Ahmed Medhat Hegazy*1 , Marwa I. Khalifa2
, Marwa I. Khalifa2 and Soad M. Nasr3
and Soad M. Nasr3
1Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Aswan University, Sahari, Airport Way, Post Box 81528, Aswan, Egypt.
2Department of Food Hygiene, Faculty of Veterinary Medicine, Aswan University, Sahari, Airport Way, Post Box 81528, Aswan, Egypt.
3Department of Parasitology and Animal Diseases, National Research Centre, 33 Bohouth Street, Post Box 12622, Dokki, Giza, Egypt.
Corresponding Author E-mail: ahmed_medhat012@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1658
Abstract
These investigations were conducted to detect the residues of polycyclic aromatic hydrocarbons, lead, and cadmium in the raw milk samples of lactating cows grazing around the Sugar Cane Factory. One hundred raw milk samples were collected from apparent healthy lactating cows during the rest and work periods of the Sugar Cane Factory. Detection of polycyclic aromatic hydrocarbons residue in milk samples was performed using a gas chromatography. Lead and cadmium levels in the milk samples were determined after digestion. Results revealed that benzo(a)anthracene and indeno(1,2,3-cd)pyrene (carcinogenic), and acenaphthylene and phenanthrene non-carcinogenic were detected only in the raw milk samples during the work period, while fluoranthene and benzo(a)pyrene were detected only during the rest period. However, chrysene and benzo(b) fluoranthene (carcinogenic) were detected in the milk at the rest and work periods of the Sugar Cane Factory. The highest levels of lead and cadmium were detected during the work period compared to the levels of lead and cadmium at rest. In conclusion, benzo(a)anthracene, indeno(1,2,3-cd)pyrene, acenaphthylene, and phenanthrene, lead and cadmium could be detected in cows’ milk which raised around the Sugar Cane Factory. Further investigations of these pollutants must be done in water, plants, air, and soil around this factory.
Keywords
Cadmium; Cows’ Milk; Lead; Polycyclic Aromatic Hydrocarbons
Download this article as:| Copy the following to cite this article: Hegazy A. M, Khalifa M. I, Nasr S. M. Monitoring of Carcinogenic Environmental Pollutants in Raw Cows’ Milk. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Hegazy A. M, Khalifa M. I, Nasr S. M. Monitoring of Carcinogenic Environmental Pollutants in Raw Cows’ Milk. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2FtdTTt |
Introduction
Milk is considered a natural anti-toxin, and it can be used to reduce the severity of poisonousness in the body. It contains natural potent antioxidants such as conjugated linoleic acid (CLA), fat-soluble vitamin A, b-carotene, coenzyme Q10 and proteins of high nutritional value. It helps in the fight against pollutants and toxic substances by reducing the inflammation and stimulating high levels of antioxidant activity.1
The environmental toxic pollutants are very important health concern, which may accumulate in the food chain. This high level of pollution is expected to contaminate water and soil.2 Many dangerous pollutants (e.g. hydrocarbons, pesticides, and heavy metals) may end up along the food chain.3
One of environmental pollutants is a group of polycyclic aromatic hydrocarbons (PAHs). It consists of two or more fused rings which formed as a result of the organic materials incomplete combustion during industrial and human activities as processing of coal, vehicle traffic, cooking, and tobacco smoking.4 Breathing the air near coal-tar, asphalt production or applications, cigarette smoke, wood smoke, vehicle exhausts, fumes from chimneys, eating grilled or charred meats and any food with PAHs deposited on them during processing are the main sources of PAH exposure.5 In 2001, PAHs ranked 9th on the list of most threatening compounds to human health.6
PAHs can be absorbed by the lung and gastrointestinal tract tissues and skin. It accumulates in the internal organs containing fat.7 Fats and oils may contain high levels of PAH compounds because of their lipophilic nature. It was demonstrated that milk may contain different PAHs concentrations according to environmental conditions. 8
Heavy metals are persistent as a contaminant in the environment. The industrial and agricultural processes have increased the concentration of heavy metals in water, air, and soil which are taken by plants and/or animals into the food chain.
