Anna N. Berlina , Anastasia V. Bartosh
, Anastasia V. Bartosh , Anatoly V. Zherdev
, Anatoly V. Zherdev , Sergei A. Eremin
, Sergei A. Eremin and Boris B. Dzantiev
and Boris B. Dzantiev
A. N. Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Moscow 119071, Russia.
Corresponding Author E-mail: dzantiev@inbi.ras.ru
DOI : https://dx.doi.org/10.13005/bpj/1609
Abstract
Detection of antibiotics in the blood is necessary for characterizing their common or individual pharmacokinetics. This has increased the need in rapid detection techniques, such as lateral flow immunoassay, for the on-site control of antibiotics. The present study characterized factors influencing the analytical parameters of lateral flow immunoassay to increase its sensitivity for detecting tetracycline in human serum samples. Assay sensitivity was increased by altering the concentrations of immunoreagents and surfactant and the number of interaction stages in the assay with indirect labeling a specific antibody. The optimal assay conditions reduced the limit of visual detection of tetracycline from 100 to 10 ng/mL. The developed assay allowed us to detect tetracycline in both two-fold diluted and undiluted human serum samples within 15 min. Our results suggest that the developed assay can be used to screen patients under antibiotic treatment.
Keywords
Antibiotics; Gold Nanoparticles; Immunochromatography; Limit of Detection; Pharmacokinetics; Rapid Tests
Download this article as:| Copy the following to cite this article: Berlina A. N, Bartosh A. V, Zherdev A. V, Eremin S. A, Dzantiev B. B. Management of Factors for Improving Antigen–Antibody Interaction in Lateral flow Immunoassay of Tetracycline in Human Serum Samples. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Berlina A. N, Bartosh A. V, Zherdev A. V, Eremin S. A, Dzantiev B. B. Management of Factors for Improving Antigen–Antibody Interaction in Lateral flow Immunoassay of Tetracycline in Human Serum Samples. Biomed Pharmacol J 2019;12(1). Available from: http://biomedpharmajournal.org/?p=25751 |
Introduction
Tetracyclines are a group of broad-spectrum antibiotics that exert a bacteriostatic effect on bacterial cells. Tetracycline (Tet) and its related compounds bind to the 30S subunit of bacterial ribosomes to block protein synthesis in bacterial cells.1 Tetracyclines are widely used in human and veterinary medicine. Despite the synthesis, and clinical investigation of various novel broad-spectrum antibiotics, Tet progenitors are widely used for treating infections caused by sensitive bacteria.2 Tet and doxycycline are the drugs of choice for treating bone and joint infections and for use in suppressive antibiotic therapy of patients at home (under out-of-hospital conditions).3,4 However, an increase in bacterial resistance in previous years has highlighted the need to regulate antibiotic treatment carefully. Different approaches have been used in modern medicine to prevent the development of antibiotic resistance and to increase the effectiveness of antibiotic therapy. An understanding of the specificity of an infectious agent does not guarantee the sensitivity of this agent to the antibiotic chosen for treatment.5 Investigations performed worldwide indicate that many factors influence the effectiveness of antibiotic therapy.6 Microbiological methods used worldwide help in understanding reasons underlying inflammation and in selecting appropriate antibiotics for medication.7 Adequate dosage of antibiotics is important because it decreases the intensity of side effects and affects the ability of bacteria to develop antibiotic resistance [8, 9]. High doses of antibiotics reduce bacterial survival more effectively than increased duration of antibiotic exposure.10 Problems concerning the personalization of medicine are actual, and development of personalized medicine is important.11,12
These factors highlight the need to control Tet level in the serum of humans under treatment. This can be achieved using different methods. Traditional chromatographic methods are expensive because of the high cost of equipment and complications associated with sample pretreatment.13,14 In contrast, immunochemical methods are highly specific because they involve antibody–antigen interaction and do not require sample pretreatment or preparation.15 Among the various immunochemical methods available, immunochromatography is a simple and rapid technique that can be used under out-of-laboratory conditions. Quantitative analysis to estimate analyte concentration can be performed using portable readers.16,17 Thus far, many developments have been described for the rapid analysis of different analytes in serum samples.18-20 However, specific features of the serum influence antibiotic detection.
