Elsharabasy F. S1, Metwally N. S2, Mahmoud A. H2, Soliman M. S2, Youness E. R*3, Farrag, A. H4 and Sherifa Arafa5
1Department of Chemistry, Prince Sattam Bin Abdul-Aziz University, Saudi Arabia and Chemistry of Natural and Microbial Products Department, National Research Center, Dokki, Egypt.
2Therapeutic Chemistry Department, Pharmaceutical Industries Research Division, National Research Center, Dokki, Egypt.
3Medical Biochemistry Department, Medical Research division, National Research Center, Dokki, Egypt.
4Pathology Department, National Research Center, Dokki, Egypt.
5Department of Biology, Prince Sattam Bin Abdulaziz University, Saudi Arabia and Department of Botany, Faculty of Science, Cairo University, Egypt.
Corresponding Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1620
Abstract
The objective of this study is to investigate the chemical constituents of Suaeda monoica and Suaeda pruinosa and evaluate their effects on rat liver intoxicated by over dose of paracetamol. Qualitative analysis of (80%) methanol in water fraction revealed the presence of alkaloids, terpenoids, steroids, tannins, quinones, saponins, flavonoids and phenols from two mangrove species of S. monoica and S. pruinosa was carried out. The compounds were identified from the 80% methanol-H2O fraction as Rutin, quercetin, Syringic acid, Coffeic acid, Catechin, Coumaric acid, Vanillin, Gallic acid, Cinnamic acid. Amino acids analysis for the methanolic extract from the aerial parts of Suaeda monoica and Suaeda pruinosa showed the presence of thirteen amino acids and absence of valine, Isoleucine, Phenylalanine. Fatty acid analysis of lipids showed high percentage of long chain fatty acids.TLC of the lipoidal matter for each plant showed the presence β-amyrin, β-sitosterol and stigmasterol. Toxicity was stimulated by administration of a single oral dose of paracetamol (3 g/kg body weight). The extract of the aerial parts of plants (100 mg/kg) was utilized on a pre-and post-treatment basis. Both extracts significantly improved liver and kidney function with prophylactic or therapeutic treatments. Histopathological and histochemical studies showed parallel effects with the biochemical measured parameters.
Keywords
Hepatoprotection; Paracetamol; Secondary Metabolites; Suaeda Sp.
Download this article as:| Copy the following to cite this article: Elsharabasy F. S, Metwally N. S, Mahmoud A. H, Soliman M. S, Youness E. R, Farrag A. H, Arafa S. Phytoconstituents and Hepatoprotective Effect of Suaeda Monoica Forssk and Suaeda Pruinosa Lange. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Elsharabasy F. S, Metwally N. S, Mahmoud A. H, Soliman M. S, Youness E. R, Farrag A. H, Arafa S. Phytoconstituents and Hepatoprotective Effect of Suaeda Monoica Forssk and Suaeda Pruinosa Lange. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2HUMmvB |
Introduction
A mangrove and mangrove associate plants are proved to have rich of high value secondary metabolites viz, saponins, alkaloids, polyphenols which possess antibacterial, antifungal, antiplasmodial and hepatoprotective activities.1
Suaeda (Chenopodiaceae) is a genus of plants also known as seep weeds,includes annual and perennial herbs or rarely small trees. There are about 110 species in the Suaeda genus (S.).2 They are distributed on coasts, deserts, lakeside and saline and alkaline land all over the world.
A number of species have been found to be valuable feed for livestock in arid area like Suaeda pruinosa Lange, S. vera and S. vermiculata,3 while others have been utilized to desalinize irrigated farmlands like Suaeda maritime.4 In Mexico, some species such as Suaeda pulvinata are cooked in traditional dishes known as romeritos. Suaeda species are commonly used in folk medicine.5-6 Some species possess hypoglycemic, anti-inflammatory, hypolipidemic, cardiotonic, antioxidant, antimicrobial and anticancer activity.7-8
In Saudi Arabia it is represented by 9 species according to Boulos.9 Five species were found in Al Jouf area represented by; S. aegyptica, S. vera and S. vermiculata, S. fruiticosa and S. mollis.10
Suaeda vera subspecies pruinosa (Lange) Bolòs and Vigo, (1974), are facultative halophytes that tolerate moderate salt soils, dry soils, nitrified and saline (halonitrófilos) in very sunny places arid climate during May to October.11 The specific epithet ” pruinosa ” refers to the presence of pruina, which is kind of thin waxy coating on stems and leaves, which offers a glaucous appearance. In the area south of Alicante has been used to make the ” stone soda ” and was said to be as good as that made with fine barrilla (Halogetum sativus). The nutritive value of forage species (crude protein ranges) in Suaeda pruinosa Lange is 27.17%.
Suaeda monoica Forssk. ex J. F. Gmel is a salt marsh mangrove herb growing in hypersaline soils. It is smaller in size, simple leaves which are edible. Traditionally, the leaf from S. monoica is known to use as a medicine for hepatitis12 and scientifically it is reported to be used as ointment for wounds13 and possess antiviral activity7 because of the presence of triterpenoids and sterols.5
Methanolic extracts of S. monoica, exhibits moderate sun protective activity and a safe, effective and affordable sun screen formulation.14 Endophytic fungi were isolated from the leaves and stems of S. monoica , the isolation was according to the method described by Suryanarayanan et al.(2003).15 Phytochemical screening of methanolic extracts from S. monoica, showed the presence of flavonoids, tannins, phenols , polyphenols, steroids and Alkaloids.16
A polysaccharide extracted from the leaves of S. monoica showed positive activity against human immune deficiency viruses.7, 13
Therefore, the present attempt has been made to identify the phytoconstituent of S. monoica and Suaeda pruinosaextracts and to find out the anti- inflammatory effect of each extract on the rat liver intoxicated by paracetamol overdose.