The presence of high levels of lead (Pb) in diet results in anemia and kidney, liver, heart, immune system, reproductive system, digestive system, and central nervous system disorders.9
Cadmium (Cd) is a carcinogenic agent that causes tumors in the prostate.10 Cadmium also causes failure in kidneys, bones, lungs, liver, heart, and vessels.11 The effects of Cd contamination on pregnant women are malformations, fetal weight reduction, and abnormality in the baby’s DNA and proteins as well as abortion.12
Heavy metals and PAHs contaminated milk and dairy products can cause a health risk to human especially children.13
Thus, this work was to investigate the residue levels of PAHs, Pb and Cd in milk samples of lactating cows raised around the Sugar Cane Factory located in Kom-Ombo city, Aswan governorate, Egypt.
Material and Methods
The Institutional Animal Ethics Committee approved all experimental protocols (No. 135 at 11 October 2015) conducted at Benha University, Egypt.
Locality
Kom-Ombo city, Aswan governorate, Egypt was the study area. Where, the Sugar Cane Factory is starting to work at the beginning of the year from January to May, followed by the rest period until the end of the year.
Sampling
One hundred of raw cows’ milk samples were collected from apparent healthy lactating cows which are raised in the area around the Sugar Cane Factor during the rest and work periods (50 samples, each). The first period was during the rest of the factory (from August to December 2015). The second period was during the work of the factory (from January to May 2016). Sampling collection was carried out in the early morning to the first milking of a day. From each lactating cow, 50 mL of milk was collected into cleaned sterilized Falcon tubes, previously washed with diluted nitric acid and then rinsed with double distilled water. All samples were stored at –20°C until analytical process.
Analytical Assays
Determination of Polycyclic Aromatic Hydrocarbons (PAHs)
Detection of PAHs residue in milk was performed according to the method of Hegazy et al.14 as the following:
Extraction of PAHs
The PAHs from each milk sample (10 mL) was extracted using 10 mL mixture of hexane /acetone (1:1, V/V) then filtered by filter paper with 2 g anhydrous sodium sulfate. The extract was reduced to about 3 mL.
Saponification
The extract was transferred to a flask (round bottom) then added 100 mL of an aqueous methanol potassium hydroxide [KOH (30 g) dissolved in methanol (270 mL) and distilled water (30 mL)]. This mixture refluxed in water bath for 3 h. The flask was rinsed with 100 mL mixture of methanol/distilled water (4:1 V/V). The mixture was transferred to a separating funnel and 50 mL of hexane was added and shaking for 3 min. The aqueous layer was drained into a beaker, while organic layer collected into another one. The aqueous layer was repeated twice with hexane (50 mL). Then, the aqueous layer was discarded. The organic layer was evaporated in a rotary evaporator (3 mL).
Aluminum Oxide and Silica Gel Activation
For Column Chromatography, aluminum oxide 90 and silica gel 60 were kept at 200°C for 4 h. Then transferred to a closed container to reach ambient temperature and stored in a well-sealed glass bottle.
Adsorption Column Chromatography
Ten grams of AlO3 was weighed to pack glass column (22×1.5 ID). Another ten grams of silica gel loaded over the AlO3 layer. Before the sample was loaded, the column topped with 2 g sodium sulfate anhydrous then filed with hexane (20 mL). Aliphatic hydrocarbons were eluted with hexane (50 mL) and then aromatic hydrocarbons were eluted with 50 mL toluene. PAHs were reduced to 4ml then transferred to the glass tube (screw-capped) and were kept at –20°C until analyzed.
PAHs Standard
The standard curve of PAHs was including naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo (a) anthracene, chrysene, benzo (b) fluoranthene, benzo (a) pyrene, indeno (1,2,3-cd) pyrene, and benzo (g,h,i) perylene as shown in figure 1.