Antibody labeling in lateral flow immunoassay (LFIA) can be performed using two methods. The first method is a traditional method involving the direct adsorption of an antibody on the surface of gold nanoparticles. The second method involves the use of a native specific antibody in combination with a conjugate of antispecies antibody and gold nanoparticles. Previous studies have shown that the direct immobilization of a specific antibody on the surface of gold nanoparticles results in the inefficient binding of the target analyte to the conjugate without decreasing an analytical signal.21 A comparison of competitive immunoassays involving both direct and indirect labeling has shown that the prevention of inefficient binding by the indirect labeling significantly improves assay sensitivity.22-25 Thus, the present study investigated the most efficient method for performing the immunochromatographic detection of Tet with indirect labeling.
Materials and Methods
Materials
Mouse anti-Tet monoclonal antibody (mAb) and hapten-protein conjugate (Tet conjugated with bovine serum albumin [BSA]; Tet-BSA), as described previously,26 were provided by Prof. C. Xu of Jiangnan University (Wuxi, China). Goat anti-mouse polyclonal (antispecies) antibodies were purchased from Arista Biologicals (Allentown, PA, USA). Compounds for preparing and storing gold nanoparticles (sodium azide, Tween-20, and chloroauric acid) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Triton X-100 was obtained from Panreac Química (Barcelona, Spain), a Tet base was obtained from Applichem (Darmstadt, Germany), and BSA was obtained from Boval Biosolutions (Cleburne, TX, USA). All other reagents were of analytical-grade purity or higher.
All solutions were prepared using deionized water obtained using Milli-Q system (18 MΩ·cm at 25°C; Simplicity Millipore, Billerica, MA, USA). Working nitrocellulose membrane CNPC15 was obtained from MDI Membrane Technologies (Ambala Cantt, India). CFSP223000 adsorption pads and fiberglass macroporous CFCP203000 conjugate pads were obtained from Millipore (Bedford, MA, USA). IsoFlow dispenser (Imagene Technology, Hanover, NH, USA) was used to apply the reagents onto the membranes, and Index Cutter-1 (A-Point Technologies, Gibbstown, NJ, USA) was used to cut multimembrane composites onto individual test strips.
Methods
Synthesis of Gold Nanoparticles and Their Conjugation with Antibody
Gold nanoparticles were synthesized using a standard protocol for synthesizing 30-nm nanoparticles, as described previously.27 First, 0.2 mL 5% HAuCl4 was added to 97.5 mL water, and the solution was boiled. Next, 1.5 mL 1% sodium citrate was added to this solution, and the resulting mixture was boiled for 22 min, cooled, and stored at 4°C–6°C. Additional details of this method are given in a previous study.28
The anti-mouse IgG antibody (aMAb) dialyzed against 10 mM Tris-HCl buffer (pH 8.6) was diluted using 0.2 M K2CO3 solution and was added to the gold nanoparticle solution whose pH was preadjusted to 8.6. For this, 8 mL gold nanoparticle solution was added to 150 µL aMAb solution (concentration, 1 mg/mL) in a glass flask, and the mixture was incubated with stirring at room temperature for 45 min. Next, 200 µL 10% aqueous BSA solution was added to the mixture, followed by incubation with stirring at room temperature for 15 min. The obtained conjugate was separated from unbound antibodies through centrifugation at 10,000 × g and 4°C for 15 min, and supernatant obtained was decanted. Next, 1 mL 50 mM potassium phosphate buffer (pH 7.4) supplemented with 0.1 M NaCl (PBS) containing 0.25% BSA, 0.25% Tween-20, 1% saccharose, and 0.05% NaN3 was added to the precipitate obtained after centrifugation and was used for subsequent analysis. Optical density of the obtained conjugate was determined using Libra S60 spectrophotometer (Biochrom, Cambridge, UK).
