Omar M. E. Abdel-Salam1 , Amany A. Sleem2, Marawan Abd El-Baset Mohamed Sayed2, Eman R. Youness3
, Amany A. Sleem2, Marawan Abd El-Baset Mohamed Sayed2, Eman R. Youness3 and Nermeen Shaffie4
and Nermeen Shaffie4
1Department of Toxicology and Narcotics, National Research Centre, Cairo, Egypt.
2Department of Pharmacology, National Research Centre, Cairo, Egypt.
3Department of Medical Biochemistry, National Research Centre, Cairo, Egypt.
4Department of Pathology, National Research Centre, Cairo, Egypt.
Corresponding Author E-mail: omasalam@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1610
Abstract
Anandamide (N-arachidonoylethanolamine) is an endogenous cannabinoid receptor CB1 ligand that exhibits neuroprotective effects in the brain. In this study, the effect of exogenously given anandamide on pentylenetetrazole (PTZ)-induced chemical kindling oxidative stress and brain damage in rats was studied. Rats were intraperitoneally (i.p.) injected with 35 μg/kg PTZ once every 48 hours for 12 times to induce seizures. Anandamide was i.p. given. 30 min prior to PTZ injection at 100 or 200 μg/kg. Injections of PTZ induced significant increase in brain lipid peroxidation (malondialdehyde: MDA), and nitric oxide associated with marked decrease in brain reduced glutathione (GSH). There were also significant decrements in acetylcholinesterase (AChE) concentration, butyrylcholinesterase (BChE) and paraoxonase-1 (PON-1) activities in brain tissue of PTZ injected rats. Meanwhile, there was no significant effect for PTZ on the concentration of brain neutrophil elastase. Anandamide administered at 100 and 200 μg/kg significantly decreased MDA and increased GSH contents and at 200 μg/kg significantly decreased nitric oxide in brain of PTZ-treated rats. The drug also caused significant increments in AChE concentration and PON-1 activity but had no significant effect on BChE or neutrophil elastase in rats treated with PTZ. Anandamide given at the dose of 200 μg/kg significantly decreased the mean seizure scores over the study period by 22.3% and the frequency of myoclonic jerks and rearing (stage 3) by 56.7% compared with the vehicle-treated group. Anandamide given at 100 and 200 μg/kg completely inhibited the development of generalized tonic-clonic seizures (stage 5). It is concluded that in the PTZ-induced seizures, the cannabinoid receptor CB1 agonist anandamide decreases brain oxidative stress, neuronal injury, and exerts an antiepileptic activity.
Keywords
Anandamide; Endocannabinoids; Kindling; Neurodegeneration; Oxidative Stress; Pentylenetetrazole
Download this article as:| Copy the following to cite this article: Abdel-Salam O. M. E, Sleem A. A, Sayed M. A. E. M, Youness E. R, Shaffie N. Neuroprotective Effects of Low Dose Anandamide in Pentylenetetrazole-Induced Kindling in Rats. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Abdel-Salam O. M. E, Sleem A. A, Sayed M. A. E. M, Youness E. R, Shaffie N. Neuroprotective Effects of Low Dose Anandamide in Pentylenetetrazole-Induced Kindling in Rats. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2Fjr7ku |
Introduction
Cannabis sativa L. (family Cannabidaceae) is well known for its recreational uses. The principal psychoactive constituent in cannabis was determined to be the cannabinoid Δ9-tetrahydrocannabinol (Δ9-THC).1 Other cannabinoids eg., cannabidiol, cannabigerol, Δ9-tetrahydrocannabivarin, cannabidivarin and cannabichromene are also present.2 These are not psychoactive and displays anti-inflammatory3,4 and neuroprotective effects.5 Cannabis-based medicines have been advocated for the treatment of a number of neurological disorders such as Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, Tourette’s syndrome, multiple sclerosis, and Chorea.6,7 Sativex, an oromucosal spray of whole plant extract containing Δ9-THC and cannabidiol (CBD) [THC:CBD=1:1] is in use in the UK, New Zealand, and Spain to treat spasticity in multiple sclerosis.7 Cannabis preparations are also being used to treat epilepsy. In their study, Hamerle et al.8 found that 63/310 (20.3%) of their sample of epilepsy outpatients have consumed cannabis after the diagnosis of epilepsy. In a study by Sulak et al.,9 epilepsy patients using cannabis mostly reported the use of cannabidiol-enriched artisanal preparations. Δ9-THC and tetrahydrocannabinolic acid (THCA) might be used in addition. There are also data to suggest a benefit from the use of cannabis to treat refractory epilepsy in children.10 In surveys on the use of oral cannabis extracts in these children, parents reported a decrease in seizure frequency or even complete absence of seizures on treating their children with cannabidiol-enriched extracts.11,12 There are in addition case reports suggesting a favorable impact on seizure frequency from using cannabidiol-enriched strain of cannabis in Dravet syndrome13 or pure cannabidiol in an infant with malignant migrating partial seizures.14 Cannabidiol-enriched extracts, are however, not without side effects and somnolence, fatigue, convulsions and status epilepticus have been reported among others.11,12
In preclinical models of epilepsy, a proconvulant effect has been reported for cannabis extracts rich in Δ9-THC in PTZ-epileptic seizures in rats.15 In mice, Δ9-THC increased kindling caused by picrotoxin, PTZ or electroshock.16 Δ9-THC or the synthetic cannabinoid agonist JWH-018 caused the development of electrographic seizures in mice via CB1-receptor mechanism.17 In contrast, cannabidiol decreased seizures induced by PTZ in mice18 while a cannabidivarin-cannabidiol extract showed anticonvulsant effect in PTZ-induced seizures and suppressed the pilocarpine convulsions.19
Cannabinoids exert their effects by acting on the cannabinoid CB1 and CB2 receptors. CB1 receptors are found principally in the brain on presynaptic neuronal membranes and modulate the release of neurotransmitters and are also present on brain astrocytes. CB2 receptors on the other hand are primarily found in the periphery on immune cells and tissues.20 There exist also endogenous cannabinoids eg., arachidonoyl ethanolamide or anandamide and arachidonoylglycerol. These are produced on demand by enzymatic cleavage of membrane lipid precursors. The endocannabinoid anandamide binds with high affinity to CB1 receptors and thus is expected to mimic the exogenously given Δ9-THC in its pharmacological actions.21 It also binds to CB2 receptors acting as partial agonist or antagonist.22,23 Anandamide exerted neuroprotective effect in models of neuronal excitotoxicity24-26 via CB1 receptor mechanism and showed antioxidant effects in hippocampal neuron cells exposed to hydrogen peroxide in vitro.27 Anandamide is subject to hydrolysis by fatty acid amide hydrolase yielding arachidonic acid and ethanolamine.28 It has been shown that decreased degradation of the endenogenous cannabinoids by inhibition of the enzyme fatty acid amide hydrolase, exerted anti-convulsive effect and reduced EEG epileptiform activity induced by PTZ in rats.29 Moreover, treatment with fatty acid amide hydrolase inhibitor was found to abolish seizures induced by cocaine in mice.30 This suggests that endogenous cannabinoids increase seizure threshold.
