each variable. In Figure 1, the results indicated that there was a significant differences between either AST or ALT measurement groups (P = 0.001). However, there was only no significant difference between T1 and T2 group (P = 0.180) for the bacterial colonies measurement (Figure 2). Based on this result, it can be concluded the treatment provided for T1 and T2 group to the bacterial colonies was no significant difference.
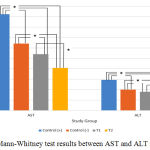 |
Figure 3: Mann-Whitney test results in the bacterial counts group. |
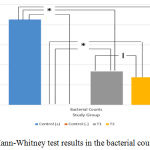 |
Figure 4: Mann-Whitney test results in the bacterial counts group.
|
Based on Table 4, this resultindicates that there is a decrease in the levels of AST, ALT, and bacterial colonies on treatment 1 and 2 compared with the positive and negative control groups. In addition, the observed variables (AST, ALT, and bacterial colonies) also resulted in a decline in enzyme levels and colonies.
Table 4: The mean comparison of AST, ALT, and number of bacterial colonies between groups.
| Variable | Control positive | Control negative | T1 group | T2 group | Description |
| AST | 184.7 U/L | 128.1 U/L | 107.8 U/L | 81.4 U/L | ↓ Levels |
| ALT | 58.6 U/L | 39.8 U/L | 35.6 U/L | 25.9 U/L | ↓ Levels |
| Σ Bacterial colonies | 21.28 x 10-1 | 0 | 8.38 x 10-1 | 6.89 x 10-1 | ↓ Σ Colonies |
Discussion
Typhoid fever is a foodborne disease caused by Salmonella enterica serovartyphi, a genus of gram-negative bacteria. It remains one of the endemic diseases in Indonesia, based on data from the World Health Organization (2003).3 This infection triggers a release of proinflammatory cytokines fromthe liver parenchymal cells damage and membrane permeability that is resulting in the AST and ALT enzymes production. Both of them are known as a good indicator for detecting liver damage since these enzymes will increase significantly formerly.17AST is a chemical parameter which has a 90% of sensitivity but only has a specificity of 18% on liver damage.18 AST levels will increase in the acute infection or hepatic injury as well as in a person with typhoid fever.19,20 In addition, ALT is similar with AST as an enzyme which is abundant in the liver cells (also present in other organs such as the kidneys, heart muscle, pancreas but in the very slight levels) despite having better sensitivity and specificity for biomarkers ofliver cell damage.21
Regarding hepatoprotective activity of earthworm extracts, it seems that these substances not only exert antioxidative properties but also may regulate the expression of specific genes.Earthworm (L. rubellus) extract is known containing Polyphenolic which has anti-oxidation and anti-inflammatory properties, G-90 glycoprotein, and also fibrinolytic enzyme. Dewi NWS found that the ethanolic extract of L. rubellus powder had total phenolic content of 1016.31 mg/100 g garlic acid equivalent (GAE) and exhibited IC 50% of 12.33 mg/ml as antioxidant capacity.22 A study conducted by Popovic et al showed that G-90 glycoproteins had antimicrobial properties where 10 mg / mL concentrations were able to inhibit the growth of facultative pathogenic bacteria such as S.enteritidis, S.aureus, and S.pyogenesalso exerts anticoagulative and fibrinolytic activities.6 Furthermore, it is also notedin the study of Tasiemski (2006)that L.rubellus also contained Lumbricin I as abroad-spectrum antimicrobial compound against both gram-positive and gram-positive bacteria.9
The comparative analysis results using Kruskal-Wallis testof ASTand ALT levels in 4 treatment groups (positive control, negative control, treatment 1 and treatment 2), showed that there were significant differences between treatment groups which receiving L.rubellus extract (P = 0.001).This study found that the treatment groups (1 and 2) had lower results of AST and ALT levels compared with positive and negative control groups. It is presumably due to the hepatoprotective effect activity of the compound found in earthworm (L.rubellus). A previous study conducted by Muchtaromahdemonstrated that there were antimicrobial effects either in vitro or in vivo in earthworms of 60% dosages of L. rubellus earthworms where reducing the transaminase enzyme levels within 14 days.23