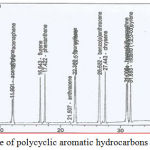 |
Figure 1: Standard curve of polycyclic aromatic hydrocarbons concentrations.
|
Detection of PAHs Residue in Milk
A gas chromatograph (Hewlett-Packard HP 5890 series II Plus) was used. It equipped with a flame ionization detector and HP-1 analytical capillary column (30 m×0.53 mm), the film thickness (0.88 µm), cross-linked methyl silicone gum for the separation of PAHs. The operational conditions of gas chromatography were detector temperature (280°C), injection port temperature (200°C), column initial temperature (100°C), initial time (2 min), and rate of temperature increase (6°C min-1), upper temperature (280°C), and upper time (10 min). The flow rate of the carrier gas nitrogen was 4 mL min-1 and the injection volume was 1 µL through out.
Determination of Lead and Cadmium
Lead and cadmium were determined according to the method of Ismail et al.15 Milk sample of one ml was placed in clean, dry screw-capped test tube. A mixture of 10 mL nitric/perchloric acid (4:1, V/V) was added. Initial digestion was at room temperature overnight. Followed by careful heating in a water bath at 40 °C – 45 °C with gentle shaking until digestion completed (become clear and transparent). The digested materials were allowed to cool at room temperature, and then diluted up to 20 mL with double distilled water and filtered through ashless filter paper. Perkin-Elmer 2380 Atomic absorption spectrophotometer was used for determination of lead and cadmium levels.
Statistical Analysis
The data were presented as means ± standard errors. Student t-test was used for evaluation of the data. Differences were considered significant at P<0.0516 using SPSS version 20 computer program.
Results
Polycyclic Aromatic Hydrocarbons
PAHs concentrations in milk samples were shown in table (1).
Table 1: Polycyclic aromatic hydrocarbons (PAHs) compounds concentration in cows’ milk (µg L-1) collected during the rest and work period of the Sugar Cane Factory. (Mean ± SE).
| PAHs | At rest | At work | P-value |
| Naphthalene | ND | ND | — |
| Acenaphthylene | ND | 3.254 ± 0.626* | 0.007 |
| Acenaphthene | ND | ND | — |
| Fluorene | ND | ND | — |
| Phenanthrene | ND | 0.223 ± 0.093 | 0.075 |
| Anthracene | ND | ND | — |
| Fluoranthene | 0.128 ± 0.053 | ND | 0.075 |
| Pyrene | ND | ND | — |
| Benzo(a)anthracene# | ND | 0.005 ± 0.001* | 0.007 |
| Chrysene# | 0.015 ± 0.003 | 0.028 ± 0.005 | 0.087 |
| Benzo(b)fluoranthene# | 0.056 ± 0.024 | 0.048 ± 0.005 | 0.763 |
| Benzo(a)pyrene# | 0.006 ± 0.001* | ND | 0.007 |
| Indeno(1,2,3-cd)pyrene# | ND | 0.081 ± 0.030 | 0.056 |
| Benzo(g,h,i)perylene# | ND | ND | — |
| Total non-carcinogenic | 0.128 ± 0.053 | 3.477 ± 0.720* | 0.010 |
| Total carcinogenic | 0.077 ± 0.028 | 0.162 ± 0.038 | 0.142 |
| Total PAHs | 0.205 ± 0.026 | 3.639 ± 0.690* | 0.008 |
# = Carcinogenic PAHs US-EPA (2000). ND = not detected. * = Significant at P<0.05.
In the raw milk samples, the carcinogenic PAHs; benzo(a)anthracene (0.005 ± 0.001 µg L-1) and indeno(1,2,3-cd)pyrene (0.081 ± 0.030 µg L-1), and the non-carcinogenic PAHs; acenaphthylene (3.254 ± 0.626 µg L-1), and phenanthrene (0.223 ± 0.093 µg L-1), were detected only during the work period. While, fluoranthene (0.128 ± 0.053 µg L-1) and benzo(a)pyrene (B(a)P) (0.006 ± 0.001 µg L-1) were detected only during the rest period. However, carcinogenic PAHs; chrysene (0.015 ± 0.003 and 0.028 ± 0.005 µg L-1) and benzo(b)fluoranthene (0.028 ± 0.005 and 0.048 ± 0.005 µg L-1) were detected in milk at the rest and work periods, respectively. On the other hand, naphthalene, acenaphthene, fluorene, anthracene, pyrene, and benzo(g,h,i)perylene were not detected in milk samples during both periods. The total non-carcinogenic PAHs and the total PAHs were significant high during the work period than in the rest period. There were no statistical differences between the total carcinogenic PAHs during the rest and work periods of the Sugar Cane Factory.