In this study, the effect of anandamide on brain oxidative stress and the development of seizures in rats subjected to PTZ-kindling was investigated. We in addition examined the effect of anandamide on the histopathological changes induced by PTZ in brain.
Materials and methods
Animals
Male Sprague-Dawley rats weighing 180-200 g were used in the study. Rats were group-housed under temperature- and light-controlled conditions and allowed standard laboratory rodent chow and water ad libitum. The experiments were done at 9 O’clock to avoid changes in circadian rhythm. The study was done in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85–23, revised 1985) and the institutional ethics committee. Seven to eight rats were used per group.
Drugs and Chemicals
Pentylenetetrazole (PTZ) and anandamide (N-arachidonoylethanolamine) were purchased from Sigma (St. Louis, MO, USA). Other chemicals were from Sigma.
Study Design
Rats were randomly divided into three groups (7-8 rats each). Rats were treated with saline (group 1), PTZ at 35 μg/kg, i.p once every 48 hours for 12 times alone (group 2) or together with anandamide at 100 and 200 µg/kg i.p given 30 min prior to PTZ injection (groups 3 & 4, respectively). Seizures were recorded for 20 minutes. Two hours after the last PTZ injection, rats were quickly euthanized by decapitation, their brains removed on ice cold glass plate, stored at -80°C until the biochemical assays. One half of each brain was kept in 10% formol saline for histopathological processing.
Biochemical Assays
Determination of Lipid Peroxidation
Malondialdehyde (MDA), a product of lipid peroxidation was determined in tissue homogenates according to the method of Nair and Turne.31 In this assay thiobarbituric acid reactive substances (TBA) react with thiobarbituric acid to form TBA-MDA adduct which can be measured colorimetrically at 532 nm.
Determination of Nitric Oxide
Nitric oxide was determined using colorimetric assay where nitrate is converted to nitrite via nitrate reductase. Griess reagent then act to convert nitrite to a deep purple azo compound that can be determined using spectrophotometer.32
Determination of Reduced Glutathione
Reduced glutathione (GSH) was determined in tissue homogenates using the procedure of Ellman et al.33 The assay is based on the reduction of Ellman´s reagent (DTNB; 5, 5’-dithiobis (2-nitrobenzoic acid)) by the free sulfhydryl group on GSH to form yellow colored 5-thio-2-nitrobenzoic acid which can be determined using spectrophotometer at 412 nm.
Determination of Acetylcholinesterase
Acetylcholinesterase (AChE) concentration was determined in supernatants using an ELISA kit purchased from NOVA (Bioneovan Co., Ltd, DaXing Industry Zone, Beijing, China) according to the manufacture instructions.
Determination of Butyrylcholinesterase
Butyrylcholinesterase (BchE) activity in the brain tissue supernatants was determined by a colorimetric method using butrylcholinesterase diagnostic kit (Biodiagnostic, Egypt). In this assay, BChE hydrolyzes butyrylcholine to butyrate and thiocholine. The latter reacts with 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) forming 5-mercapto-2-nitrobenzoic acid (5-MNBA). The rate of 5-MNBA formation, measured spectrophotometrically, is proportional to the enzymatic activity of BChE in the sample.
Determination of Paraoxonase-1
The arylesterase activity of PON1 was determined by a colorimetric method using phenyl acetate as a substrate. In this assay, PON1 catalyzes the cleavage of phenyl acetate resulting in phenol for-mation. The rate of formation of phenol was meas-ured by monitoring the increase in absorbance at 270 nm at 25°C. The working mix consisted of 20 mM Tris/HCl buffer, pH 8.0, containing 1 mM CaCl2 and 4 mM phenyl acetate as the substrate. Samples diluted 1:3 in buffer were added to the above mix and the changes in absorbance were recorded following a 20 s lag time. One unit of arylesterase activity is equal to 1 μmole of phenol formed per minute. The PON1 activity is expressed in kU/L, based on the extinction coefficient of phenol of 1310 M‒1cm‒1. Blank samples containing water were used to correct for the spontaneous hydrolysis of phenyl acetate.34
Determination of Neutrophil Elastase
The level of neutrophil elastase was determined using an ELISA kit purchased from NOVA (Bioneovan Co., Ltd, DaXing Industry Zone, Beijing, China) according to the manufacture instructions.
Chemical Kindling
The kindling procedure was induced by repeated i.p. injection of 35 μg/kg pentylenetetrazole (PTZ) (Sigma, St. Louis, USA). Pentylenetetrazole was given once every 48 hours for 12 times. After each PTZ treatment, rats were placed separately under glass funnels, and their convulsive behavior was recorded during individual observations for 20 minutes. The resultant seizure behaviors were classified into 5 stages and scored as follows: 0: no response; 1: ear and facial twitching; 2: convulsive waves through the body; 3: myoclonic jerks, rearing; 4: turn over onto one side position; 5: turn over onto back position, generalized tonic-clonic seizures.35 The daily average seizure scores and the total scores over the study period were determined and compared between the PTZ control and anandamide groups. In addition, the mean total scores for each stage, the mean number of incidents of each stage (frequency of events in each stage) as well as the % of seizure free animals in each stage for all groups were determined.