Assembly of the Multimembrane Composite and Preparation of the Test Strips
The Tet-BSA conjugate and antispecies (RAMI) antibodies at concentrations of 2 and 0.5 mg/mL, respectively, were applied to a nitrocellulose working membrane at a rate of 0.1 μL per 1 mm of the membrane. Next, the membrane was dried for 20 h at 37°C and was then glued to an absorbing fiberglass pad such that the fiberglass pad in the multimembrane composite was first in contact with the sample and then in contact with the absorbent. The resulting sheet was cut into 3.3-mm wide test strips by using an automatic guillotine cutter. The test strips were placed in a plastic foil pack together with a desiccant (0.6 g silica gel in bags) and were sealed. Cutting and packaging were performed at 20°C–22°C in a special room maintained at a relative humidity of not more than 30%.
Lateral flow Immunoassay
The spiked serum samples, working buffer PBS containing 0.05% Triton X-200 (PBST) solution, and test strips were maintained at room temperature (20°C–25°C). Next, the test strips were vertically immersed into a serum sample containing different concentrations of Tet, specific antibody, and 5 μL 20% Tween-20 (solution 1) for 7 min. Next, the strips were transferred to the PBST solution for 3 min and were dipped in the conjugate suspension (PBST supplemented with the aMAb conjugate with an optical density of 0.5 at 520 nm [A520] for 3 min. Next, the test strips were dried by placing them horizontally and after 2 min were scanned using CanoScan 9000F MarkII (Canon, Tokyo, Japan).
Data Processing
Color intensity was assessed by processing scanned digital images of the test strips by using TotalLab software (Nonlinear Dynamics, Newcastle upon Tyne, UK) with 1D regimen. Calibration curves were obtained by plotting the relationship between color intensity in the test zone and Tet concentration in a standard solution by using Origin 9.0 software (OriginLab, Northampton, MA, USA). Sigmoid function was used for fitting based on the following four-parametric equation: y = (A – D)/(1 + [x/C]B) + D.
Results and Discussion
Development of LFIA Involving the Indirect Labeling of Antibody
Our study focused on factors that influenced the analytical parameters of LFIA (importantly, the limit of detection [LOD]) for determining the target analyte. First, concentrations of the anti-Tet antibody and Tet-BSA conjugate were considered. Previous characterization of these immunoreactants suggested that the mAb interacted with the target analyte and its structurally related compounds in food matrixes (e.g., milk).26 The present study was performed to characterize Tet detection in human serum samples by performing LFIA and to determine conditions influencing LFIA results.
The initial concentration of the hapten-protein conjugate (Tet-BSA) was chosen based on our previous experience29 concerning Tet determination. The slightly high concentration of the unlabeled specific antibody was chosen based on the results obtained for a buffer solution22 because of the high viscosity of the serum sample. Optical density of the gold conjugate was chosen as its commonly applied dilution in competitive immunoassays.30 Thus, the initial parameters were as follows: 1 mg/mL of Tet-BSA loaded onto the CNPC15 membrane, 300 ng/mL of the specific antibody adsorbed onto the fiberglass pad, and the gold conjugate with A520 of 1.0. Human serum samples containing different Tet concentrations and diluted two times with the working buffer solution were used as the sample solution. However, comparison of the buffer solution and serum samples indicated that the serum samples delayed the interaction and resulted in background staining (Figure 1). The LOD of Tet was set to >30 ng/mL.
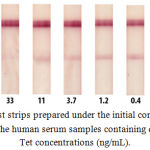 |
Figure 1: Test strips prepared under the initial conditions after testing the human serum samples containing different Tet concentrations (ng/mL).
|
Methods for Decreasing Background Staining
Several approaches are available for decreasing unspecific staining in the test zone, including varying the concentration of immunoreagents,31 use of additives that influence antigen–antibody interaction30 and pretreatment of membrane or sample.32,33 Factors affecting assay results were determined using undiluted and diluted serum samples.
