Ketut Dewi Kumara Wati*1,2 , Endah Dhyanti Pratiwi3, Yanni Dirgantara3, Cynthia Retna Sartika3, Meita Dhamayanti1,4
, Endah Dhyanti Pratiwi3, Yanni Dirgantara3, Cynthia Retna Sartika3, Meita Dhamayanti1,4 , Budi Setiabudiawan1,4
, Budi Setiabudiawan1,4 and Ida Parwati1,5
and Ida Parwati1,5
1Faculty of Medicine Universitas Padjadjaran, Bandung, Indonesia.
2Department of Child Health, Faculty of Medicine, Udayana University / Sanglah Hospital Denpasar, Bali, Indonesia.
3Prostem laboratory, Jakarta, Indonesia.
4Department of Child Health, and Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
5Department of Clinical Pathology and Laboratory Medicine, Dr. Hasan Sadikin General Hospital, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
Corresponding Author E-mail: dewi_kumara@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1614
Abstract
Problem in growth might need to be sought deeper inside the cells to enhance better management. There was a lack of study done in chondrocytes to determine growth problem mechanism. We sought to see the chondrocyte’s life span by observing the cells characteristic in a small container over long period. Human mesenchymal stem cell-derived MSC underwent chondrocyte differentiation procedure in a 96 wells-plate using chondrocyte/osteocyte differentiation medium followed by basal medium. A morphological observation and MTT assay was done to observe apoptosis in chondrocyte in the different wells. MTT assay absorbance was used to determine number of apoptosis. Statistical analysis was done to assess the different of mean absorbance values on different days of observation using Anova folowed by post hoc analysis in SPSS IBM package version 24 , with significance determined at P<0.05. Chondrocytes was confirmed with 1% Alcian blue staining and showed characteristic larger then its parent MSC. The MTT assay showed that chondrocyte absorbance reduce from day 3 through day 14, which was different significantly (anova, P=0.03) and day 14 was the most differed compared to day 7 and day 10 (P=0.02, P=0.017 and P=0.013, respectively). The 2D monolayer chondrocyte underwent total apoptosis on day 14 which can set the limit for observation in further long-term observational cell study.
Keywords
Chondrocytes; Monolayer; Osteocyte
Download this article as:| Copy the following to cite this article: Wati K. D. K, Pratiwi E. D, Dirgantara Y, Sartika C. R, Dhamayanti M, Setiabudiawan B, Parwati I. Life Span of the Chondrocytes from Human Umbilical Cord Derived-Mesenchymal Stem Cell Differentiation. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Wati K. D. K, Pratiwi E. D, Dirgantara Y, Sartika C. R, Dhamayanti M, Setiabudiawan B, Parwati I. Life Span of the Chondrocytes from Human Umbilical Cord Derived-Mesenchymal Stem Cell Differentiation. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2Cp9zma |
Introduction
Linear growth or longitudinal growth achieved in human life, is determined by a process of differentiation, proliferation and maturation of the chondrocytes in the growth plate.1,2
This process take place intra uterine and continued in the earliest period of life, mostly during the first year, during the first five year, slower thereafter and rapidly during puberty before entirely stop around 18 years. Some individual might continue to have the process until 21 years of age. We can see the result of the process with an objective measurement in form of body height attainment at the end of the process or any time during the life.3
Even though genetic potentials contribute to the final height attainment; human might experienced some interference during the process, which affect the result. Mostly the external factors come from disease and nutrition especially during childhood. However, nutrition state can also be influenced by a disease, hence need to be carefully assessed, in order to know the primary cause and allow the more proper management.3
In children with HIV, the long-term inflammation corrupt the long-term nutrition status is widely accepted as a mechanism to explain growth failure or stunting.4-8 The fact that stunting continue to occur even though viral load is in control brought the possibility that stunting is a result of disturbance in the process of chondrocyte’s differentiation, proliferation, maturation.9,10
As such, chondrocytes for such research should be made available. Here we report the life span of the chondrocytes from differentiation of mesenchymal stem cell derived from human umbilical cord. Knowing the life span will allow researcher to determine the duration for observation.