Materials and methods
Plant material and preparation of extract
Suaeda sp. were collected during months of June, July, 2016-2017 from El Doubia at El Riyadh- El Dallamroad. Air-dried and powdered aerial parts of Suaeda monoicaand Suaeda pruinosa were extracted with petroleum ether. The solvent was evaporated under reduced pressure then extracted with methyl alcohol in H2O(80%), the solvent was stripped off under reduced pressure gave 50 g. The petroleum ether extract 10 g was subjected to subsequent investigation.
Extract of Amino Acids
Lyophilized tissues were ground in a mortar and extracted with MCW (methanol:chloroform:water 12:5:3 v/v/v), following the procedure described by Marur et al.(1994)17. A sample of ground material (100 mg) was extracted with 10 mL of MCW for 3 days in a closed tube in a refrigerator. After centrifugation at 1200 rpm for 5 min on a bench centrifuge, the supernatant was collected and the residue extracted with a further 6 mL of MCW. After centrifugation the supernatants were combined (16mL) and shaken with chloroform (4mL) followed by water (6 mL). On standing, the resulting separation of phases enabled the removal of chlorophyll and the aqueous phase was taken to dryness under a stream of N2 gas. This material was resuspended in water and used for amino acid determination.
Preparation of Fatty Acid Methyl Esters
This method is a modified Folch procedure,18 utilizing 2:1 (v/v) chloroform/methanol as the organic solvent.
Chemicals and Reagents
Amino Acids Analysis
Methanol (HPLC grade),Chloroform (HPLC grade) chloroform/methanol (HPLC grade)2:1 (v/v), 0.88% (8.8 g/liter) KCl in distilled H2O, Nitrogen gas: high purity (<5 mg O2/kg), Centrifuge tube with spout or 50-ml glass tube, Polytron PT-3100 homogenizer equipped with a PT-DA 3012/2EC aggregate(probe) or equivalent, Whatman no. 40 filter paper,15- and 50-ml test tubes with Teflon-lined screw cap, Test tube shaker, Table top centrifuge, Vacuum aspirator,N-EVAP model 112 nitrogen evaporator (Organomation) with moisture trap in line from N2 source, Glass pipets, Glass beakers.
Organic Solvent for Preparation of Lipid Sample
Nitrogen gas: high purity (<5 mg O2/kg), 0.5 N NaOH: 2 g NaOH diluted to 100 ml in methanol; store up to 6 months at 2° to 8°C 10% to 14% boron trifluoride (BF3) in methanol (Sigma or Supelco; also see recipe).
Isooctane or hexane, Anhydrous sodium sulfate: store at 100°C N-EVAP model 112 nitrogen evaporator (Organomation) with moisture trap in line from N2 source (Fig. D1.2.1), 15-ml test tubes with Teflon-lined screw caps 100°C heating block, Test tube shaker,Tabletop centrifuge, GC sample vials with Teflon caps.
Methods
Separation of the amino acids was carried out on a Superpac (Pharmacia) ODS-2 column coupled to an LKB dual pump HPLC system (model 2150), controlled by a gradient generator, model 2152. Knowing the concentration of amino acids in the sample, an appropriate volume was taken for the preparation of o-phthaldialdehyde (OPA)19-20 and 9-fluorenylmethyl-chloroformate (FMOC-Cl) derivatives21 prior to separation and analysis by reverse-phase HPLC. The analysis of proline was carried out by a second analysis using FMOC-Cl derivatives on each sample. FMOC derivates were prepared as described by.22
Extracted Lipid Sample
Fatty acid compositional analysis of lipids carried out by gas-liquid chromatography (GLC) modified Folch procedure.18
GC Method
Column: J+W DB-23 (50% cyanopropyl) methylpolysiloxane FID: Temp: 270°C, Injection: – mode: split ratio 30:1 , – temperature: 270°C , injection volume: 1 µL
Oven: – intial temp: 140°C, – temp ramp: 10°C / min, – final temp: 260°C, Hold at final temperature for 3 minutes. For sequences run prior to 03/30/07, the final temperature was held for 5 minutes.
HPLC Conditions for Phenolic Compounds
HPLC analysis was carried out using an Agilent 1260 series. The separation was carried out using C 18 column (4.6 mm x 250 mm i.d., 5 μm). The mobile phase consisted of water (A) and 0.02% tri-floro-acetic acid in acetonitrile (B) at a flow rate 1 ml/min. The mobile phase was programmed consecutively in a linear gradient as follows: 0 min (80% A); 0–5 min (80% A); 5-8 min (40% A); 8-12 min (50% A); 12-14 min (80% A) and 14-16 min (80% A). The multi-wavelength detector was monitored at 280 nm. The injection volume was 10 μl for each of the sample solutions. The column temperature was maintained at 35°C.
Biochemical Studies
Materials and Methods
Drugs and Chemicals Paracetamol was purchased from Alexandria Company for Pharmaceuticals and Chemical Industries. Kits for determination of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP),bilirubin and urea were obtained from Biomed Diagnostics, Egypt. The interleukin-1β(IL-1β) and tumor necrosis factor alpha (TNF-α) were carried out using an ELISA reagent kits obtained from Biosource, USA.
Animals
Male rats weighing 100-120 g were obtained from theAnimal Breeding Lab of National Research Centre, Cairo, Egypt. The animals were kept under constant temperature conditions (22 ± 2°C), relative humidity (50–60%), and lighting (12 h light/dark cycle). Food and water were accessible ad libitum. The study was carried out according to the guidelines of the Ethics Committee of the National Research Centre.
Experimental Design
Six groups each of six male albino rats were selected. Group1: served as negative control. Group 2: rats received a single dose of paracetamol (positive control). Group 3 & 4: rats received Suaeda monoica and Suaeda pruinosebefore paracetamol induction. Group 5 & 6: rats received the Suaeda monoica and Suaeda pruinose extract after paracetamol induction. Twenty–four hour after treatments, the rats of all groups were anesthetized and blood samples were collected directly from retro-orbital plexus. The blood samples were allowed to clot for 20-30 min. Serum was separated by centrifugation at 37°C and used for estimation of various biochemical parameters. Animals were sacrificed by decapitation. Livers were rapidly isolated; a part of each was homogenized using cold saline to prepare a 10% homogenate that was used for estimation of malondialdehyde (MDA). The second part of the liver was preserved in 10% formalin for histopathological and histochemical examinations.