Histopathological Studies
Brain samples of all animals were dissected immediately after death. The specimens were then fixed in 10 % neutral-buffered formalin saline for 72 hours at least. All the specimens were washed in tap water for half an hour and then dehydrated in ascending grades of alcohol, cleared in xylene and embedded in paraffin. Serial sections of 5μm thick were cut and stained with haematoxylin and eosin for histopathological investigation.
Quantitative Assessment
10 fields for each section were investigated and counted for total cells in field and cells that show degenerative signs such as pyknotic nuclei for cerebral cortex and hippocampus and flattened atrophied cells in substantia nigra. The mean of cells and percentage of damaged cells were calculated.
Statistical Analysis
Results are expressed as mean ± SEM. The results of the biochemical assays analyzed using One Way ANOVA and Duncan’s multiple range test. Data of the behavioral study were analyzed by Kruskal-Wallis test using Graphpad Prism software, version 5 (inc., San Diego, USA). A probability value of less than 0.05 was considered statistically significant.
Results
Biochemical Results
Lipid Peroxidation
Rats treated with only pentylenetetrazole (PTZ) exhibited significant increase in brain malondialdehyde (MDA) by 79.2% compared with the saline control group (32.8 ± 1.7 vs. 18.3 ± 0.67 nmol/g.tissue). Malondialdehyde decreased significantly by 21.9% and 33.2% in rats treated with PTZ and anandamide at 100 and 200 µg/kg, respectively (25.6 ± 0.9 and 21.9 ± 0.7 vs. 32.8 ± 1.7 nmol/g.tissue) (Fig. 1A).
Nitric Oxide
Pentylenetetrazole injections resulted in significantly increased brain nitric oxide content compared to the saline control value (41.9 ± 1.2 vs. 24.3 ± 2.1 µmol/g.tissue). Nitric oxide decreased significantly by 21.0% following treatment with 200 µg/kg anandamide (33.1 ± 1.8 vs. 41.9 ± 1.2 µmol/g.tissue) (Fig. 1B).
Reduced Glutathione
Significant decrease in brain reduced glutathione by 31.9% was observed in PTZ-treated rats compared to the saline control (2.8 ± 0.06 vs. 4.11 ± 0.1 µmol/g.tissue). Reduced glutathione increased significantly by 20.0% and 39.3% after treatment with 100 and 200 µg/kg anandamide, respectively (3.36 ± 0.09 and 3.9 ± 0.11 µmol/g.tissue) (Fig. 1C).
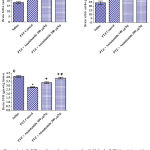 |
Figure 1: A-C. Effect of anandamide on malondialdehyde (MDA), nitric oxide, and reduced glutathione (GSH) in brain of rats subjected to pentylenetetrazole (PTZ)-induced kindling.
|
*: p< 0.05 vs. vehicle-treated group. +: p< 0.05 vs. PTZ control group. #: p< 0.05 vs. PTZ + anandamide 100µg/kg group.
Acetylcholinesterase
Repeated PTZ injections resulted in significant decrease in brain acetylcholinesterase (AChE) concentration by 69.3% relative to the saline control (0.98 ± 0.04 vs. 3.19 ± 0.08 ng/ml). Treatment with anandamide (100 and 200 µg/kg) significantly increased AChE by 85.7% and 203.1%, respectively (1.82 ± 0.08and 2.97 ± 0.19 vs. 0.98 ± 0.04 ng/ml) (Fig. 2A).
Butyrylcholinesterase
Significant inhibition of brain butyrylcholinesterase (BChE) activity by 23.0% was observed after PTZ injections (136.3 ± 6.5 vs. 177.0 ± 3.14 U/l). The administration of anandamide had no significant effect on BChE activity in PTZ-treated rats (Fig. 2B).
Paraoxonase-1
Pentylenetetrazole resulted n significant inhibition of paraoxonase-1 activity by 37.8% compared with the saline control value (7.62 ± 0.3 vs. 12.26 ± 0.79 kU/l). Paraoxonase-1 activity increased by 26.4% in rats treated with 200 µg/kg anandamide (9.63 ± 0.16 vs. 7.62 ± 0.3 kU/l) (Fig. 2C).
Neutrophil Elastase
No significant effect was observed for PTZ or PTZ + anandamide on brain neutrophil elastase concentration (Fig. 2D).
 |
Figure 2: A-D. Effect of anandamide on acetylcholinesterase, butyrylcholinesterase, paraoxonase-1 and neutrophil elatase in brain of rats subjected to pentylenetetrazole (PTZ)-induced kindling.
|
*: p< 0.05 vs. vehicle-treated group and between different groups as shown in the graph. +: p< 0.05 vs. PTZ control group. #: p< 0.05 vs. PTZ + anandamide 100µg/kg group.
Kindling Results
Compared with the PTZ only group, treatment with anandamide 200 μg/kg produced significant decrease of the mean seizure scores after the 6th, 7th, 8th and 9th PTZ injections during seizure development. This inhibitory effect of anandamide on the development of seizures, however, ceased by the 10th injection, suggesting the development of tolerance to some scores (Figure 3). Figure 4 shows the mean total seizure scores over the study period. The mean seizure score was 3.01 ± 0.24 in the PTZ only group, 2.61 ± 0.25 in the PTZ- anandamide (100 μg/kg) group and 2.34 ± 0.26 in the PTZ anandamide (200 μg/kg). The mean seizure score was significantly decreased by 22.3% in the PTZ + 200 μg/kg anandamide group with respect to the PTZ control group (p<0.05). Figure 5 shows that anandamide (200 μg/kg) significantly decreased the frequency of myoclonic jerks and rearing (stage 3) with respect to both the PTZ control and PTZ + 100 μg/kg anandamide by 56.75% and 52.85%, respectively. Also, the occurrence of generalized tonic-clonic seizures (stage 5) was completely inhibited by either dose of anandamide. Figure 6 shows that the administration of 200 μg/kg anandamide decreased the mean total score of myoclonic jerks and rearing (stage 3), with respect to both PTZ control and PTZ + 100 μg/kg anandamide by 63.84 % and 42.72 %, respectively. In addition, anandamide given at 100 μg/kg or 200 μg/kg completely inhibited the generalized tonic-clonic seizures (stage 5) as compared to the PTZ control group.