In treatment 1 (T1) where bacterial infection and earthworm extract were given on the first day up to the 18th day of treatment, AST and ALT levels were higher than treatment 2 (T2). However, both groups were still had lower levels of AST and ALT compared with positive and negative control group. This shows that earthworm extract has a hepatoprotective effect on S.typhimurium infection. The hepatoprotective effect is better in treatment 2 where bacterial infection performed on the 8th day after administration of the extract on day 1-7 of treatments, followed by administration of the extract on days 10-18. This results was supported by Salzet et al which stated that L.rubellus earthworm had peptide compounds as the first defense against microbes with their own anti-microbial properties.7 The hepatoprotective effect was also reported by Balamurugan et alwhere a decrease in ALP, AST, ALT, and Bilirubin enzyme levels in Rats occurred in the hepatic cellular injury exposed to substances.24
In typhoid fever due to S.typhi bacterial infection, patients who have recovered still possible to spread the S.typhi called as a carrier. Carrier is a person who has recovered from typhoid fever and still able to secrete S.typhi bacteria in feces and urine.25 Patients who did not receive appropriate treatment then will have a possibility for bacteria still be remain in the organs of the reticuloendothelial system and able to do re-proliferated and re-secreted by the patient.25 The appropriate treatment for this issue is by giving specific antibiotics. Antibiotics are substances that kill or inhibit bacteria, e.g., growth, by any of several mechanisms that specifically target the bacterial cell. However, Kelanit et aldemonstrated that MDR was found to be resistant to 18 antibiotics for S.typhi infection in Jayapura, Papua, such as 8 isolates resistant to Amoxicillin 100%, 75% for Cefazolin (6 isolates), and 75% for ampicillin (6 isolates).26 In this regards, our study is trying to figure out the antibacterial properties belong to L.rubellus extract using bacterial colonies counts. The bacterial colonies measurement using the TPC (Total Plate Count) method in rat feces shown that there were significantly differences between treatment groups by Kruskal-Wallis test (P < 0.05). The decrease number of bacterial colonies indicates that the antibacterial properties possessed by the L.rubellus earthworm work against S.typhimurium infection. GlycoproteinG-90 and Lumbricin I antibacterial molecules in L.rubellusextract are known highly sensitive to gram-positive bacteria, but it can also work against gram-negative bacterial infections such as S.typhimurium.7,27 G-90 glycoprotein is also known to be a stimulant of proliferation, anti-inflammation, and antimicrobial.28 Chauhan et al demonstrated that the earthworm extracts were able to inhibit the P.aeruginosa bacteria growth similar to the inhibition properties produced by Streptomycin.29 Purwaningroom using L.rubellus and Pheretima aspergillum flours also suggested that the L.rubellus earthworm flour was better and significantly different in inhibiting the S.typhi bacteria growth in vitro with the processing temperature of 50oC.11
However, multivariate analysis using Mann-Whitney test demonstrated no significant difference only between T1 and T2 groups for anumber of bacterial colonies (P = 0.180). In the treatment group 2 (T2), L.rubellus earthworm extract was administered for 7 days of treatment, then infected with S.typhimurium bacteria for the following days, in contrast to treatment group 1 (T1) where infection and administration of the extract were performed at the same time. These results suggested that despite L.rubellus extract has anti-inflammatory and hepatoprotective properties; the treatment differences show no significant impacton the number of bacterial colonies observed. Both either the L.rubellusextract or infection given simultaneously or separated, its effect on the inhibition of bacterial colony count was not significantly different in rats.