Optimization of The Concentration of Immunoreagents
The first approach method to optimize LFIA condition was to optimize Tet-BSA and mAb/Tet concentrations. We used three concentrations of Tet-BSA, namely, 0.5, 1.0, and 1.5 mg/mL, and three concentrations of mAb/Tet, namely, 100, 200, and 300 ng/mL. Low Tet-BSA concentration decreased test zone coloration and prevented background staining. However, the signal in the test zone decreased in the absence of a competitor at the same time (data not shown). An increase in Tet-BSA concentration to 1.0 mg/mL increased test zone coloration, which allowed the visual distinction of staining in the absence and presence of the analyte. A further increase in Tet-BSA concentration increased test zone coloration in the presence of high Tet concentration (100 ng/mL). Therefore, Tet-BSA concentration of 1.0 mg/mL was chosen as the optimal concentration in subsequent experiments.
The principle of competitive LFIA indicates that the concentration of the specific antibody is one of the most important parameters. We observed that 100 ng/mL specific antibody in combination with 1.0 mg/mL Tet-BSA provided an adequate analytical signal (approximately 25 arbitrary units). A further increase in the antibody concentration increased unspecific staining by more than two fold. Therefore, 100 ng/mL was chosen as the optimal concentration of the specific antibody in subsequent experiments.
The Order of Interaction and the Number of Stages
Indirect labeling of the antibody was performed in two ways. The first method involved the separation of immunological stages. After the competitive stage for a sample containing Tet, immobilized Tet-BSA, and mAb/Tet, a washing step was added to the protocol, followed by incubation with the aMAb conjugate. The results are shown in Fig. 2, tests 1–3. The second method involved the use of a mixture of all the reagents in one stage, i.e., mixing the serum sample with the specific antibody solution and the aMAb conjugate, and the obtained results were compared. We observed that the separation of the antigen–antibody interaction (competitive stage) and the specific antibody (i.e., the antispecies antibody conjugated with gold nanoparticles) decreased unspecific staining and allowed a clearer visualization of the test zone. At the same time, the use of a single-stage interaction without the washing step decreased the signal in the test zone (Fig. 2, tests 4 and 5). Thus, the washing step and separation of immunochemical interaction can also influence assay results. Moreover, we observed that the separation of the competitive and staining stages was preferable when an undiluted serum sample was used. Addition of the washing step to the protocol removed the excess serum proteins from the membrane and stabilized the flow of the gold conjugate (Fig. 2, tests 3 and 5 for comparison).
Alteration of Surfactant Concentration
The human serum is a complicated sample containing high concentration of proteins and having high viscosity. Therefore, one task in the present study was to achieve a uniform flow of the serum sample along the membrane. Membrane type was chosen based on our previous experience34,35 and recommendations of manufacturers.36 A surfactant was added to the sample to achieve the necessary flow rate and to decrease sample viscosity. Tween-20 was chosen as the surfactant based on the results of previous studies involving viscous samples.34,35 We examined various Tween-20 concentrations (0.25%, 0.5%, and 1.0%). We observed that the use of 0.25% Tween-20 did not provide the necessary flow rate and produced a signal in the test zone within only 30 min (Table 1). An increase in the surfactant concentration increased the lateral flow rate; however, the achieved flow rate was still low. However, the use of 1% Tween-20 provided sufficient movement of the sample and uniform distribution of the conjugate.
Table 1: Parameters of LFIA for detecting Tet in serum samples containing different Tween-20 concentrations.
| Parameter | Concentration of Tween-20 | ||
| 0.25% | 0.5% | 1.0% | |
| Arbitrary units | 30.4 | 35.6 | 41.2 |
| Conjugate elution time | Low (>25 min) | Low (20 min) | Normal (7 min) |
A further increase in Tween-20 concentration in the sample (to 2.5% or 5%) decreased the flow rate, thus preventing the generation of a staining signal in the test zone (Fig. 2). Furthermore, initially used optical density of aMAb conjugate (A520=1.0) has been decreased by more than two fold. This factor also allowed in the improved distinction of signal-to-noise ratio because of a decrease in unspecific staining.