Biochemical Analyses
The activities of AST and ALT were determined according to the method of Reitman and Frankel,(1957).23 Alkaline phosphatase was determined using the method described by Demetriouet al. (1974).24 Bilirubin was carried out by themethod described by Young (2000).25 Creatinine was measured by the method of Bartelsand Bohmer (1971).26 Urea was calculated by the method of Tabaccoet al. (1979).27 Arylesterase activity of paraoxonase 1 (POX-1) was measured spectrophotometrically using phenylacetate as a substrate.28-29 IL-1βwas carried out using an ELISA reagent kit and expressed as pg/ml. TNF-αwas determined by ELISA using kit (Biosource International, USA) and microtiter plate reader (Fisher Biotech, Germany). Lipid peroxidation was assayed by measuring the level of malondialdehyde (MDA) in the tissue homogenates using the method of Ruiz-Larrea et al. (1994).30
Histopathological and Histochemical Studies
After the experimental period, animals were sacrificed, liver removed immediately, sliced and washed in saline. Liver pieces were preserved in 10% formalin for histopathological studies. Sections were taken and stained with hematoxylin and eosin (H&E)31 and then they were examined for histopathological changes and photographed. The histochemical study was performed by periodic acid-Schiff method32 for visualization of the polysaccharidein the liver. These materials were demonstrated in sections with 5 μ m thickness.
Statistical Analysis
The protection percent is calculated by 1-(T-V/C-V)x100. Data were expressed as mean ± SD. The data were analyzed by one-way ANOVA followed by Duncanۥs multiple tests, using SPSS software (SAS Institute Inc., Cary, NC, USA). A probability value of less than 0.05 was considered statistically significant.
Results and Discussion
Preliminary Phytochemical Analysis
Plant materials were screened using the methods previously described33. Phytochemical analysis gave positive test for alkaloids, terpenoids, steroids, tannins, saponins, flavonoids and phenols. The identification of major chemical groups was carried out by thin layer chromatography (TLC) on silica gel. TLC was developed in chloroform/methanol/water (26:14:3), spots were visualized with sulfuric acid 20% in methanol solution under 254 and 356 nm gave yellow spots for Flavonoids, and purple color suggested the existence of triterpene, pink color for sterols and brown color for monoterpenes. Data shown that the main compounds were observed in S. monoica and S. pruinosaphenolic and terpenes /or sterols.
Amino Acids Analysis
Separation of the amino acids for methanolic extracts of each plant was carried out on HPLC system revealed that, the highest percentage of Methionine in S. pruinosa (0.999) while S. monoica (0.889) and Histidine was (0.216) in S. monoica and (0.167) in S.pruinosa extracts. Valine, Isoleucine and Phenylalanine were absent from the extracts of S. monoica and S. pruinosa (table1).
Table 1:Amino acids identified in the methanol extract from the aerial parts of each S. monica and S.pruinosa.
| Identified amino acids | µmol/mg dry wt | |
| S. monica | S. pruinosa | |
| Histidine | 0.216 | 0.167 |
| Arginine | 0.137 | 0.130 |
| Threonine | 0.121 | 0.115 |
| Alanine | 0.091 | 0.084 |
| Proline | 0.082 | 0.077 |
| Tyrosine | 0.105 | 0.081 |
| Valine | ND | ND |
| Methionine | 0.889 | 0.999 |
| Cysteine | 0.151 | 0.143 |
| Isoleucine | ND | ND |
| Leucine | 0.122 | 0.116 |
| Phenylalanine | ND | ND |
| lysine | 0.312 | 0.297 |
| Aspartic acid | 0.111 | 0.086 |
| Glutamic acid | 0.035 | 0.027 |
| Glycine | 0.128 | 0.122 |
GC of Fatty Acid Methyl Esters in the Petroleum Ether Extract
Fatty acid analysis of lipids carried out by gas-liquid chromatography (GC)34 showed high percentage of long chain fatty acids, the percentage of unsaturated fatty acids was (polyunsaturated fatty acids linolenic acid was (38.96%) followed by linoleic acid (59.84%) and monounsaturated fatty acid, oleic was 71.69% and the percentage of saturated fatty acids 65.26%for S. pruinosa, while the high percentage of linolenic acid (33.72%) followed by linoleic acid (52.42% ) and oleic acid (40.71%) and 77.80 saturated fatty acids for S. monoica (table 2).
Table 2: GC of fatty acid methyl esters in the petroleum ether extract from the aerial parts of S. monoica and S. pruinosa.
| Identified fatty acids
(mg/100 g D.W) |
Plant samples | |
| S. monica | S. pruinosa | |
| Area% | Area% | |
| Lauric acid (C12:0) | 15.78 | 10.49 |
| Myristic acid(C14:0) | ND | ND |
| Myristoleic (C14:1) | 7.55 | 8.27 |
| Pentadecylic acid (C15:0) | ND | ND |
| Palmitic acid (C16:0) | 39.41 | 33.39 |
| Palmitoleic acid (C16:1) | 20.65 | 25.17 |
| Stearic acid (C18:0) | 22.61 | 21.38 |
| Oleic acid (C18:1) | 40.71 | 38.25 |
| Linoleic acid (C18:2) | 52.42 | 59.84 |
| Linolenic acid (C18:3) | 33.72 | 38.96 |
HPLC of Phenolic Compounds
HPLC of phenolic compounds in Methanolic extract from the aerial parts of S.monoica and S. pruinosa revealed that, the identified compounds in methanolic extract of S. pruinosa were nine while methanolic extract of S. monoica showed eight compounds. The extract of each plant contained Gallic Acid, Catechin, Coffeic Acid, Syringic Acid, Rutin, Coumaric Acid, Vanillin, Quercetin but cinnamic acid found in methanolic extract of S. pruinosa only (table 3).