Lead and Cadmium
Lead and cadmium were detected in milk samples during the rest and work periods during the experiment (Table 2). The levels of lead and cadmium were significant (P<0.05) increased in milk samples (0.218 ± 0.021 µg L-1 and 0.161 ± 0.016 µg L-1) respectively during the work period than in the rest period of the Sugar Cane Factory.
Table 2: Lead and cadmium levels in cows’ milk (µg L-1) collected during the rest and work periods of the Sugar Cane Factory. (Mean ± SE).
| At rest | At work | P-value | |
| Lead | 0.065 ± 0.023 | 0.218 ± 0.021* | 0.011 |
| Cadmium | 0.052 ± 0.010 | 0.161 ± 0.016** | 0.007 |
* = Significant at P<0.05. ** = Significant at P<0.01.
Discussion
Environmental risk factors have a contribution to the burden of many diseases.17 Many public health concerns are identified due to bioaccumulation of pollutants and high stability in nature. Animals feed plants absorb such pollutants from the soil lead to contamination of animal products as a residue in meat and milk. It may lead to adverse effects on consumer’s health.
The present study revealed that different concentrations of PAHs were detected in milk samples collected from lactating cows raised around the study area during the rest and the work seasons of the Sugar Cane Factory.
Fluoranthene and B(a)P were detected during the rest period of the Factory. While, acenaphthylene, phenanthrene, benzo (a) anthracene and indeno(1,2,3-cd)pyrene were detected during the work period. On the other hand, naphthalene, acenaphthene, fluorene, anthracene, pyrene and benzo(g,h,i)perylene were not detected during both rest and work periods. Total PAHs and non-carcinogenic PAHs significantly increased during the work season. Total carcinogenic PAHs show no statistical differences between the two seasons of the investigation. These results agree with.18 The PAHs are made into a whole or made part of a whole in fats of milk owing to their lipophilic nature. After chronic exposure of lactating animals to PAHs, the PAHs’ the ring cycles number and its molecular weight appear to be determining factors for PAHs’ transferability into the milk.19 The low molecular weight of PAHs contain two benzene ring (naphthalene) and three rings (acenaphthylene, acenaphthene, fluorene, phenanthrene and anthracene) and the high molecular weight PAHs contain 4 rings (fluoranthene, pyrene, benzo (a) anthracene and chrysene), five rings (benzo (b) fluoranthene and [B(a)P] and six rings (indeno(1,2,3-cd)pyrene). The low molecular weight of PAHs were not detected in milk samples collected during the period of investigation may referee to their lower stability in the environment (its high volatility) in comparison with high molecular weight of PAHs that characterized by their stability and resist the environmental degradation. So the animals live in such polluted area as in the present study (around the Sugar Cane Factory) exposed to PAHs especially the compounds remain in the environment for long period and resist the environmental degradation.20
PAHs found in the environment as the form of a mixture. The present study revealed that the total PAHs were significant high during the work period due to the large amount of PAHs liberated in the environment from the chimney of the Sugar Cane Factory and subsequent more exposure of living creatures in the adjacent areas to these pollutants. On the other hand, during the rest period, the total PAHs were significant low that may referee to other sources of PAHs in the study area as the ignition of wood and fuel in automobiles. Lutz et al21 stated that PAHs with less than 5 rings are transferred to the milk as native compound. Moreover, PAHs are transferred as metabolites, possibly including those of the high molecular mass. The parent PAH is quickly metabolized and its compounds concentrations are mostly dependent on different metabolic rates. The main routes of PAH exposure to animals are fed intake, respiration and percutaneous intake.20
The PAHs absorption decreases with its molecular weight.22 Also, there are alternative sources of milk PAHs such as field drinking water, breathing air and soil ingested with grass.23
Cereals were considered the largest contribution to the total dietary intake of PAHs, followed by oils and fats, while meat and milk made minor contributions in the United Kingdom.24 However, in the Italian diet, the largest contribution to dietary PAH intake were cereals, milk products, meats, vegetables, and fruits.25
Human exposure to PAHs arises predominantly from dietary sources. So, further studies will be necessary to establish the trend for PAH concentrations in foodstuffs.