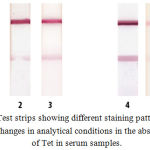 |
Figure 2: Test strips showing different staining patterns based on changes in analytical conditions in the absence of Tet in serum samples.
|
Tests 1–3: analysis using the separation of immunochemical stages. Tests 4 and 5: one-stage analysis. Test 1: working buffer solution supplemented with 0.25% Tween-20 and 100 ng/mL mAb/Tet; test 2: undiluted serum sample, 0.25% Tween-20, and 100 ng/mL mAb/Tet; test 3: undiluted serum sample, 1.0% Tween-20, and 100 ng/mL mAb/Tet; test 4: working buffer solution supplemented with 0.25% Tween-20 and 200 ng/mL mAb/Tet; and test 5: undiluted serum sample, 1.0% Tween-20, and 200 ng/mL mAb/Tet. The duration of analysis for the tests 4 and 5 was 7 min.
Based on these findings, the optimal concentrations of Tet-BSA, mAb/Tet, and Tween-20 were set to 1.0 mg/mL, 100 ng/mL, and 1%, respectively. The recommended dilution of aMAb conjugate accords to A520 = 0.5.
Calibration Curve and Analytical Characteristics of the Optimized LFIA
Test strips corresponded to different concentrations of Tet were obtained for the (a) twice-diluted and (b) undiluted spiked serum samples under optimal conditions. Fig. 3 shows the absence of differences between the the obtained assay results. Both the methodscan be used to determine Tet concentration in human serum samples. Fig. 3 also shows the absence of unspecific staining, which was a disturbing factor at the beginning of this optimization. For improved perception, two calibration curves have been plotted for Tet determination in the undiluted human serum sample (Fig. 4). The curve (a) presents the data from Fig. 1. The visual LOD of Tet was approximately 100 ng/mL under non-optimized conditions (see Fig. 1). The choice of reagent concentration helped in decreasing the visual LOD of Tet by 10 fold to 10 ng/mL (at the disappearance of the test line; Fig. 3). Optimization of assay conditions also highlighted a possibility of increasing the signal-to-noise ratio through simple manipulations.
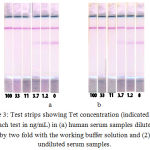 |
Figure 3: Test strips showing Tet concentration (indicated under each test in ng/mL) in (a) human serum samples diluted by two fold with the working buffer solution and (2) undiluted serum samples.
|
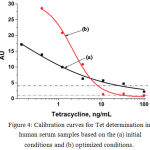 |
Figure 4: Calibration curves for Tet determination in human serum samples based on the (a) initial conditions and (b) optimized conditions.
|
The dash-dot lines indicate the level of non-specific staining observed by the naked eye.
Conclusion
The LFIA conditions for detecting Tet in human serum samples by performing indirect labeling of the specific antibody were optimized by chosing the concentrations of immunoreagents and surfactant (Tween-20) and separating the immunochemical interaction stage. This analysis of factors influencing the parameters of LFIA helped in decreasing the visual LOD of Tet by 10 fold to 10 ng/mL. The versatility of the described approach indicates that it can be used to optimize the interaction between other antibodies and antigens.
Acknowledgments
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (agreement no. 14.613.21.0061, 07.17.2017). The unique identification number of this project is RFMEFI61317X0061.
Conflict of Interest
There is no conflict of interest.
Funding Source
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (agreement no. 14.613.21.0061, 07.17.2017). The unique identification number of this project is RFMEFI61317X0061.
Ethics
The local Ethical Committee of the Centre has approved this investigation, and that the subjects gave informed consent to the work. The experiment was conducted in accordance with the legal guidelines of the concerning authority.
References
- Brodersen D. E., Clemons W. M., Carter A. P., Morgan-Warren R. J., Wimberly B. T and Ramakrishnan V. The Structural Basis for the Action of the Antibiotics Tetracycline, Pactamycin, and Hygromycin B on the 30S Ribosomal Subunit. Cell. 2000;103:1143-1154.
CrossRef - Chen A., Smith K. P., Whitfield B. A., Zucchi P. C., Lasco T. M., Bias T. E., Kirby J. E and Hirsch E. B. Activity of minocycline against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae clinical isolates with comparison to doxycycline and tigecycline. Diagn. Microbiol. Infect. Dis. 2017;88:365-367.