Conclusion
The Lumbricus rubellus earthworm extract has hepatoprotective and antibacterial properties through its effect on decreasing levels of AST, ALT, and number of S.typhimurium bacterial colonies in male Wistar rats.
Acknowledgment
We thank to I Dewa Made Sukrama, Associate Professor from Clinical Microbiology Department, Sanglah General Hospital, Bali, Indonesia for helping us in the preparation of Salmonella thypimuriumbacteria.
Ethics Approval
The animal model used in this study has been approved by the Ethical Commission of Faculty of Medicine Udayana University/Sanglah General Hospital
Conflict of Interest
The authors declare that there is no conflict of interest regarding this article
Funding
The study is a self-funding with no other source of funding
References
- Bhuta Z. A. Current concepts in the diagnosis and treatment of typhoid fever. BMJ. 2006;333:78-82.
CrossRef - Brooks G. F., Butel S. J and Morse S. A. Jawetz, Melnick & Adelberg’s Medical Microbiology twenty-second edition Lange Medical Books/ Mc Graw-Hill Companies Inc. Medical Publishing Division. 2004.
- World Health Organization. Background document the diagnosis, treatment and prevention of typhoid fever. Geneva: Immunity and Immunization. Department of Vaccines and Biologicals. 2003. Available at http://www.who.int/irir/handle/10665/68122.
- Morgenstern R., Hayes P. C. The liver in typhoid fever always affected not just a complication. Am J Gastroenterol. 1991;86(9):1235-9.
- Olsen S.J., Michele Y., Meghan F. D., Marshal D., Ben H., Larry I et al. Multidrug-resistant Salmonella Typhimurium Infection from Milk Contaminated after Pasteurization. Emerg Infect Dis. 2004;10(5):932-5.
CrossRef - Popovic M., Grdisa M and Hrzenjak T. M. Glycolipoprotein G-90 obtained from the earthworm Eisenia foetida exerts antibacterial activity. Veterinarski Arhiv. 2005;75:119-128.
- Salzet M., Tasiemski A., Cooper E. Innate immunity in Lophotrochozoans the annelids. Curr Pharm Des. 2006;12(24):3043-50.
CrossRef - Kalac Y., Kimiran A., Ulakoglu G., Cotuk A. The role of opsonin in phagocytosis bycoelomocytes of earthworm Dendrobaena veneta. J Cell Mol Biol. 2002;1:7-14.
- Tasiemski A., Schikorski D., Marrec-Croq F. L. E., Camp C. P. V et al. Hestidin: A novel antimicrobial peptide containing bromotryptophan constitutively expressed in the NK cells-like of the marine annelid, Nereis diversicolor. Dev Comp Immunol. 2006;31:749-762.
CrossRef - Istiqomah L., Sofyan A., Damayanti E., Julendra H. Amino acid profile of earthworm and earthworm meal (Lumbricus rubellus) for animal feedstuff. J Indonesian Trop Anim Agric. 2009; 34(4):253-257.
CrossRef - Purwaningroom D. L. Uji In Vitro pengaruh Jenis Teung Cacing tanah (Lumbricus rubellus) dan Pheretima Aspergillum) denganvariasisuhupengolahan (50,60,70‘C) terhadappenghambatanpertumbuhanbakterityphi. Skripsi. Malang: UIN Maulana Malik Ibrahim. 2010.
- Ratriyani W. Uji Sensitivtas typhiterhadapekstrakcacingtanahsecara in vitro. Skripsi. Semarang: Universitas Diponegoro. 2000.
- Rosenberger C. M., Scott M. G., Gold M. R., Hancock R. E., Finlay B. B. Salmonella typhimurium infection and lipopolisacharyde stimulation induce similar change in machropage gene expresion. J Immunol. 2000;164(11):5894-904.
CrossRef - Sardini S. Penentuan aktivitas enzim GOT dan GPT dalam serum dengan metode reaksi kinetik enzimatik sesuai IFCC (International federation of clinical chemistry and laboratory medicine). Pusat Teknologi keselamatan dan Metrologi Radiasi-BATAN. Jakarta. 2007.