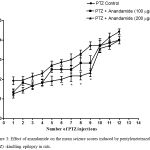 |
Figure 3: Effect of anandamide on the mean seizure scores induced by pentylenetetrazole (PTZ) -kindling epilepsy in rats.
|
Rats were i.p. injected with PTZ once every 48 hours for 12 times to induce seizures. Anandamide was i.p. given 30 min before each PTZ injection. Each point in the line represents the mean ± S.E. of 7-8 experiments. Data were analyzed by Kruskal-Wallis test. Asterisks indicate significant change from the PTZ control group (P <0.05).
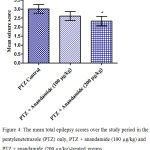 |
Figure 4: The mean total epilepsy scores over the study period in the pentylenetetrazole (PTZ) only, PTZ + anandamide (100 μg/kg) and PTZ + anandamide (200 μg/kg)-treated groups.
|
Rats were injected with i.p. pentylenetetrazole (PTZ) only or given PTZ with anandamide (30 minutes before PTZ). Data represents mean ± S.E. of 7-8 experiments. Data were analyzed by Kruskal-Wallis test. * P <0.05 vs. PTZ control.
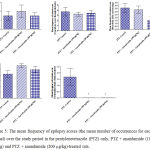 |
Figure 5: The mean frequency of epilepsy scores (the mean number of occurrences for each animal) over the study period in the pentylenetetrazole (PTZ) only, PTZ + anandamide (100 μg/kg) and PTZ + anandamide (200 μg/kg)-treated rats.
|
Each bar represents mean ± S.E. of 7-8 experiments. Kruskal-Wallis test. *P <0.05 vs. PTZ control. @P <0.05 vs. PTZ anandamide 100 μg/kg.
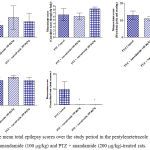 |
Figure 6: The mean total epilepsy scores over the study period in the pentylenetetrazole (PTZ) only, PTZ + anandamide (100 μg/kg) and PTZ + anandamide (200 μg/kg)-treated rats.
|
Each bar represents mean ± S.E. of 7-8 experiments. Kruskal-Wallis test. *P <0.05 vs. PTZ control. @P <0.05 vs. PTZ anandamide 100 μg/kg.
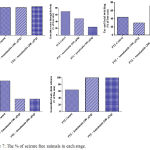 |
Figure 7: The % of seizure free animals in each stage.
|
Histopathologicl Results
Epilepsy induced by PTZ affected the hippocampal area as many of the neurons in this area became flattened with pyknotic nuclei, although there was no reduction in their number (Fig 8B) if compared with normal control section (Fig. 8A). The administration of anandamide at 100µg/kg did not ameliorate this damaging effect of PTZ (Fig. 8C). The higher dose, however, markedly improved the neurons in this area (Fig. 8D). Examination of cerebral cortex sections from saline-treated rats clarified the normal structure of this area (Fig. 9A). Epilepsy increased number of neurons that showed variable signs of degeneration (Fig. 9B). Low dose of anandamide did not ameliorate the damaged neurons (Fig. 9C) while the high dose markedly decreased the number of cells with signs of degeneration (Fig. 9D). Sections of substantia nigra area showed that injections of PTZ markedly decreased the size and number of pigmented neurons (Fig. 10B) if compared with sections from the saline-treated control (Fig. 10A). Anandamide ameliorated the damaging effect of epilepsy in a dose dependent manner (Fig. 10C & D).
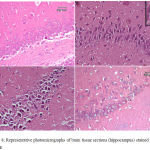 |
Figure 8: Representative photomicrographs of brain tissue sections (hippocampus) stained with Hx & E
|
(A) hippocampus of a control -ve rat. (B) Epileptic rat shows most of the hippocampal neurons are flattened with dark pyknotic nuclei. (C) Epileptic rat treated with anandamide at 100 µg/kg shows no noticeable amelioration of hippocampal area. (D) Epileptic rat treated with anandamide at 200 µg/kg shows most of the neurons appear normal with large rounded vesicular nuclei. Only a very few cells show pyknotic nuclei.
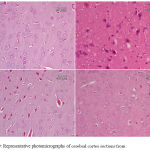 |
Figure 9: Representative photomicrographs of cerebral cortex sections from
|
(A) Saline control rat. (B) Epileptic (PTZ-treated) rat shows many neurons with dark pyknotic nuclei. (C) Epileptic rat treated with annandamide at 100 µg/kg shows many cells still show signs of degeneration in the form of pykotic nuclei. (D) Epileptic rat treated with annandamide at 200 µg/kg shows only a few cells with degenerative signs.
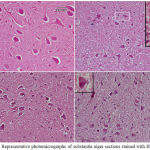 |
Figure 10: Representative photomicrographs of substantia nigra sections stained with Hx & E:
|
(A) Saline control rat. shows normal structure of substantia nigra area. (B) Epileptic (PTZ-treated) rat hows decrease in neurons’ number. The higher magnified part shows a karyolitic neuron (the upper one), while the lower one is atrophied with pyknotic nucleus. (C) Epileptic rat treated with annandamide at 100 µg/kg shows ) shows noticeable increase in size and number of substantia nigra neurons. (D) Epileptic rat treated with annandamide at 200 µg/kg shows shows close to normal structure of the area.
Quantitative Results
For normal cerebral cortex 7 cells only showed variable levels of damage out of 404 cells counted (1.7%), while in epileptic rat 176 cells showed signs of degeneration out of 537 cells (32.8%). Treating epileptic rats with anandamide at 200 µg/kg decreased the damaged cells to 96 out of 663 (14.4%). The lower dose of 100 µg/kg gave less effect as cells with degenerative signs were 148 out of 592 with (25%).
Results obtained from hippocampus clarified the effect of annandamide in treating epilepsy. Cells with damage signs in normal control rat were 4 out of 400 (1%), while the epileptic rat showed 112 out of 314 neurons (35.6%). Anandamide at 200 µg/kg decreased damaged cells to 60 out of 643 (9.3%). The low dose decreased the damaged cells to 95 out of 584 (16%).