CrossRef - Darley E. S. R and MacGowan A. P. Antibiotic treatment of Gram-positive bone and joint infections. J. Antimicrob.Chemother. 2004;53:928-935.
CrossRef - Pradier M., Robineau O., Boucher A., Titecat M., Blondiaux N., Valette M., Loïez C., Beltrand E., Nguyen S., Dézeque H., et al. Suppressive antibiotic therapy with oral tetracyclines for prosthetic joint infections: a retrospective study of 78 patients. Infection. 2018;46:39-47.
CrossRef - Speer B. S., Shoemaker N. B and Salyers A. A. Bacterial resistance to tetracycline: mechanisms, transfer, and clinical significance. Clin. Microbiol. Rev. 1992;5:387-399.
CrossRef - Li J., Xie S., Ahmed S., Wang F., Gu Y., Zhang C., Chai X., Wu Y., Cai J and Cheng G. Antimicrobial Activity and Resistance: Influencing Factors. Front. Pharmacol. 2017;8. doi: 10.3389/fphar.2017.00364. eCollection 2017.
CrossRef - Balouiri M., Sadiki M and Ibnsouda S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016;6:71-79.
CrossRef - Baker C. M., Ferrari M. J and Shea K. Beyond dose: Pulsed antibiotic treatment schedules can maintain individual benefit while reducing resistance. Sci. Rep. 2018;8:5866.
CrossRef - Ahmad A., Græsbøll K., Christiansen L. E., Toft N., Matthews L and Nielsen S. S. Pharmacokinetic-pharmacodynamic model to evaluate intramuscular tetracycline treatment protocols to prevent antimicrobial resistance in pigs. Antimicrob. Agents.Chemother. 2015;59:1634-1642.
CrossRef - Brauner A., Fridman O., Gefen O and Balaban N. Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016;14:320-330.
CrossRef - McAlister F. A., Laupacis A and Armstrong P. W. Finding the right balance between precision medicine and personalized care. Can. Med. Assoc. J. 2017;189:E1065-E1068.
CrossRef - Schuetz P. Personalized medicine of patients with respiratory infections through the measurement of specific blood biomarkers: fact or fiction? Expert Rev. Respir. Med. 2017;11:605-607.
CrossRef - Junior L. B., Pugens A. M., Pritsch M. C., Mantovani P. B., dos Santos M. B and Manfio J. L. Development and validation of a liquid chromatographic/tandem mass spectrometric method for determination of tetracycline in human plasma: application to bioequivalence study. J AOAC Int. 2008;91:731-738.
- Haidara H., Saffaj T., Tan A., Bentama A., Outmoulait W., Benchekroun Y. H and Ihssane B. Full validation using β-content, γ-confidence tolerance interval: Application for LC-MS/MS determination of Doxycycline in human plasma. Chemom. Intell. Lab. Syst. 2017;168:89-95.
CrossRef - Sotnikov D. V., Zherdev A. V and Dzantiev B. B. Theoretical and experimental comparison of different formats of immunochromatographic serodiagnostics. Sensors. 2018;18:36.
CrossRef - Mak W. C., Beni V and Turner A. P. F. Lateral-flow technology: From visual to instrumental. Tr. Anal. Chem. 2016;79:297-305.
CrossRef - Eltzov E., Guttel S., Adarina L. Y. K., Sinawang P. D., Ionescu R. E and Marks R. S. Lateral flow immunoassays – from paper strip to smartphone technology. Electroanalysis. 2015;27:2116–2130.
CrossRef - Tüdős A. J., Besselink G. A. J and Schasfoort R. B. M. Trends in miniaturized total analysis systems for point-of-care testing in clinical chemistry. Lab Chip. 2001;1:83-95.
CrossRef - Hu J., Wang S., Wang L., Li F., Pingguan-Murphy B., Lu T. J and Xu F. Advances in paper-based point-of-care diagnostics. Biosen. Bioelectron. 2014;54:585-597.
CrossRef - Sajid M., Kawde A. N and Daud M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015;19:689-705.