The current study also revealed that there were different levels of Pb and Cd detected in milk samples collected from the study area, during the rest and the work periods.
The level of Pb detected in all milk samples during the rest period was below the permissible limit (0.1 ppm) for Pb in Egyptian Standards 1993 for heavy metals residues in the milk of cattle.26 However, during the work period this level exceeded this permissible limit. The concentration of Cd detected in all milk samples during the rest season was nearly the permissible limit (0.05 ppm) for cadmium for Egyptian Standards 1993 for heavy metals residues in the milk of cattle. While during the work season the level of Cd exceeded this permissible limit. These results agree with.27
Lactating cows exposed to high levels of Pb and Cd lead to accumulation of these metals in their milk that become a health hazard to consumers.28 The presence of Pb in milk samples could be attributed to fodder and water contamination, cows graze along roads, climatic factors, such as winds. The present findings agree with29 who found that raw milk contained higher than permissible limits of Pb and Cd that collected from urban shops and dairy farms near wastewater drains. Moreover, high levels of Pb were recorded in raw milk samples collected from cows grazed near roads with heavy traffic, iron and steel production and coal combustion.30 Also, metallic utensils may be another source. Lead toxicity reduces the functions of brain, liver and kidney due to affects the membrane permeability and necrosis. Lead has a large affinity to thiol and phosphate containing enzymes. So it causes anemia due to inhibit the heme biosynthesis.9,31 Exposure to Pb in vitro and during infancy- causes irreversible affects for the development of the nervous system which leads to reduce learning disabilities.32
Cow’s milk contaminated with high level of Cd may be resulted from consumption of contaminated rations and water with Cd.33 It originates from fertilizers which contaminate crops and soil, inhalation of dust and fumes resulted from the industrial activities.28 Also, Cd-lined metal equipment used in commercial food processing such as pottery glazes, kitchenware enamel, and plastics containing Cd.34 Cadmium has estrogenic effects and increased the cancer incidence in mice.35 Chronic exposure to Pb and Cd associated with reduced kidney function in infants and kidney damage in adults.36
Conclusion
Acenaphthylene, phenanthrene, benzo (a) anthracene and indeno(1,2,3-cd)pyrene, Pb and Cd could be detected in cows’ milk which raised around the Sugar Cane Factory during the working period. Further investigations of these pollutants should be done in water, plants, air, and soil around this Factory.
Recommendations
It should give sanitary protection of surface water against the discharges and effluents of factories that contain PAHs and heavy metals. Purchased milk must be far from such contaminated areas. Regular analysis of milk for PAHs and heavy metals should be evaluated according to the international programs in order to protect the consumer life. The consumer should be aware about environmental pollution, sources, health hazards and control. In the contaminated areas, the diet of consumers must be rich in proteins, antioxidants and vitamins that may play an important role in decreasing the toxicity.
Acknowledgments
The authors are greatly indebted to Prof. Dr. Elam Abd-Elmonem Elshewy Head of Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Benha University, for her cooperation and help during this work.
Conflict of interest
There is no conflict of interest.
References
- Grazyna C., Hanna C., Adam A and Magdalena B. M. Natural antioxidants in milk and dairy products. Int J Dairy Technol. 2017;70(2):165-178.
- Arianejad M., Alizadeh M., Bahrami A and Arefhoseini S. R. Levels of Some Heavy Metals in Raw Cow’s Milk from Selected Milk Production Sites in Iran: Is There any Health Concern? Health Promot Perspect. 2015;5(3):176-182.
- Tajkarimi M., Faghih M. A., Poursoltani H., Nejad A. S., Motallebi A. A and Mahdavi H. Lead residue levels in raw milk from different regions of Iran. Food Control. 2008;19(5):495-498.