In substantia nigra sections, the cells with signs of degeneration in control normal rat were 3 out of 72 (4.2%), while epileptic rat showed 57 damaged cells out of 131(43.5%). Anandamide at 200 µg/kg decreased the damaged cells to be 21 out of 98 (21.4%), while the low dose decreased the damaged cells to only 34 out of 110 (32%).
Table 1: Count and % of neurons with degenerative changes (deg. Neu) in PTZ-treated rats and the effect of anandamide.
| Cerebral cortex | Hippocampus | Substantia nigra | |||||||
| deg. Neu | total | % | deg. Neu | total | % | deg. Neu | total | % | |
| Saline | 7 | 404 | 1.7 | 4 | 400 | 1 | 3 | 72 | 4.2 |
| PTZ | 176 | 537 | 32.8 | 112 | 314 | 35.6 | 57 | 131 | 43.5 |
| PTZ + Anandamide 100 µg/kg | 148 | 592 | 25 | 95 | 584 | 16 | 35 | 110 | 32 |
| PTZ + Anandamide 200 µg/kg | 96 | 663 | 14.4 | 60 | 643 | 9.3 | 21 | 98 | 21.4 |
Discussion
The main findings in this study were that kindling induced in the rat by repeated injection of the epileptogen pentylenetetrazole (PTZ) resulted in brain oxidative stress, inhibition of the enzymes AChE, BChE, and PON-1 in brain. Histologicaly, PTZ induced neuronal injury in the cerebral cortex, hippocampus and also the substantia nigra, indicating widespread brain injury by the epileptogen. Anandamide, an endocannabinoid, administered prior to PTZ was able to decrease oxidative stress, nitric oxide levels and to increase AChE concentration and PON-1 activity. This occurred along with a suppressive effect for anandamide on the development of seizures by PTZ. Additionally, anandamide exerted marked neuroprotective effect as indicated by the histological examination of the brain tissue.
When injected into rats, PTZ led to oxidative stress as indicated by the increase in the level of the lipid peroxidation end product malondialdehyde (MDA), thereby, suggesting free radicals attack on cell membrane lipids.36 Significant decrease in reduced glutathione, an important brain antioxidant and a free radical scavenger37 was also found. These findings agree with previously published observations in the PTZ-induced kindling in the rat.15,38 Other researchers showed significant increase in lipid peroxidation products and decreased activities of the antioxidant enzymes Cu-Zn superoxide dismutase and catalase as well as decreased reduced glutathione levels in erythrocytes after a single injection of 50 μg/kg PTZ in the rat.39 Abbott et al.40 in addition, reported decreased concentrations of reduced glutathione in the occipital cortex in a genetic mouse model of epilepsy. Moreover, in their study, Pence et al.38 found that the increase in MDA in brain of rats treated with PTZ could be reduced by administering glutathione, which would suggest that reduced glutathione decreases in the brain of PTZ-treated rats due to consumption by increased free radicals. Increased brain lipid peroxidation and a decrease in brain reduced glutathione have also been reported in epileptic patients.40,41 Increased lipid peroxidation has been demonstrated in plasma of epileptic children who showed nuclear magnetic resonance changes.42 In their study, Mueller et al.41 reported significant reduction in glutathione/water ratio in the parieto-occiptal region in both cerebral hemispheres. This finding was independent of seizure activity.
This study in addition demonstrates marked increase in brain nitric oxide content after treatment with PTZ. Other studies indicated that in PTZ-induced seizures in rats, inhibitors of NOS suppressed the generalized tonic extension and delayed the latency to develop myoclonic jerks and clonic seizures.43 In slices from the hippocampal and entorhinal cortex, the application of non-specific inhibitors of nitric oxide synthase (NOS) or a selective neuronal NOS prevented low Mg++-induced epileptic activity. Seizure activity was re-induced by the addition of nitric oxide donor.44,45 It has been suggested that during PTZ-induced seizures, neuronal NOS induces endoplasmic reticulum stress and oxidative tissue damage; this effect being mediated by peroxynitrite.46
Our results also provide evidence for an inhibitory effect for PTZ on AChE, BChE in brain of treated rats. The function of the enzymes AChE and BChE is the hydrolysis of acetylcholine (ACh) and thus to terminate its actions at the neuronal synapse, cholinergic nerve endings and motor end-plate.47 Cholinesterases differ in that AChE hydrolysis ACh faster than BChE and is more specific in this respect whereas BChE is less specific and hydrolysis butyrylcholine and ester containing drugs.48,49 The inhibition of AChE by PTZ has been previously reported.15 Increased ACh in brain might have a role in the genesis of seizures. Green et al.50 using immunohistochemical techniques reported loss of AChE fibers in Ammon’s horn in resected temporal lobes from epileptic patients. In addition, gene mutation and loss of function of neuronal nicotinic ACh receptors was found in patients with a genetic form of epilepsy.51 The present study also confirms our previous observations indicating inhibition of PON-1 activity in brain of PTZ kindled rats.15 The function of PON-1 is to hydrolyse organophosphates and other xenobiotics.52 This enzyme has important antioxidative and anti-inflammatory actions53,54 and a decrease in its activity has been found in a number of neurological disorders.55