Table 3: HPLC of phenolic compounds in Methanol extract from aerial parts of each S. monoica and S. pruinosa.
| Compounds | S. monoica | S. pruinosa | ||||
| Area | Conc. (µg/ml) | Conc. (µg/g) | Area | Conc. (µg/ml) | Conc. (µg/g) | |
| Gallic Acid | 250.08 | 13.43 | 268.60 | 160.71 | 8.63 | 172.61 |
| Catechin | 132.56 | 25.58 | 511.61 | 155.22 | 29.95 | 599.08 |
| Coffeic Acid | 165.36 | 6.13 | 122.50 | 159.21 | 5.90 | 117.95 |
| Syringic Acid | 362.51 | 18.86 | 377.23 | 285.90 | 14.88 | 297.51 |
| Rutin | 474.19 | 65.43 | 1308.50 | 374.03 | 51.61 | 1032.12 |
| Coumaric Acid | 83.54 | 1.86 | 37.20 | 97.57 | 2.17 | 43.45 |
| Vanillin | 209.57 | 6.37 | 127.32 | 253.38 | 7.70 | 153.94 |
| Quercetin | 89.57 | 8.45 | 168.95 | 12.56 | 1.18 | 23.70 |
| Cinnamic Acid | 0.00 | 0.00 | 0.00 | 17.27 | 0.16 | 3.28 |
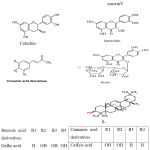 |
Scheme 1: Chemical Structures of compounds identified in methanolic extract from the aerial parts of S.monoica and S.pruinosa.
|
Table 4: Effect of Suaeda. Sp. extract on rat liver and kidney treated with over dose paracetamol.
| Parameter Group name | ALT | AST | ALP | Total bilirubin | urea | Creatinine |
| 1- Clean control | 12.13± 1.17
(2,3,4,5,6) |
24.74± 2.23
(2,3,4) |
92.61± 2.93
(2,3,4,5,6) |
0.92± 0.15
(2,3,4,5,6) |
28.97± 1.95
(2,3,4,5,6) |
1.15± 0.19
(2,3,4,5,6) |
| 2- Positive control | 60.11± 2.41
(1,3,4,5,6) |
92.56± 2.78
(1,3,4,5,6) |
261.98± 4.07
(1,3,4,5,6) |
1.65± 0.18
(1,3,4,5,6) |
66.49± 2.57
(1,3,4,5,6) |
4.20± 0.59
(1,3,4,5,6) |
| 3-S. monoica Pre- treated | 41.21± 3.44
(1,2,4,5,6) |
45.99± 2.39
(1,2,5,6) |
226.39± 1.99
(1,2,5,6) |
1.46± 0.03
(1,2,5,6) |
52.95± 1.94
(1,2,5,6) |
3.48± 0.27
(1,2,5,6) |
| 4- S. pruinosa Pre -treated | 49.07± 2.93
(1,2,3,5,6) |
46.64± 2.83
(1,2,5,6) |
228.89± 3.70
(1,2,5,6) |
1.49± 0.03
(1,2,5,6) |
55.98± 1.13
(1,2,5,6) |
3.48± 0.38
(1,2,5,6) |
| 5- S. monoicaPost-treated | 30.59± 2.03
(1,2,3,4) |
28.44± 1.58
(1,2,3,4) |
179.73± 2.72
( 1,2,3,4) |
1.37± 0.03
(1,2,3,4) |
39.54± 2.06
(1,2,3,4) |
2.29±0.20
(1,2,3,4) |
| 6- S. pruinosa Post-treated | 31.93± 2.17
(1,2,3,4) |
27.87± 2.25
(1,2,3,4) |
166.62± 2.42
( 1,2,3,4) |
1.38± 0.05
(1,2,3,4) |
39.87± 2.097
(1,2,3,4) |
2.54± 0.23
(1,2,3,4) |
Values represent mean of six animals ± SE.
Significant change at P < 0.05.LSD: Least significance difference.
Table 5: Effect SU. Sp. extract on antioxident of rat treated with over dose paracetamol.
| Parameter Group name | 1-Clean control | 2- Positive control | 3– S. monica Pre- treated | 4– S. pruinosa Pre -treated | 5– S. monicaPost-treated | 6– S. pruinosa Post-treated |
| MDA | 19.91± 1.65
(2,3,4,5,6) |
59.59± 2.43
(1,3,4,5,6) |
48.46± 1.86
(1,2,5,6) |
49.36± 1.72
(1,2,5,6) |
37.09± 1.75
(1,2,3,4) |
38.02± 2.09
(1,2,3,4) |
| POX-1 | 26.61± 1.70
(2,3,4,5,6) |
13.18± 1.14
(1,3,4,5,6) |
18.11± 1.84
(1,2) |
18.54±1.29
(1,2) |
18.54±1.29
(1,2) |
18.94± 1.63
(1,2) |
Values represent mean of six animals ± SE.
Significant change at P < 0.05. LSD: Least significance difference.
Table 6: Effect Suaeda Sp. extract on immune response in rat treated with over.
| Parameter Group name | 1-Clean control | 2- Positive control | 3– S. monica Pre- treated | 4– S. pruinosa Pre -treated | 5– S. monicaPost-treated | 6– S. pruinosa Post-treated |
| IL-1β (Pg/ml) | 29.00± 1.58
(2,3,4,5,6) |
93.46± 2.78
(1,3,4,5,6) |
77.17± 1.74
(1,2,5,6) |
71.55± 2.49
(1,2,5,6) |
54.65± 2.24
(1,2,3,4) |
54.89± 1.92
(1,2,3,4) |
| TNF (Pg/ml) | 17.79± 1.18
(2,3,4,5,6) |
45.46± 1.63
(1,3,4,5,6) |
38.251± 1.77
(1,2,5,6) |
39.53± 2.08
(1,2,5,6) |
30.94± 1.59
(1,2,3,4) |
30.44± 2.38
(1,2,3,4) |
Values represent mean of six animals ± SE.