- Mottier P., Parisod V and Turesky R. J. Quantitative determination of polycyclic aromatic hydrocarbons in barbecued meat sausages by gas chromatography coupled to mass spectrometry. J Agric Food Chem. 2000;48(4):1160-1166.
- Veyrand B., Brosseaud A., Sarcher L., Varlet V., Monteau F., Marchand P., Andre F and Le Bizec B. Innovative method for determination of 19 polycyclic aromatic hydrocarbons in food and oil samples using gas chromatography coupled to tandem mass spectrometry based on an isotope dilution approach. J Chromatogr A. 2007;1149(2):333-344.
- King S., Meyer J. S and Andrews A. R. Screening method for polycyclic aromatic hydrocarbons in soil using hollow fiber membrane solvent microextraction. J Chromatogr. A. 2002;982(2):201-208.
- Guillen M and Sopelana P. Polycyclic aromatic hydrocarbons in diverse foods, In: JPF D’Mello (ed.) Food Safety: Contaminants and Toxins. CABI Publishing: Oxon, UK. 2003;175–198.
- Hampikyan H and Colak H. Investigation of polycyclic aromatic hydrocarbons in foods. Asian Journal of Chemistry. 2010;22(8):5797.
- Carocci A., Catalano A., Lauria G., Sinicropi M. S and Genchi G. Lead toxicity, antioxidant defense and environment. Rev Environ Contam Toxicol. 2016;238:45–67.
- Rapisarda V., Miozzi E., Loreto C., Matera S., Fenga C., Avola R and Ledda C. Cadmium exposure and prostate cancer: insights, mechanisms and perspectives. Front Biosci (Landmark Ed). 2018;23:1687-1700.
- Bernard A. Cadmium & its adverse effects on human health. Indian J Med Res. 2008;128(4):557-564.
- Blum J. L., Edwards J. R., Prozialeck W. C., Xiong J. Q and Zelikoff J. T. Effects of maternal exposure to cadmium oxide nanoparticles during pregnancy on maternal and offspring kidney injury markers using a murine model. J Toxicol Environ Health Part A. 2015;78(12):711-724.
- Caggiano R., Sabia S., D’Emilio M., Macchiato M., Anastasio A., Ragosta M., and Paino S. Metal levels in fodder, milk, dairy products, and tissues sampled in ovine farms of Southern Italy. Environ Res. 2005;99(1):48-57.
- Hegazy A. M., Bakry H. H., El-Shawarby R. M., Abou-Salem M. E., Abd El-Aleem N. M and Nasr S. M. Effects of benzo (a) pyrene on blood components, tumor markers, and oxidative status in mice. Toxicological and Environmental Chemistry. 2012;94(1):136-145.
- Ismail A., Riaz M., Akhtar S., Goodwill J. E and Sun J. Heavy metals in milk: global prevalence and health risk assessment. Toxin Reviews. 2017;1-12. https://doi.org/10.1080/15569543.2017.1399276.
- Snedecor G and Cochran W. Analysis of variance: the random effects model. Statistical Methods. Iowa State University Press, Ames, IA. 1989;237-252.
- Lim S. S., Vos T., Flaxman A. D., Danaei G., Shibuya K., Adair-Rohani H., AlMazroa M. A., Amann M., Anderson H. R and Andrews K. G. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions. 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380(9859):2224-2260.
- Grova N., Feidt C., Crépineau C., Laurent C., Lafargue P. E., Hachimi A and Rychen G. Detection of polycyclic aromatic hydrocarbon levels in milk collected near potential contamination sources. Journal of Agricultural and Food chemistry. 2002;50(16):4640-4642.
- Grova N., Feidt C., Laurent C and Rychen G. [14C] Milk, urine and faeces excretion kinetics in lactating goats after an oral administration of [14C] polycyclic aromatic hydrocarbons. Int Dairy J. 2002;12(12):1025-1031.
- Ciganek M and Neca J. Polycyclic aromatic hydrocarbons in porcine and bovine organs and tissues. Vet Med. 2006;51(5):239-247.