In this study, we have demonstrated that the endocannabinoid anandamide given at doses as low as 100 and 200 µg/kg exerted significant antioxidant effect and suppressed the development of seizures induced by PTZ administration in rats. In particular, anandamide completely inhibited the development of generalized tonic-clonic seizures. Other researchers used inhibitors of the enzyme fatty acid hydrolase so as to increase brain anandamide level, and reported an anticonvulsant effect against seizures evoked by PTZ29 or cocaine.30 Anandamide (and also2-arachydonoylglycerol) reduced excitatory postsynaptic currents in hippocampal slices from mice with temporal lobe epilepsy via CB1 receptor mechanism.56 Several mechanisms are likely to account for the seizure suppressive effect of anandamide. The endocannabinoid acts as a partial agonist at CB1 receptor.21 This site of action has been reported to account for the neuropotective effects of anandamide.57 It also binds to CB2 receptors and acts as a partial agonist or an antagonist22,23 and binds to transient receptor potential channel vanilloid receptor 1 (TRPV1).20 This capsaicin sensitive non-selective cation channel is involved in pain, heat, inflammation.58,59 The TRPV1 channel, however, has been implicated in increasing the susceptibility to PTZ seizures by hyperthermia in mice60 and its blockade by capsazepine led to decreased epileptic activity in an acute temporal lobe model of epilepsy in mice.61 The endocannabinoid might also regulate neuronal excitability by inhibition of T-type calcium channels.62 Anandamide’s ability to inhibit the development of seizures could also be due to an antioxidant effect as shown in the present study. In hippocampal neurons treated with H2O2, anandamide was reported to prevent the increase in intracellular reactive oxygen metabolites and the decrease in superoxide dismutase and reduced glutathione.27
The endogenous cannabinoid, anandamide, a derivative of arachidonic acid, is synthesized “on demand” from its membrane lipid precursor28 and as a component of the endocannabinoid system (along with 2-arachidonoyl glycerol) is likely to be involved in maintaining neuronal integrity by protecting neurons from excitotoxic injury.63 Thus, anandamide (10 μg/kg, i.p.) was shown to reduce neuronal excitotoxic injury following intracerebral injection of ouabain in neonatal rats.64 It protected striatal cells in culture cells against quinolinic acid excitotoxicity24 and retinal amacrine neurons against AMPA-induced excitotoxicity in vivo via CB1 receptor mechanism.25 Anandamide (and also 2-arachidonoyl-glycerol) protected prefrontal cortex neurons in culture against excitotoxicity and neuronal death (caused by human immunodeficiency virus type 1 toxin, transactivator of transcription) via CB1 receptor mechanism.26 In mice subjected to transient middle cerebral artery occlusion, prior treatment with anandamide (i.p. injection of 10 μg/kg ) resulted in a significant decrease in infarct volume.65 Moreover, inhibition of fatty acid hydrolase which enhanced brain anandamide level protected against neurodegeneration and behavioral deficits in traumatic brain injury in mice.66 Our data also indicated that anandamide decreased neuronal injury caused by PTZ in different brain regions.
In conclusion, in a model of human epilepsy induced by PTZ injection in the rat, the administration of low doses of the endogenous cannabinoid anandamide reduced brain oxidative stress, seizure development and reduced brain damage.
Acknowledgements
This work was not supported by research grants.
Conflict of Interest
There is no conflict of interest.
References
- Mechoulam R., Gaoni Y. Recent advances in the chemistry of hashish. Fortschr Chem Org Naturst 1967;25:175–213.
CrossRef - ElSohly M. A., Radwan M. M., Gul W., Chandra S and Galal A. Phytochemistry of Cannabis sativa L. In: Kinghorn A. D., Falk H., Gibbons S., Kobayashi J (eds). Phytocannabinoids Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa. Progress in the Chemistry of Organic Natural Products. Switzerland, Springer International Publishing. 2017;1-36.
CrossRef - Izzo A. A., Capasso R., Aviello G., Borrelli F., Romano B., Piscitelli F.,Gallo L., Capasso F., Orlando P., Di Marzo V. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br J Pharmacol 2012;166(4):1444-60. doi: 10.1111/j.1476-5381.2012.01879.x.
CrossRef - Borrelli F., Fasolino I., Romano B., Capasso R., Maiello F., Coppola D., Orlando P., Battista G., Pagano E., Di Marzo V., Izzo A. A. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol. 2013;85(9):1306-16. doi: 10.1016/j.bcp. 2013;01:017.
CrossRef - Hind W. H., England T. J., O’Sullivan S. E. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br J Pharmacol. 2016;173(5):815-25. doi: 10.1111/bph. 13368.
CrossRef - Hill A. J., Williams C. M., Whalley B. J., Stephens G. J. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther. 2012;133(1):79-97. doi: 10.1016/j.pharmthera. 2011.09.002.
CrossRef - Kaur R., Ambwani S. R., Singh S. Endocannabinoid System: A multi-facet therapeutic target. Curr Clin Pharmacol. 2016;11(2):110-7.
CrossRef - Hamerle M., Ghaeni L., Kowski A., Weissinger F., Holtkamp M. Cannabis and other illicit drug use in epilepsy patients. Eur J Neurol. 2014;21(1):167-70. doi: 10.1111/ene. 12081.
CrossRef - Sulak D., Saneto R., Goldstein B. The current status of artisanal cannabis for the treatment of epilepsy in the United States. Epilepsy Behav. 2017;70(Pt B):328-333. doi: 10.1016/j.yebeh. 2016;12:32.
- Reddy D. S., Golub V. M. The pharmacological basis of cannabis therapy for epilepsy. J Pharmacol Exp Ther. 2016;357(1):45-55. doi: 10.1124/jpet. 115.230151.
CrossRef - Porter B. E., Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013;29(3):574-7. doi: 10.1016/j.yebeh. 2013;08:037.
CrossRef - Press C. A., Knupp K. G., Chapman K. E. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav. 2015;45:49-52. doi: 10.1016/j.yebeh. 2015;02:043.
- Maa E., Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55(6):783-6. doi: 10.1111/epi. 12610.
- Saade D., Joshi C. Pure cannabidiol in the treatment of malignant migrating partial seizures in infancy: a case report. Pediatr Neurol. 2015;52(5):544-7. do0i: 10.1016/j.pediatrneurol. 2015.02.008.
- Abdel-Salam O. M. E., Sleem A. A., Sayed M. A.B. M., Khadrawy Y. A., Morsy F. A. Cannabis sativa increases seizure severity and brain lipid peroxidation in pentylenetetrazole-induced kindling in rats. Biomed Pharmacol J. 2018;11(3);1187-1197.
CrossRef - Karler R., Calder L. D., Sangdee P., Turkanis S. A. Interaction between delta-9-tetrahydrocannabinol and kindling by electrical and chemical stimuli in mice. Neuropharmacology. 1984;23(11):1315-20.
CrossRef - Malyshevskaya O., Aritake K., Kaushik M. K., Uchiyama N., Cherasse Y., Kikura-Hanajiri R., Urade Y. Natural (∆9-THC) and synthetic (JWH-018) cannabinoids induce seizures by acting through the cannabinoid CB1 receptor. Sci Rep. 2017;7(1):10516. doi: 10.1038/s41598-017-10447-2.