Methods
Mesenchymal stem cell isolation and expansion as describe elsewhere.11-16 The differentiation was done at the end of the third passages when we have 1.385.000 cells on harvest. As much as 3.2×104 cells/cm2 were cultured in a 9.2cm dish with a StemproTM Chondrocyte/osteocyte differentiation medium (Thermo Fisher Scientific).17 This differentiation medium was changed on day 7th. and replaced with basal medium with DMEM glutamax, enriched with Platelet rich plasma lysate and antibiotics Pen Strep GibcoTM containing Streptomycin Sulfate 100 μG/mL and Penicillin G Sodium Sulfate 100 μ/mL (Thermo Fisher Scientific). The medium was changed as the proliferation rate of the chondrocytes was satisfying and was clearly observed in the culture.
We did the culture in a 6 well’s plate to observe the ability of the MSC to differentiate into a chondrocyte. To know the ability of the chondrocyte maintained it’s viability capacity in a small container; we did the observation in a 96-wells-plate.
The incubation was done in humidifier incubator with 5% CO2 and temperature 37°C. Chondrocyte was identified with Alcian blue. We did the observation on day 1, 3, 7, 10 and 14, under inverted microscope (Z2 Zeiss-Germany) and captured result in the computer monitor using Axio vision 3.4. software. We observed the morphological changes under a phase-contrast microscope also doing MTT (Methyl Tetrazolium) assay on day 1, 3, 7, 10 and 14.
We prepare MTT solution (Abcam-US) in a 5 mg/mL solution in PBS and mix by vortexing followed by filter sterilize solution after adding MTT. We discard media from cell cultures and add 50 µL of serum-free media and 50 µL of MTT solution into each well, then incubate the plate at 37°C for 3 hours. After incubation, we add 150 µL of MTT solvent into each well then wrap plate in foil and shake on an orbital shaker for 15 minutes. We also pipetting the liquid m to fully dissolve the MTT formazan. We read absorbance at OD=590 nm within 15 minutes. This procedure was done repeatedly on day 1, 3, 7, 10 and 14 to acquire an impression how the chondrocyte proliferated or gone through apoptosis over longer observations.
We start this process using 3.2 x 105 cells/cm2 in a 6 wells culture plate following manufacture recommendation (Thermo Fisher). In a 96 wells, we accommodated 1 x 104 cells/cm2.
The statistical analysis was done to assess difference of MTT absorbance through day 1,3, 7,10 and 14 using Anova on SPSS IBM package version 24.
Ethical clearance was derived from Faculty of Medicine Universitas Padjadjaran no. 205/UN6/KEPK/EC/2018.
Results
The MSC went through the differentiation showed a positive staining on 1% Alcian blue which became more apparent on day 7 as shown in figure 1.
 |
Figure 1: Chondrocytes in a 96 wells-plate under 1% Alcian blue staining reflecting production of glycosaminoglycans.
|
There was a difference of the chondrocytes morphology in a 96 wells-plate on day 7 as shown in figure 2, compared to the morphology on day 14 as shown in figure 3.
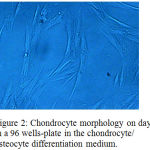 |
Figure 2: Chondrocyte morphology on day 7 in a 96 wells-plate in the chondrocyte/ osteocyte differentiation medium.
|
The observation showed that the chondrocytes went through a high proliferation capacity on day 7, hence we only changed the differentiation medium once on day 7 and using the basal medium thereafter while observing the apoptosis every three days. On day 14 while chondrocytes in a 96 wells underwent total apoptosis on day 14th.
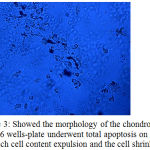 |
Figure 3: Showed the morphology of the chondrocytes in a 96 wells-plate underwent total apoptosis on day14, in which cell content expulsion and the cell shrinked.
|
This morphology changes was in accordance to a dynamic of MTT absorbance as shown in figure 4.
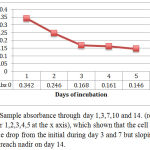 |
Figure 4: Sample absorbance through day 1,3,7,10 and 14. (represented by number 1,2,3,4,5 at the x axis), which shown that the cell MTT absorbance drop from the initial during day 3 and 7 but sloping on day 10 to reach nadir on day 14.
|
In Anova test, it is found that data sample absorbance was homogenous and there was a significant difference between sample absorbance from day 1 through 14 (P=0.003), with day 14 showed the most significant different from the other day to day 1, 3, 7 and 10 (Post hoc HSD, P=0.002).