- Lutz S., Feidt C., Monteau F., Rychen G., Le Bizec B and Jurjanz S. Transfer of polycyclic aromatic hydrocarbons and their principle metabolites to milk after chronic exposure of dairy cows to contaminated soil. in Abstract from Symposium New Methods for Assessing Human Exposure to PAHs Toronto. 2005.
- Zhang G., Pan Z.,Wang X.,Mo X and Li X. Distribution and accumulation of polycyclic aromatic hydrocarbons (PAHs) in the food web of Nansi Lake, China. Environ Monit Assess. 2015;187(4): 173. doi: 10.1007/s10661-015-4362-4.
- Grova N., Laurent C., Feidt C., Rychen G., Laurent F and Lichtfouse E. Gas chromatography-mass spectrometry study of polycyclic aromatic hydrocarbons in grass and milk from urban and rural farms. Eur J Mass Spectrom. 2000;6(5):457-460.
- Falco G., Domingo J. L., Llobet J. M., Teixido A., Casas C and Müller L. Polycyclic aromatic hydrocarbons in foods: human exposure through the diet in Catalonia, Spain. J Food Prot. 2003;66(12):2325-2331.
- Santonicola S., Albrizio S., Murru N., Ferrante M. C and Mercogliano R. Study on the occurrence of polycyclic aromatic hydrocarbons in milk and meat/fish based baby food available in Italy. Chemosphere. 2017;184:467-472.
- Malhat F., Hagag M., Saber A and Fayz A. E. Contamination of cows milk by heavy metal in Egypt. Bull. Environ. Contam Toxicol. 2012;88(4):611-613.
- Chary N. S., Kamala C and Raj D. S. S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol Environ Safety. 2008;69(3):513-524.
- Norouzirad R., González-Montaña J. R., Martínez-Pastor F., Hosseini H., Shahrouzian A., Khabazkhoob M., Ali Malayeri F., Moallem Bandani H., Paknejad M., Foroughi-Nia B and Fooladi Moghaddam A. Lead and cadmium levels in raw bovine milk and dietary risk assessment in areas near petroleum extraction industries. Sci Total Environ. 2018;635:308-314.
- Younus M., Abbas T., Zafar M., Raza S., Khan A., Saleem A. H., Idrees M. A., Nisa Q. U., Akhtar R and Saleem G. Assessment of heavy metal contamination in raw milk for human consumption. S Afr J Anim Sci. 2016;46(2):166-169.
- Kim D. G., Kim M., Shin J. Y and Son S. W. Cadmium and lead in animal tissue (muscle, liver and kidney), cow milk and dairy products in Korea. Food Addit Contam Part B Surveil. 2016;9(1):33-37.
- Förstner U and Wittmann G. T. Metal pollution in the aquatic environment. Springer Science & Business Media. 2012.
- Tripathi R. M., Raghunath R., Sastry V. N and Krishnamoorthy T. M. Daily intake of heavy metals by infants through milk and milk products. The Science of the total environment. 1999;227(2-3):229-235.
- Abd El-Salam M. M., Farahat M. F., Abu-Zuid G. I and Saad S. G. Application of in-plant control measures in some Egyptian micro-scale dairy enterprises and its impact on heavy metal contents of their products. Environ Monit Assess. 2017;189(10):486. doi: 10.1007/s10661-017-6214-x.
- Lu Y., Song S., Wang R., Liu Z., Meng J., Sweetman A. J., Jenkins A., Ferrier R. C., Li H and Luo W. Impacts of soil and water pollution on food safety and health risks in China. Environ Int. 2015;77: 5-15.
- Johnson M. D., Kenney N., Stoica A., Hilakivi-Clarke L., Singh B., Chepko G., Clarke R., Sholler P. F., Lirio A. A. and Foss C. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med. 2003;9(8):1081-1084.
- Navas-Acien A., Tellez-Plaza M., Guallar E., Muntner P., Silbergeld E., Jaar B and Weaver V. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol. 2009;170(9):1156-1164.