CrossRef - Vilela L. R., Lima I. V., Kunsch É. B., Pinto H. P. P., de Miranda A. S., Vieira É. L. M., de Oliveira A. C. P., Moraes M. F. D., Teixeira A. L.., Moreira F. A. Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: Pharmacological mechanisms, electroencephalographic profile and brain cytokine levels. Epilepsy Behav. 2017;75:29-35. doi: 10.1016/j.yebeh. 2017;07:014.
CrossRef - Hill T. D., Cascio M. G., Romano B., Duncan M., Pertwee R. G., Williams C. M., Whalley B. J., Hill A. J. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism.Br J Pharmacol. 2013;170(3):679-92. doi: 10.1111/bph. 12321.
CrossRef - Pacher P., Bátkai S., Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389-462.
CrossRef - Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992 18;258(5090):1946-9.
- Chaperon F., Thiébot M. H. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13(3):243-81.
CrossRef - Gonsiorek W., Lunn C., Fan X., Narula S., Lundell D and Hipkin R. W. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol. 2000;57:1045–1050.
- Rangel-López E., Colín-González A. L., Paz-Loyola A. L., Pinzón E., Torres I., Serratos I. N., Castellanos P., Wajner M., Souza D. O., Santamaría A. Cannabinoid receptor agonists reduce the short-term mitochondrial dysfunction and oxidative stress linked to excitotoxicity in the rat brain. Neuroscience. 2015;285:97-106. doi: 10.1016/j.neuroscience. 2014;11:016.
- Kokona D., Thermos K. Synthetic and endogenous cannabinoids protect retinal neurons from AMPA excitotoxicity in vivo, via activation of CB1 receptors: Involvement of PI3K/Akt and MEK/ERK signaling pathways. Exp Eye Res. 2015;136:45-58. doi: 10.1016/j.exer. 2015;05:007.
CrossRef - Xu C., Hermes D. J., Nwanguma B., Jacobs I. R., Mackie K., Mukhopadhyay S., Lichtman A. H., Ignatowska-Jankowska B., Fitting S. Endocannabinoids exert CB1 receptor-mediated neuroprotective effects in models of neuronal damage induced by HIV-1 Tat protein. Mol Cell Neurosci. 2017;83:92-102. doi: 10.1016/j.mcn. 2017;07:3.
- Jia J., Ma L., Wu M., Zhang L., Zhang X., Zhai Q., Jiang T., Wang Q., Xiong L. Anandamide protects HT22 cells exposed to hydrogen peroxide by inhibiting CB1 receptor-mediated type 2 NADPH oxidase. Oxid Med Cell Longev. 2014;2014:893516. doi: 10.1155/2014/893516.
CrossRef - Grotenhermen F. Cannabinoids. Curr Drug Targets CNS Neurol Disord. 2005;4(5):507-30.
CrossRef - Vilela L. R., Medeiros D. C., Rezende G. H., de Oliveira A. C.,Moraes M. F., Moreira F. A. Effects of cannabinoids and endocannabinoid hydrolysis inhibition on pentylenetetrazole-induced seizure and electroencephalographic activity in rats. Epilepsy Res. 2013;104(3):195-202. doi: 10.1016/j.eplepsyres.2012.11.006.
CrossRef - Vilela L. R., Gomides L. F., David B. A., Antunes M. M., Diniz A. B., Moreira A. F., Menezes G. B. Cannabidiol rescues acute hepatic toxicity and seizure induced by cocaine. Mediators Inflamm. 2015;2015:523418. doi: 10.1155/2015/523418.
CrossRef - Nair V., Turner G. A. The thiobarbituric acid test for lipid peroxidation: structure of the adduct with malondialdehyde. Lipids. 1984;19:804-805.
CrossRef - Archer S. Measurement of nitric oxide in biological models. FASEB J. 1993;7(2):340-360.
CrossRef - Ellman G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70-77.
CrossRef - Higashino K., Takahashi Y., Yamamura Y. Release of phenyl acetate esterase from liver microsomes by carbon tetrachloride. Clin Chim Acta. 1972;41:313‒20.
CrossRef - Sefil F., Kahraman I., Dokuyucu R., Gokce H., Ozturk A., Tutuk O., Aydin M., Ozkan U., Pinar N. Ameliorating effect of quercetin on acute pentylenetetrazole induced seizures in rats. Int J Clin Exp Med. 2014;7(9):2471-7. eCollection 2014.
- Halliwell B., Gutteridge J. M. Free Radicals in Biology and Medicine. 2nd edition. Clarendon Press, Oxford, UK. 1989.
- Pence S., Erkutlu I., Kurtul N., Bosnak M., Alptekin M., Tan U. Antiepileptogenic effects of glutathione against increased brain ADA in PTZ-induced epilepsy. Int J Neurosci. 2009;119(5):616-29. doi: 10.1080/00207450802055440.
CrossRef - Bains J. S., Shaw C. A. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25(3):335‒58.
CrossRef - Akbas S. H., Yegin A., Ozben T. Effect of pentylenetetrazol-induced epileptic seizure on the antioxidant enzyme activities, glutathione and lipid peroxidation levels in rat erythrocytes and liver tissues. Clin Biochem. 2005;38(11):1009-14.
CrossRef - Abbott L. C., Nejad H. H., Bottje W. G., Hassan A. S. Glutathione levels in specific brain regions of genetically epileptic (tg/tg) mice. Brain Res Bull. 1990;25(4):629-31.
CrossRef - Mueller S. G., Trabesinger A. H., Boesiger P., Wieser H. G. Brain glutathione levels in patients with epilepsy measured by in vivo (1)H-MRS. Neurology. 2001;57(8):1422-7.
CrossRef - Turkdogan D., Toplan S., Karakoc Y. Lipid peroxidation and antioxidative enzyme activities in childhood epilepsy. J Child Neurol. 2002;17:673-676.
CrossRef - Osonoe K., Mori N., Suzuki K., Osonoe M. Antiepileptic effects of inhibitors of nitric oxide synthase examined in pentylenetetrazol-induced seizures in rats. Brain Res. 1994;663(2):338-40.