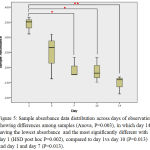 |
Figure 5: Sample absorbance data distribution across days of observation showing differences among samples (Anova, P=0.003), in which day 14 having the lowest absorbance and the most significantly different with day 1 (HSD post hoc P=0.002), compared to day 1vs day 10 (P=0.013) and day 1 and day 7 (P=0.013).
|
Discussion
This study shown the ability of the MSC from our previous research to differentiate into chondrocytes, confirmed by the positive 1% Alcian blue staining. This staining giving information about the ability of the chondrocytes to produce glycosaminoglycans, the characteristic matrix product of the chondrocytes. We also shown that the chondrocytes experienced different rate of apoptosis over a long period. The longer the observation the more chondrocytes went through apoptosis.
The MTT assay was a well known technique to assess apoptosis or proliferation rate of the cells culture. The MTT reagent formed a formazan crystals which produced yellow or purple color. The intensity of color absorbed by the substrate determined the amount of the cells that remained viable or, in the other hand, apoptosis. Our statistical analysis supported the morphological evidence, showing the apoptosis was mostly occurred on day 14. Having apoptosis on day 14 in a 96 wells-plate, indicates the maximum duration that can be provided for further assay in a 96 wells-plate monolayer.
This research is beneficial to validate life span of chondrocyte in small container. Validation is normal process during chondrocyte differentiation and culture, although many researchers would do this procedure for other purpose. Most of the validation process was done to derive standard cells to be used in purpose for treatment.19
We use the chondrocyte in monolayer to assess proliferative capacity as also done by other researcher, however we directed our activity to use chondrocyte for research purpose. We use human umbilical tissue derived MSC as source for chondrocyte which considered less exposed from unwanted environmental hazard as it is used immediately after birth.
The ability of the chondrocyte to absorb MTT can be used to calculate proliferative capacity for further use. We perform the calculation in different part of the study as we primarily intended to show the total apoptosis during long period of observation. This is due to the use of 2D monolayer culture in an attempt to measure any process in the chondrocyte of children to mimick the chondrocyte in the growth plate. Unlike in the adult where providing healthy donor for cartilage is possible, in the children population, deriving chondrocyte from cartilage or growth plate would considered unethical as this might lead to growth arrest. As such, human umbilical cord MSC was a more rational option. This tissue can be collected without an invasive procedure, although in many tradition in our country, the umbilical cord was an important aspect for the baby including after birth. As such an informed consent was absolute before collecting the cord.
Mimicking chondrocytes in the growth plate invitro has been attempted by other researcher in which found that apoptosis would be occur on day 23-37. The researcher used chondrocytes from animal. This findings was different from our findings, in which we intended to observe until day 21, however, with regards to the small container, we fail to observe until day 21. The study was done using fetal bovine epiphyseal chondrocyte cartilage and attempted to show the fate of cartilage that experienced apoptosis after hypertrophic state.20
In the other part of our study, we also documented that chondrocytes underwent hypertrophic state before apoptosis. As conclusion, we have shown the life span of monolayer chondrocytes in small container for 14 days. This life span is shorter compared to chondrocyte derived from adult animal chondrocyte, but sufficiently informative for future research done in monolayer chondrocyte.
Acknowledgements
We would like to thanks donors of the umbilical cords.
Conflict of Interest
There is no conflict of interest.
Fundings Source
Faculty of Medicine, Udayana University, under Grants DIPA/PNBP year 2018, NO. 61/UN14.2.2/UPPM/2018.
References
- Kozhemyakina E., Lassar A. B., Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015;142(5):817-31.
CrossRef - Emons J., Chagin A. S., Savendahl L., Karperien M., Wit J. M. Mechanisms of growth plate maturation and epiphyseal fusion. Horm Res Paediatr. 2011;75(6):383-91.
CrossRef - Stein A. D., Wang M., Martorell R., Norris S. A., Adair L. S., Bas I., et al. Growth patterns in early childhood and final attained stature: data from five birth cohorts from low- and middle-income countries. Am J Hum Biol. 2010;22(3):353-9.