- Efek Phylanthus niruri L pada prosentase neutrofil, koloni bakteri limpa, dan histopatologi hepar mencit Balb/C yang diinfeksi Salmonella typhimurium. Tesis. Semarang: Universitas Diponegoro. 2007.
- Cappucino J. G., Sherman N. Microbiology: A Laboratory Manual. 6th Pearson Education Inc. San Fransisco. 2002:15-224.
- Kaur J., Jain S. K. Role of antigens and virulence factors of salmonella eneterica serovar typhi in its pathogenesis. Microbiol Res. 2012;167(4):199-210.
CrossRef - Gorosia J., Kamariya C., Vachanni U. Requirement of newer parameters to replace conventional Liver Function test for Differentiation of Liver disease from Non-Liver disease. International Journal of Scientific and Research publication. 2013;8(3):1-3.
- Ndukaku O. Y., Emmanuel E. U., Mercy E. A., Caroline N. O. Evaluation of the serum liver enzyme markers, lipid profile, and kidney function parameters in typhoid patients. International Journal of Tropical Disease and Health. 2015;8(2):79-89
CrossRef - Shamin A., Shamin A., Hussain B. Study of biochemical change and elevated levels of enzymes in salmonella typhi infected patients in pakistani population. Int J Bioautomation. 2012;16(1):33-42.
- HalL P., Cash J. What is the real function of the liver ‘function’ tests? Ulster Med J. 2012;81(1):30-36.
- Dewi N. W. S., Mahendra A. N., Putra G. W. K., Jawi I. M., Sukrama D. M., Kartini N. L. Ethanolic extract of the powder of red earthworm (Lumbricus rubellus) obtained from several organic farmlands in Bali, Indonesia: Analysis of total phenolic content and antioxidant capacity. Bali Medical Journal. 2017;3(3):S80-S83.
CrossRef - Muchtaromah B. Effect of earthworm (Lumbricus rubellus) flour against histological profile of several organs and transaminase enzyme level in rat that infected by Salmonella typhi. Workshop on Plant Products Chemistry and International Symposium on Medicinal-Aromatic Plants. Izmir, Turkey. 2013.
- Balamurugan M., Partasarapthi K., Ranganathan L. S., Cooper E. L. Hypotetical mode of action of eartworm extract with hepatoprotective and antioxidant properties. J Zhejiang Univ Sci B. 2008;9(2):141-7.
CrossRef - Gunn J. S., Marshall J. M., Baker S., Dongol S., Charles R. C., Ryan E. T. Salmonella chronic carriage epidemiology diagnosis and gallbladder persistence. Trends Microbiol. 2014;22(11):648-655.
CrossRef - Kelanit R. S., Runtuboi D. Y. P., Gunaedi T. Uji Resistensi dan Deteksi Gen Plasmid Inc HI1 Salmonella typhi isolat Jayapura. Jurnal Biologi Papua. 2006;8:48-56.
- Cho J. H., Park C. B., Yoon Y. G., Kim S. C. Lumbricin I, a novel proline-rich antimcrobial peptide from earthworm: purification, cDNA cloning and molecular characterization. Biophys. ACTA. 1998;1408:67-76.
- Chang Y. M., Shih Y. T., Chen Y. S., Liu C. L., Fang W. K et al. Cell Migration Induced by Earthworm Extract via Activation of PAs and MMP2/9 Mediated through ERK1/2 and p38. Evid Based Complement Alternat Med. 2011;2011:395458.
CrossRef - Chauhan P. S., Tomar J., Prasad G. B. K. S., Agrawal O. P. Evaluation of antimicrobial activity of earthworm Eudrilus eugeniae tissue extract. J Chem Pharm Res. 2014;6(8):28-38.