Significant change at P < 0.05. LSD: Least significance difference.
Histopathological Studies
At the end of the experiment, and after blood samples were obtained liver samples were taken, washed with normal saline and processed for histopathological study. Initially the materials were fixed in 10% buffered neutral formalin and paraffin sections were taken at 5 µm thickness processed in alcohol-xylene series and was stained with alum hematoxylin and eosin, then were examined for histopathological changes.
The microscopic examination of liver of control rats shows the normal hepatic lobules and portal areas structure. The central vein is surrounded by the hepatocytes with eosinophilic cytoplasm and distinct nuclei. The hepatic sinusoids are shown between the hepatocytes (Fig. 1.A, B).
Histopathological investigations of liver of rat administered a single dose of paracetamol (3 g/kg/b.w) showed widespread swelling and ballooning degeneration and focal necrosis that associated with inflammatory infiltration in the hepatocytes (Fig. 1.C). In some rats dilated and congested portal tracts (Fig. 1.D).
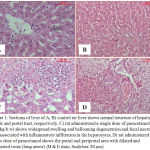 |
Figure 1: Sections of liver of A, B) control rat liver shows normal structure of hepatic lobule and portal tract, respectively, C) rat administered a single dose of paracetamol (2 g/kg/b.w) shows widespread swelling and ballooning degeneration and focal necrosis that associated with inflammatory infiltration in the hepatocytes, D) rat administered a single dose of paracetamol shows the portal and periportal area with dilated and congested veins (long arrow) (H & E stain, Scale bar: 20 µm).
|
In rats given Sum extract and paracetamol, examination showed the hepatic lobules that appeared more or less like control (Fig. 2.A). In somerats, hydrobic degeneration and congested portal tracts were shown (Fig. 2.B).Histopathological investigation of liver of rats given Sup extract and paracetamol showed the hepatic lobules that appeared more or less like control (Fig. 2.C).In somerats hydrobic degeneration and congested portal tracts were noticed (Fig. 2.D).
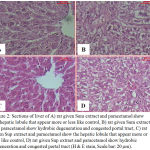 |
Figure 2: Sections of liver of A) rat given Sum extract and paracetamol show the hepatic lobule that appear more or less like control, B) rat given Sum extract and paracetamol show hydrobic degeneration and congested portal tract, C) rat given Sup extract and paracetamol show the hepatic lobule that appear more or less like control, D) rat given Sup extract and paracetamol show hydrobic degeneration and congested portal tract (H & E stain, Scale bar: 20 µm).
|
Sections of liver of rats given paracetamol and Sum, and Sup extracts showed the hepatic lobules that appeared more or less like control (Fig. 3A, B). But in rats given paracetamol and Sup extract few foci of necrosis in the hepatocytes were showed (Fig. 3.B).
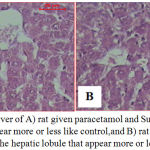 |
Figure 3: Sections of liver of A) rat given paracetamol and Sum extract show the hepatic lobule that appear more or less like control,and B) rat given paracetamol and Sup extract show the hepatic lobule that appear more or less like control.
|
Notice few foci of necrosis in the hepatocytes (H & E stain, Scale bar: 20 µm).
Examination of control liver sections stained by PAS reaction shows the abundance of glycogen in the cell of hepatic lobule. The glycogen particles appear accumulated at one side of the cytoplasm leaving the other side almost devoid of such materials (Fig. 4.A). Histochemical investigations of liver of rats administered a single dose of paracetamol dose (3 g/kg body weight) and were sacrificed after 24 h showed markeddiminution in glycogen distribution in the hepatocytes as compared with control group(Fig. 4.B). Examination of sections of liver that stained with PAS in rat orally given paracetamol after 7 days of Sum and Sup plants extracts administration (prophylactic groups, 100 mg/kg b.w) indicated an increase in glycogen contents as compared with paracetamol only. The distribution of this inclusion was more or less like normal control (Fig. 4C, D). On the other hand, in case of therapeutic groups in which paracetamol was conducted once before Sum and Sup plant extracts administration (100 mg/kg b.w) daily for 7 days showed heterogeneous distribution of glycogen, where the healthy zones exhibited normal distribution and the injured zones exhibited a decrease in this inclusion (Fig. 4E, F).
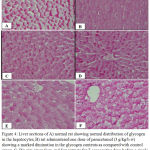 |
Figure 4: Liver sections of A) normal rat showing normal distribution of glycogen in the hepatocytes, B) rat administered one dose of paracetamol (3 g/kg/b.w) showing a marked diminution in the glycogen contents as compared with control group, C, D): rats given Sum and Supextracts for 7 consecutive days before a single dose paracetamol showing glycogen contents in the hepatic lobule appeared more or less like control, E, F) rats given Sum or Supextracts for successive days before paracetamol showing heterogeneous distribution of glycogen.
|
The healthy zones exhibite normal distribution and the injured zones exhibite a decrease in this inclusion (PAS stain, Scale bar: 20 μm).
Biochemical Studies
Liver regulates many important metabolic functions and contains a host of enzymes. Any injury in this organ causes distortion of these metabolic functions. The present work aimed to compare the possible hepatoprotective impact of Suaeda Sp. administered for one week prior to and after a single acetaminophen toxic dose (3g/kg body weight) in male rats.