CrossRef - Schuchmann S., Albrecht D., Heinemann U., von Bohlen und Halbach O. Nitric oxide modulates low-Mg2+-induced epileptiform activity in rat hippocampal-entorhinal cortex slices. Neurobiol Dis. 2002;11(1):96-105.
CrossRef - Kovács R., Rabanus A., Otáhal J., Patzak A., Kardos J., Albus K., Heinemann U., Kann O. Endogenous nitric oxide is a key promoting factor for initiation of seizure-like events in hippocampal and entorhinal cortex slices. J Neurosci. 2009;29(26):8565-8577. DOI: https://doi.org/10.1523/JNEUROSCI.2009;5698-08
- Zhu X., Dong J., Han B., Huang R., Zhang A., Xia Z., Chang H., Chao J and Yao H. Neuronal nitric oxide synthase contributes to PTZ kindling epilepsy-induced hippocampal endoplasmic reticulum stress and oxidative damage. Front Cell Neurosci. 2017;11:377. doi: 10.3389/fncel. 2017;00377. eCollection 2017.
- Mileson B. E., Chambers J. E., Chen W. L., Dettbarn W., Ehrich M., Eldefrawi A. T., Gaylor D. W., Hamernik K., Hodgson E., Karczmar A. G., Padilla S., Pope C. N., Richardson R. J., Saunders D. R., Sheets L. P., Sultatos L. G., Wallace K. B. Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol Sci. 1998;41(1):8-20.
- Chatonnet A., Lockridge O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem J. 1989;260(3):625–34.
CrossRef - Silman I., Sussman J. L. Acetylcholinesterase: ‘classical’ and ‘non-classical’ functions and pharmacology. Curr Opin Pharmacol. 2005;5(3):293–302. doi: 10.1016/j.coph. 2005;01:014.
CrossRef - Green R. C., Blume H. W., Kupferschmid S. B., Mesulam M. M. Alterations of hippocampal acetylcholinesterase in human temporal lobe epilepsy. Ann Neurol. 1989;26(3):347–351.
CrossRef - Bertrand D. Neuronal nicotinic acetylcholine receptors: their properties and alterations in autosomal dominant nocturnal frontal lobe epilepsy. Rev Neurol (Paris) .1999;155(6-7):457-62.
- La Du B. N. Human serum paraoxonase: arylesterase. In: Kalow W (ed) Pharmacogenetics of drug metabolism. Pergamon, Elmford. 1992;51–91.
- Costa L. G., Furlong C. E. Paraoxonase (PON1) in Health and Disease: Basic and Clinical Aspects. Kluwer Academic Publishers, Norwell, MA. 2002.
CrossRef - Marsillach J., Camps J., Ferré N., Beltran R., Rull A., Mackness B., Mackness M., Joven J. Paraoxonase-1 is related to inflammation, fibrosis and PPAR delta in experimental liver disease. BMC Gastroenterol. 2009;9:3.
CrossRef - Menini T., Gugliucci A. Paraoxonase 1 in neurological disorders. Redox Rep. 2014;19(2):49–58. doi: 10.1179/1351000 213 Y .0000000071.
- Bhaskaran M. D., Smith B. N. Cannabinoid-mediated inhibition of recurrent excitatory circuitry in the dentate gyrus in a mouse model of temporal lobe epilepsy. PLoS One. 2010;5(5):10683. doi: 10.1371/journal.pone.0010683.
CrossRef - Van der Stelt M., Veldhuis W. B., van Haaften G. W., Fezza F., Bisogno T., Bar P. R., Veldink G. A., Vliegenthart J. F., Di Marzo V., Nicolay K. Exogenous anandamide protects rat brain against acute neuronal injury in vivo. J Neurosci. 2001;21(22):8765-71.
CrossRef - Szolcsányi J. Capsaicin and sensory neurones: a historical perspective. In: Abdel-Salam OME. (ed.) Capsaicin as a Therapeutic Molecule. Progress in Drug Research. 2014;68:1-37.
CrossRef - Nagy I., Friston D., Valente J. S., et al. Pharmacology of the capsaicin receptor, transient receptor potential vanilloid type-1 ion channel. In: Abdel-Salam OME. Capsaicin as a Therapeutic Molecule. Progress in Drug Research, Springer, Basel. 2014;68:39-76.
CrossRef - Kong W. L. Role of TRPV1 in susceptibility to PTZ-induced seizure following repeated hyperthermia challenges in neonatal mice. 2014;31:276–280.
- Carletti F., Gambino G., Rizzo V., Ferraro G., Sardo P. Involvement of TRPV1 channels in the activity of the cannabinoid WIN 55,212-2 in an acute rat model of temporal lobe epilepsy. Epilepsy Res. 2016;122:56-65. doi: 10.1016/j.eplepsyres. 2016.02.005.
CrossRef - Chemin J., Monteil A., Perez-Reyes E., Nargeot J., Lory P. Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J. 2001;20(24):7033-40.
CrossRef - Marsicano G., Goodenough S., Monory K., Hermann H., Eder M., Cannich A., Azad S. C., Cascio M. G., Gutiérrez S. O., van der Stelt M., López-Rodriguez M. L., Casanova E., Schütz G., Zieglgänsberger W., Di Marzo V., Behl C., Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302(5642):84-8.
CrossRef - Neuroprotection by the endocannabinoid anandamide against in vivo neuronal injury in the rat. Veldhuis W. B., van der Stelt M., van Haaften G. W., Veldink G. A., Vliegenthart J. F. G., Di Marzo V., Nicolay K. Proc Intl Soc Mag Reson Med. 2002;10.
- Schomacher M., Müller H. D., Sommer C., Schwab S., Schäbitz W. R. Endocannabinoids mediate neuroprotection after transient focal cerebral ischemia. Brain Res. 2008;1240:213-20. doi: 10.1016/j.brainres. 2008.09.019.
- Tchantchou F., Tucker L. B., Fu A. H., Bluett R. J., McCabe J. T., Patel S., Zhang Y. The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology. 2014;85:427-39. doi: 10.1016/j.neuropharm. 2014.06.006.