CrossRef - Hansudewechakul R., Sirisanthana V., Kurniati N., Puthanakit T., Lumbiganon P., Saphonn V., et al. Antiretroviral Therapy Outcomes of HIV-Infected Children in the TREAT Asia Pediatric HIV Observational Database. J Acquir Immune Defic Syndr. 2010;55(4):503-9.
CrossRef - Padmapriyadarsini C. P. N., Chandrasekaran K., Subramanyan S., Thiruvalluvan C., Bhavani P. K., Swaminathan S. Prevalence of Underweight, Stunting, and Wasting among Children Infected with Human Immunodeficiency in South India. Intl J Pediatr. 2009;2009:5.
CrossRef - Aurpibul L. P. T., Taecharoenkul S., Sirisanthana T., Sirisanthana V. Reversal of growth failure in HIV-infected Thai children treated with non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. AIDS patient care STDs. 2009;29(12):1067-71.
CrossRef - McGrath C. J., Diener L., Richardson B. A.,Peacock-Chambers E., John-Stewart G. C. Growth reconstitution following antiretroviral therapy and nutritional supplementation: systematic review and meta-analysis. AIDS. 2015;29(15):2009-23.
CrossRef - Sutcliffe C. G., van Dijk J. H., Munsanje B., Hamangaba F., Sinywimaanzi P., Thuma P. E., et al. Weight and height z-scores improve after initiating ART among HIV-infected children in rural Zambia: a cohort study. BMC Infect Dis. 2011;11:54.
CrossRef - Bunupuradah T., Kariminia A., Aurpibul L., Chokephaibulkit K., Hansudewechakul R., Lumbiganon P., et al. Final Height and Associated Factors in Perinatally HIV-infected Asian Adolescents. Pediatr Infect Dis J. 2016;35(2):201-4.
CrossRef - Wati K. D. K., Sawitri A. A. S., Vedaswari N. P. D., Satriawibawa W. E., Dhamayanti M., Setiabudiawan B., Parwati I. Time to persistent and newly stunting among children with HIV infection during Highly active antiretroviral treatment. Presented at Asia Pacific Congress of Pediatrics, Bali. 23 Juli 2018.
- Ullah I., Subbarao R. B., Rho G. J. Human mesenchymal stem cells – current trends and future prospective. Biosci Rep. 2015;35(2).
CrossRef - Boeuf S., Richter W. Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Res Ther. 2010;1(4):31.
CrossRef - Ding D. C., Shyu W. C., & Lin S. Z. Mesenchymal Stem Cells. Cell Transplantation. 2011;20(1):5–14. https://doi.org/10.3727/096368910X
CrossRef - Riekstina U., Muceniece R., Cakstina I., Muiznieks I., Ancans J. Characterization of human skin-derived mesenchymal stem cell proliferation rate in different growth conditions. Cytotechnology. 2008;58(3):153-62.
CrossRef - Secunda R., Vennila R., Mohanashankar A. M., Rajasundari M., Jeswanth S., Surendran R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology. 2014;67(5):793-807.
CrossRef - Smith J. R., Pfeifer K., Petry F., Powell N., Delzeit J & Weiss M. L. Standardizing Umbilical Cord Mesenchymal Stromal Cells for Translation to Clinical Use: Selection of GMP-Compliant Medium and a Simplified Isolation Method. Stem cells Intl. 2016;6810980.
CrossRef - Thermofisher. Useful number for cell culture. Available. at https://www.thermofisher.com/id/en/home/references/ gibco-cell-culture-basic/cell-culture-protocols/cell-culture-useful- numbers.html. Accessed on January 9th 2019.
- MTT assay protocol. Available at https://www.abcam.com/kits/mtt- assay-protocol. Accessed on January 13. 2019.
- Galeano-Garces C., Camilleri E. T., Riester S. M., et al. Molecular Validation of Chondrogenic Differentiation and Hypoxia Responsiveness of Platelet-Lysate Expanded Adipose Tissue-Derived Human Mesenchymal Stromal Cells. Cartilage. 2016;8(3):283-299.
CrossRef - Cheung J. O., Grant G. E., Hayland J. A., Freemont A. J., Hillarby M. C. Apoptosis of terminal hypertropic chondrocytes in an invitro model of endochondral ossification. J Pathol. 2003; 201(3):496-503.
CrossRef