The present study showed damage of liver due to paracetamol over dosage which is confirmed by elevated levels of all biochemical parameters measured (SGPT, SGOT, ALP, TB, urea, creatinine, IL1, TNF and MDA). The study also showed decrease in POX enzyme. These elevated enzymatic activities in serum, are indicators of cellular leakage and loss of functional integrity of cell membrane in liver.35 Aqueous extract of S. monoica and S. peruinosa contain alkaloids, terpenoids, steroids, tannins, quinones, saponins, flavonoids and phenols. They can scavenge off free radicals which are the principles for the hepatoprotective activity for protection or therapeutic effect. Decreased serum parameters level following extract treatment indicated the effectiveness of the extract in restoring normal functional status of the liver.
Suaeda had successfully reversed the PCM-induced hepatotoxic effect by its ability to reduce the elevated levels of ALT, AST, and ALP suggesting that these biochemical restorations could be due to the extract ability to inhibit the cytochrome P450 or/and ability to promote the PCM glucuronidation.36-37 Furthermore, the ability to lower the enzymes level can be associated with the ability of Suaeda to prevent lipid peroxidation of endoplasmic reticulum that is rich in polyunsaturated fatty acid by disrupting the binding of activated radicals to the macromolecules. This process can possibly be achieved via the antioxidant activity of Suaeda extracts due to the presence of high amount of α linolenic and linoleic fatty acid.38 Besides, mechanisms of protection that can take place include activation of liver regeneration by enhancing the protein and glycoprotein synthesis or accelerated detoxification and excretion,39 prevention of lipid peroxidation process, and stabilization of hepatocellular membrane.38 The two Suaeda extracts contain methionine and cysteine amino acids which act as -SH donner in synthesis of GSH and prevent APAP metabolite to interact with macromolecules. The results are confirmed with Di Pierro and Rossoni, (2013) who found that a mixture of L-cysteine, L-methionine, and L-serine was effective at a lower dose than N- acetyl cysteine (NAC) in APAP over dose and improved mouse survival rates. Moreover, the mixture significantly reduced ALT level and induced hepatic GSH synthesis and decrease MDA accumulation.40
Increase in serum level of ALP is due to increased synthesis in the presence of increasing biliary pressure41 and reflects the pathological alteration in biliary flow.42
Bilirubin, a metabolic product of hemoglobin, undergoes conjugation with glucuronic acid in hepatocytes to increase its water solubility. Determination of serum bilirubin represents an index for assessment of hepatic function, and any abnormal increase in the levels of serum bilirubin indicates hepatobiliary diseases and severe disturbance of hepatocellular function.43Decreased serum bilirubin level following extract treatment indicated the effectiveness of the extract in restoring normal functional status of the liver.
Paracetamol-induced toxicity in rats may have altered membrane structure and function as well as lipids metabolism in the liver.
Therefore, it is possible to propose that the extract/compounds exerting an anti-inflammatory activity might also demonstrate hepatoprotective activity. Interestingly, this is supported by the study of Gupta et al. 2006 who suggested that the combination of hepatoprotective effect and antioxidant activity synergistically prevents the process of initiation and progression of hepatocellular injury in prophylactic and therapeutic treatments.44
Vanillin, a phenolic aldehyde has been reported to possess antioxidant and free radical scavenging ability which could possibly account for the hepatoprotective property of this plant. The activities of antioxidant counteract the redox state precipitated intracellularly and hence ensure hepatoprotection against paracetamol-induced liver injury.45-47 The antioxidant activity of this extract may also explain the mechanism of the hepatoprotective activity of Suaeda sp. The findings of this study corroborate the effect that was reported for Homalium zeylanicum.48
Lipid peroxidation (LPO) is one of the characteristic features of oxidative stress in PCM hepatotoxicity. According to Luqman and Rizvi (2006), LPO is known to injure the cells by inactivation of membrane enzymes, decrease fluidity of the membrane and release, into cytotoxic, aldehydes such as MDA.49 The elevated level of hepatic MDA has been regarded as an indicator of cellular damage and indicates the inability of the antioxidant defense system to protect against the production of excessive free radicals. In this study, MDA increased and POX decreased as a result of paracetamol over dose. The enhanced antioxidant defense in addition to reduced LPO product in the liver is indicative for the antioxidant effect of the tested extracts.
The biochemical results were supported by the histopathological findings. The results demonstrated that rats exposed to PCM alone showed severe cellular damage that might be due to the production of free radicals by PCM and subsequent LPO. These histopathologic findings were ameliorated significantly in the groups of rat wither were pre-treated or treated with either S. monoica or S. peruisma extracts, indicating pronounced protection of hepatocytes against PCM induced hepatic damage. It can be referred to the antioxidant effect of the tested extracts that markedly decreased the oxidative stress and thereby reduced the histopathological alterations of the liver.
The present study found that two Suaeda contain cysteine and Methionine with excessive amount which considered as -SH donner for GSH synthesis. Di Pierro and Rossoni (2013) found that DDM-GSH ( amixture of L-cysteine, L-methionine, and L-serine in a weight ratio of 2:1:1) was effective at a lower dose thanN-acetylcysteine (NAC) in reducing APAP-induced hepatotoxicity; improving mouse survival rates, decreasing sALT and MDA accumulation and increase hepatic GSH synthesis in the mouse model.40
Suaeda sp. may inhibit the activity of certain cytochrome P450 enzymes whose inhibition could safeguard against APAP hepatotoxicity.37 With regard to the potential hepatoprotective effects of Suaeda, it was demonstrated that rats which were chronically supplemented with Suaeda extract (100 mg/kg) and APAP (3 g/kg ) exhibited an improvement in APAP-induced liver necrosis and increased serum ALT and AST levels.50 In the present study, paracetamol hepatotoxicity was associated with an increase in serum level of IL1β.Similar results were reported by other investigators.51-52
Signaling through the IL-1 receptor (IL-1R) was recently shown to play an important role in paracetamol-induced hepatotoxicity.53 Activation of NF-kB/Rel transcription factor leads to the increased production of various inflammatory mediators, including IL1 β and TNF-α.54
Activation of Toll-like receptors (TLRs) by pathogen-associated molecular patern results in upregulation of pro- IL1 β via NF-kB pathway. This is followed by a second signal that results in a caspase 1 mediated cleavage of pro- IL1β to release the active molecule 55.
