Abdel Razik Farrag1 , Mahmoud Nassar2
, Mahmoud Nassar2 , Zakaria El-Khayat3, Jihan Hussein*3
, Zakaria El-Khayat3, Jihan Hussein*3 , Nadia Ahmed Mohammed3, Dalia Medhat3
, Nadia Ahmed Mohammed3, Dalia Medhat3 , Abd El-Nasser El-Gendy4 and Abdelsamed Elshamy2
, Abd El-Nasser El-Gendy4 and Abdelsamed Elshamy2
1Departments of Pathology, National Research Centre, Cairo, Egypt.
2Natural Compounds Chemistry, National Research Centre, Cairo, Egypt.
3Medical Biochemistry, National Research Centre, Cairo, Egypt.
4Medicinal and Aromatic Plants Research Department, National Research Centre, Cairo, Egypt.
Corresponding Author E-mail: jihan_husein@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1615
Abstract
DNA damage is correlated to type-2 diabetes mellitus (T2DM) and its complications via oxidative stress. This study aimed to evaluate the anti-diabetic effect of Heteroxenia ghardaqensis extract on streptozotocin (STZ) induced-diabetes and how far can this extract attenuate DNA damage in this model. Forty male albino rats (160-180 g) were used in this study and divided into four groups: control, diabetic, diabetic rats received H. ghardaqensis extract (30 mg/kg body weight/day) orally for four weeks and diabetic rats received H. ghardaqensis extract (60 mg/kg body weight/day) orally for four weeks. After the experimental period, fasting blood sugar and serum cholesterol were determined. Urinary 8-hydroxyguanosine (8-OHdG) as a marker of DNA damage was estimated by reversed phase (HPLC). Liver and kidney nitic oxide (NO) and malondialdehyde (MDA) were estimated. Pancreatic tissues were histopathologicaly examined. Our results suggested that diabetes mellitus is accompanied by elevation of DNA damage that enhances the tendency to mutagens and reduce the efficacy of DNA repair. H. ghardaqensis extract appeared to be effective against the oxidative stress induced by STZ which may be due to sesquiterpenoids and diterpenes compounds that scavenge free radicals and increase the antioxidant enzymes as appeared in attenuation of DNA damage.
Keywords
Diabetes Mellitus; DNA Damage; Heteroxenia Ghardaqensis; HPLC - 8-Ohdg; Streptozotocin
Download this article as:| Copy the following to cite this article: Farrag A. R, Nassar M, El-Khayat Z, Hussein J, Mohammed N. A, Medhat D, El-Gendy A. E, Elshamy A. Heteroxenia Ghardaqensis Extract Protects Against DNA Damage in Streptozotocin-Induced Experimental Diabetes. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Farrag A. R, Nassar M, El-Khayat Z, Hussein J, Mohammed N. A, Medhat D, El-Gendy A. E, Elshamy A. Heteroxenia Ghardaqensis Extract Protects Against DNA Damage in Streptozotocin-Induced Experimental Diabetes. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2FeI1lj |
Introduction
Diabetes mellitus (DM) is a common and widespread disease affecting the citizens of both developed and developing countries (Arumugam et al., 2013).
DM characterized by either defects in insulin secretion causing chronic hyperglycemia or insulin resistance which is affected by alteration in cell membrane fatty acids and phospholipids fractions (Hussein et al., 2011).
In diabetic patients, systemic complexities consider the main matter of morbidity and mortality. Oxidative stress results in protein, lipid, and DNA alterations that cause cellular dysfunction and contribute to the pathogenesis of macro- and microvascular complications of diabetes, including diabetic nephropathy (Greenman et al., 2007). Mitochondrion and nucleus with a set of DNA repair enzymes are the mostly affected targets by oxidation (Evans et al., 2004). Excess damage causes impairment of endogenous antioxidant and DNA repair systems (Rachek et al., 2007).
DNA damage is one of the most common complications in diabetes mellitus; urinary 8-hydroxyguanosine (8-OHdG) as a marker for oxidative stress that reflects mitochondrial oxidative damage (Hussein et al., 2016).
Natural products are readily available, with very low side effects and great origin of drugs both directly and indirectly way (Cragg et al., 2013).
Soft corals (Octocorallia, Alcyonacea) comprise multiple types of secondary metabolites. Heteroxenia ghardaqensis are rich in sesquiterpenoids, ceramides, diterpenes, sterols and acylglycerols (Elshamy et al., 2015; Abdel-Razik et al., 2016) and possess many pharmacological activities such as antitumor nature, antiviral, antibacterial, anti-inflammatory, antifungal, antipyretic, hypoglycemic properties, protective effect against cadmium toxicity and antioxidant (Mohamed et al., 2012).