CrossRef - Sotnikov D. V., Radchenko A. S., Zherdev A. V and Dzantiev B. B. Determination of the composition and functional activity of the conjugates of colloidal gold and antibodies. Eurasian J. Anal. Chem. 2016;11:169-179.
- Berlina A. N., Bartosh A. V., Sotnikov D. V., Zherdev A. V., Xu C and Dzantiev B. B. Complexes of gold nanoparticles with antibodies in immunochromatography: Comparison of direct and indirect immobilization of antibodies for the detection of antibiotics. Nanotechnol. Rus. 2018;13:80-87.
CrossRef - Urusov A. E., Petrakova A. V., Gubaydullina M. K., Zherdev A. V., Eremin S. A., Kong D., Liu L., Xu C. and Dzantiev B. B. High-sensitivity immunochromatographic assay for fumonisin B1 based on indirect antibody labeling. Biotechnol. Lett. 2017;39:751-758.
CrossRef - Urusov A. E., Petrakova A. V., Zherdev A. V and Dzantiev B. B. Multistage in one touch design with a universal labelling conjugate for high-sensitive lateral flow immunoassays. Biosens. Bioelectron. 2016;86:575-579.
CrossRef - Urusov A. E., Zherdev A. V and Dzantiev B. B. Use of gold nanoparticle-labeled secondary antibodies to improve the sensitivity of an immunochromatographic assay for aflatoxin B1. Microchim. Acta. 2014;181:1939-1946.
CrossRef - Chen Y., Kong D., Liu L., Song S., Kuang H and Xu C. Development of an ELISA and immunochromatographic assay for tetracycline, oxytetracycline, and chlortetracycline residues in milk and honey based on the class-specific monoclonal antibody. Food Anal. Meth. 2016;9:905-914.
CrossRef - Byzova N. A., Zvereva E. A., Zherdev A. V., Eremin S. A., Sveshnikov P. G and Dzantiev B. B. Pretreatment-free immunochromatographic assay for the detection of streptomycin and its application to the control of milk and dairy products. Anal. Chim. Acta. 2011;701:209-217.
CrossRef - Hermanson G. Bioconjugate techniques: 2nd Edition. Academic Press, London, UK. 2008.
- Taranova N. A., Kruhlik A. S., Zvereva E. A., Shmanai V. V., Vashkevich I. I., Semyonov D. A., Eremin S. A., Zherdev A. V. and Dzantiev B. B. Highly sensitive immunochromatographic identification of tetracycline antibiotics in milk. Int. J. Anal. Chem. 2015;10. Article ID 347621.
CrossRef - Berlina A. N., Zherdev A. V., Xu C., Eremin S. A and Dzantiev B. B. Development of lateral flow immunoassay for rapid control and quantification of the presence of the colorant Sudan I in spices and seafood. Food Control. 2017;73:247–253.
CrossRef - Han S., Zhou T., Yin B and He P. A sensitive and semi-quantitative method for determination of multi-drug residues in animal body fluids using multiplex dipstick immunoassay. Anal. Chim. Acta. 2016;927:64-71.
CrossRef - Hsieh H. V., Dantzler J. L and Weigl B. H. Analytical tools to improve optimization procedures for lateral flow assays. Diagnostics. 2017;7:E29.
- Chen Y., Chen Q., Han M., Zhou J., Gong L., Niu Y., Zhang Y., He L and Zhang L. Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chem. 2016;213:478-484.
CrossRef - Berlina A. N., Taranova N. A., Zherdev A. V., Sankov M. N., Andreev I. V., Martynov A. I and Dzantiev B.B. Quantum-dot-based immunochromatographic assay for total IgE in human serum. PLoS One. 2013;8:e77485.
- Berlina A. N., Taranova N. A., Zherdev A. V., Vengerov Y. Y and Dzantiev B. B. Quantum dot-based lateral flow immunoassay for detection of chloramphenicol in milk. Anal. Bioanal. Chem. 2013; 405:4997-5000.
CrossRef - Millipore Inc. Rapid lateral flow test strips: considerations for product development, Millipore Corp.: Bedford, MA. 2013.