Pretreatment with studied extracts decreased serum IL1 β and TNF-α in rats subjected to paracetamol hepatotoxicity. It was suggested that the two extracts have inhibitory effect on NF-kB/Rel activity in rat serum.56
In summary, our present study demonstrated that S. monoica and S. peruisma possessed a protective action against paracetamol-induced liver injury. The underlying mechanisms of their hepatoprotective activity might be attributed to the presence of the amino acids, unsaturated fatty acids and phenolic compounds. These encouraging findings would be helpful for developing potential hepatoprotective agents for remedying APAP-induced liver injury.
References
- Cybulska I., Brudecki G., Alassali A., Thomsen M., Brown J. J. Phytochemical composition of some common coastal halophytes of the United Arab Emirates. Emirates journal of food and agriculture. 2014;26(12):1046-57.
CrossRef - Sage R. F., Sultmanis S. Why are there no C4 forests? Journal of plant physiology. 2016;20;203:55-68.
CrossRef - El-Shaer H. M., El-Morsy M. H. Potentiality of salt marshes in Mediterranean coastal zone of Egypt. InBiosaline Agriculture and High Salinity Tolerance. Birkhäuser Basel. 2008;207-219.
CrossRef - Choi S. C., Lim S. H., Kim S. H., Choi D. G., Kim J. G., Choo Y. S. Growth and solute pattern of Suaeda maritima and Suaeda asparagoides in an abandoned salt field. Journal of Ecology and Environment. 2012;35(4):351-8.
CrossRef - Ghosh A., Misra S., Dutta A. K., Choudhury A. Pentacyclic triterpenoids and sterols from seven species of mangrove. Phytochemistry. 1985;24(8):1725-7.
CrossRef - Subramanyam C., Rao K. B., Rao C. V., Rao B. V. Chemical examination of Suaeda monoica and Suaeda maritima. Acta Cienca Indica (C). 1992;18:7-8.
- Premnathan M., Chandra K., Bajpai S. K., Kathiresan K. A survey of some Indian marine plants for antiviral activity. Botanica marina. 1992;35(4):321-324.
CrossRef - Qasim M., Gulzar S and Ajmal M. K. Halophytes as Medicinal Plants, Chapter: Institute of Sustainable Halophyte Utilization, University of Karachi, Pakistan. 2011;21:336.
- Boulos L. Flora of Egypt,Cairo: Al Hadara Publishing. 1999;1:417.
- Al-Hassan H. O. Wild plants of the Northern Region. Camel and range research center in collaboration with FAO, Al-Jouf. 2006.
- BOLòS O. D. E & VIGO J. Notes sobre taxonomia i nomenclatura de plantes, I. But. Inst. Cat. Hist. Nat. 1974;38:61-89.
- Bandaranayake W. M. Traditional and medicinal uses of mangroves. Mangroves and salt marshes. 1998;2(3):133-48.
CrossRef - Padmakumar K., Ayyakkannu K. Antiviral activity of marine plants. Indian Journal of Virology. 1997;13(1):33-6.
- Ratnasooriya W. D., Pathirana R. N., Dissanayake A. S., Samanmali B. L., Desman P. K. Evaluation of invitro sun screen activities of salt marshy plants Suaeda monoica, Suaeda maritima and Halosarcia indica. International Journal of Pharmaceutical Research & Allied Sciences. 2016;5(2):15-20.
- Suryanarayanan T. S., Venkatesan G., Murali T. S. Endophytic fungal communities in leaves of tropical forest trees: diversity and distribution patterns. Current Science. 2003;25:489-93.
- Lakshmanan G., Rajeshkannan C., Kavitha A., Mekala B., Kamaladevi N. Preliminary screening of biologically active constituents of Suaeda monoica and Sesuvium portulacastrum from palayakayal mangrove forest of Tamilnadu. J. Pharmacog. Phytochem. 2013;2(3):149-52.
- Marur C. J., Sodek L. A., Magalhães A. C. Free amino acids in leaves of cotton plants under water deficit. Revista Brasileira de Fisiologia Vegetal. 1994;6(2):103-8.
- Folch J., Lees M.,Stanley G. H. S. A simple method for the isolation and purification of total lipides from animal tissues. J biol Chem. 1957;226(1):497-509.
- Benson J. R., Hare P. E. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proceedings of the National Academy of Sciences. 1975;72(2):619-22.
CrossRef - Jarrett H. W., Cooksy K. D., Ellis B., Anderson J. M. The separation of o-phthalaldehyde derivatives of amino acids by reversed-phase chromatography on octylsilica columns. Analytical Biochemistry. 1986;153(1):189-98.
CrossRef - Einarsson S., Josefsson B., Lagerkvist S. Determination of amino acids with 9-fluorenylmethyl chloroformate and reversed-phase high-performance liquid chromatography. Journal of Chromatography A. 1983;282:609-18.
CrossRef - Näsholm T., Sandberg G., Ericsson A. Quantitative analysis of amino acids in conifer tissues by high-performance liquid chromatography and fluorescence detection of their 9-fluorenylmethyl chloroformate derivatives. Journal of Chromatography A. 1987;396:225-36.
CrossRef - Reitman S., Frankel S. Calorimetric method for the determination of blood aminotransferase enzymatic activities. Am. J. Clin. Pathol. 1957;28:56-63.
CrossRef - Demetriou J. A., Drewes D. A., Gin J. B. Enzymes: In: Clinical chemistry, principles and technics 2nd. Henry RJ, Cannon DC, Winkelman JW. Eds.
- Young D. S. Effects of drugs on clinical laboratory tests. AACC press. 2000.
- Bartels H., Bohmer M. Microestimation of creatinine. Clinica Chimica Acta. 1971;32:81-5.
- Tabacco A., Meiattini F., Moda E., Tarli P. Simplified enzymic/colorimetric serum urea nitrogen determination. Clinical chemistry. 1979;25(2):336-7.
- Higashino K., Takahashi Y., Yamamura Y. Release of phenyl acetate esterase from liver microsomes by carbon tetrachloride. Clinica Chimica Acta. 1972;41:313-20.
CrossRef - Watson A. D., Berliner J. A., Hama S. Y., La Du B. N., Faull K. F., Fogelman A. M., Navab M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. The Journal of clinical investigation. 1995;96(6):2882-91.
CrossRef - Ruiz-Larrea M. B., Leal A. M., Liza M., Lacort M., de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59(6):383-8.
CrossRef - Durry R., Wallington E. Carlton′s Histological Techniques. 5th edition. Durry and Wallington edition. Oxforduniversity press. London and New York 1980;93-99.
- McManus J. F. Histological demonstration of mucin after periodic acid. Nature. 1946;158(4006):202.
CrossRef - Tona L., Kambu K., Ngimbi N., Cimanga K., Vlietinck A. J. Antiamoebic and phytochemical screening of some Congolese medicinal plants. Journal of Ethnopharmacology. 1998;61(1):57-65.
CrossRef - Browse J., Mc Court P. J., Somerville C. R. Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Analytical biochemistry. 1986;152(1):141-5.
CrossRef - Rajesh M. G., Latha M. S. Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. Journal of Ethnopharmacology. 2004;91(1):99-104.
CrossRef - Porchezhian E., Ansari S. H. Hepatoprotective activity of Abutilon indicum on experimental liver damage in rats. Phytomedicine. 2005;12(1-2):62-4.
CrossRef - Ebada M. E. Essential oils of green cumin and chamomile partially protect against acute acetaminophen hepatotoxicity in rats. Anais da Academia Brasileira de Ciências. 2018;90(2):2347-2358.
CrossRef - Mujeeb M., Aeri V., Bagri P., Khan S. A. Hepatoprotective activity of the methanolic extract of Tylophora indica (Burm. f.) Merill. leaves. International Journal of Green Pharmacy (IJGP). 2009;3(2).
CrossRef - Kumar G., Banu G. S., Pappa P. V., Sundararajan M., Pandian M. R. Hepatoprotective activity of Trianthema portulacastrum L. against paracetamol and thioacetamide intoxication in albino rats. Journal of Ethnopharmacology. 2004;92(1):37-40.
CrossRef - Di Pierro F., Rossoni G. An amino acids mixture improves the hepatotoxicity induced by acetaminophen in mice. Journal of amino acids. 2013;2013.
- Muriel P., Garciapiña T., Perez‐Alvarez V., Mourelle M. Silymarin protects against paracetamol‐induced lipid peroxidation and liver damage. Journal of Applied Toxicology. 1992;12(6):439-42.
CrossRef - Plaa G. L., Charbonneau M., Plante I. Detection and evaluation of chemically induced liver injury. In Hayes’ Principles and Methods of Toxicology CRC Press. 2014;10:1472-1515.
- Martin P. Assessment of liver function and diagnostic studies. H and book of liver disease. 1992:1-4.
- Gupta A. K., Chitme H., Dass S. K., Misra N. Hepatoprotective activity of Rauwolfia serpentina rhizome in paracetamol intoxicated rats. J Pharmacol Toxicol. 2006;1(1):82-8.
CrossRef - Kamat J. P., Ghosh A., Devasagayam T. P. Vanillin as an antioxidant in rat liver mitochondria: inhibition of protein oxidation and lipid peroxidation induced by photosensitization. Molecular and cellular biochemistry. 2000;209(1-2):47-53.
CrossRef - Kumar S., Priyadarsini K. I., Sainis K. B. Free radical scavenging activity of vanillin and o-vanillin using 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical. Redox Report. 2002;7(1):35-40.
CrossRef - Lirdprapamongkol K., Kramb J. P., Suthiphongchai T., Surarit R., Srisomsap C., Dannhardt G., Svasti J. Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. Journal of Agricultural and Food Chemistry. 2009; 57(8):3055-3063.
CrossRef - Shashank T., Rajkiran E., Nusrath Y., Sujatha K., Vishal K. Evaluation of hepatoprotective activity of stem bark of Homalium Zeylanicum in Rats. Int J Pharm Tech Res. 2011;3(3):1630-4.
- Luqman S., Rizvi S. I. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2006;20(4):303-6.
CrossRef - Rehman J. U., Saqib N. U., Akhtar N., Jamshaid M., Asif H. M., Sultana S., Rehman R. U. Hepatoprotective activity of aqueous-methanolic extract of Suaeda fruticosa in paracetamol-induced hepatotoxicity in rabbits. Bangladesh Journal of Pharmacology. 2013;8(4):378-381.
CrossRef - Imaeda A. B., Watanabe A., Sohail M. A., Mahmood S., Mohamadnejad M., Sutterwala F. S., et al. Acetaminophen-induced hepatotoxicity in mice dependent on Th9 and the NaL p3 inflammasome. J Clin Invest. 2009;119:305–14.
- Stirnimann G., Kessebohm K., Lauterburg B. Liver injury caused by drugs: an update. Swiss Med Wkly. 2010;140:13080.
CrossRef - Chen C. J., Kono H., Golinbock D., Reed G., Akira S., Rock K. L. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851– 856.
CrossRef - Hiscott J., Marois J., Garoufalis J., D’Addario M., Roulston A., Kwan I., et al. Characterization of a functional NF-kappa B site in the humaninterleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol . 2006;13:6231–6240.
CrossRef - Mariathasan S., Newton K., Monack D. M., Vucic D., French D. M., Lee W. P., et al., Differential activation of the inflammsome by caspase 1 adaptors ASC and Ipaf. Nature. 2004;430:213–218.
CrossRef - Manna S. K., Mukhopadhyay A., Van N. T., Aggarwal B. B. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase and apoptosis. J Immunol. 1999;163:6800–6809.