The aim of this study was to evaluate the anti-diabetic effect of Heteroxenia ghardaqensis extract on STZ induced-diabetes and how far DNA damage was attenuated during this treatment.
Materials and Methods
Marine Organism Collection
The soft coral H. ghardaqensis was collected from the Red Sea on May 2014, at a depth of 3–4 m at the front of Hurghada marine station of National Institute of Oceanography and Fisheries, Hurghada, Egypt. The soft coral was collected and identified by Dr. Hashem Madkour, National Institute of Oceanography and Fisheries, Hurghada, Egypt.
Preparation of Extract
The frozen marine organism (wet weight 800 g) was broken down into small pieces and extracted at room temperature with sufficient amount of dichloromethane/methanol (1:1) three times. After filtration, the extract was concentrated under reduced pressure at 50°C afforded dark brown gum (51 g). A part from the extract (15 g) was stored in refrigerator until starting in bioassay. The remain amount was successive fractionated with n-hexane. The hexane fraction was evaporated under reduced pressure at 50°C afforded yellow oily gum (6 gm) that stored in refrigerator until gas chromatography-mass spectroscopy analysis.
Gas Chromatography-Mass Spectroscopy of Hexane Fraction
GC–MS analysis was performed on a Varian gas chromatograph interface to SSQ 3400 coupled to mass selective detector, the columns used were a DB5, 30 M, ×0.25 mm, 0.5 Mm film thickness. Injector and ion source temperature was 220°C, the ionization energy was set at 70 eV, and the volume injected was 0.88 μl at 270°C. The oven temperature was programmed from 50°C for 32 min, isothermal, then heating by 10°C/min to 150°C, isothermal, then heating by 5°C/min to 270°C, and isothermally for 3 min at 270°C.
Chemical
8-hydroxyguanosine (HPLC grade) standard (CAS Number: 1246818-54-1) was purchased from Cayman Chemical Company, USA and streptozotosin (STZ) (CAS Number:18883-66-4) was purchased from MERCK USA. All other chemicals were HPLC grade.
Experimental Animals
Forty male albino rats (160-180 g) were used in this study. Rats were obtained from the animal house of the National Research Centre (NRC) (Cairo, Egypt) and they were housed in stainless steel cages under controlled conditions. The temperature was 23-26°C and the light/dark cycle was 12/12 hours. The animals had free access to water and a standard rodent diet. All animals received human care in compliance with guidelines of the Ethical Committee of National Research Centre (NRC), Egypt and followed the National Institutes of Health Guide Recommendations’ Care and Use of Laboratory Animals.
Induction of Diabetes Mellitus
Streptozotosin was dissolved in sodium citrate (50 mM and PH was adjusted to 4.5) solution containing NaCl (150 mM). The solution (6.0 mg/0.5 ml/100 g body weight) was subcutaneously injected into rats; after 3 days, fasting blood sugar was estimated to confirm the development of diabetes mellitus (Uchiyama and Yamaguchi, 2003).
Experimental Design
Forty male albino rats were used in this study and divided into four groups as follow: Group I (control group): healthy rats received saline. Group II (diabetic group): diabetic rats received a vehicle. Group III) treated I) diabetic rats received H. ghardaqensis extract (30 mg/kg body weight/day) orally for four weeks. Group IV (treated II) diabetic rats received H. ghardaqensis extract (60 mg/kg body weight)/ day orally for four weeks. After the experimental period, 24 h urine was collected from each animal for estimation of urinary 8-hydroxyguanosine, then fasting blood samples were withdrawn from the retro orbital venous plexus. Liver, kidney and pancreases were removed quickly from each rat and washed with ice-cold saline. Liver and kidney were homogenized in 0.1 M Tris buffer for biochemical estimations while pancreas was used in histopathological study. Blood was centrifuged at 2000 rpm for 10 minutes at 4°C using cooling centrifuge (Laborzentrifugen, 2K15, Sigma, Germany). Plasma was separated; fasting blood glucose was estimated immediately.
Preparation of Tissue Bomogenate
Liver and kidney tissues were cut into small pieces and homogenized in phosphate buffer (pH 7.4), centrifuged at 4000 rpm using cooling centrifuge for 10 min at 4°C (Hussein et al., 2016); the resulting supernatant was used for chemical parameters estimation.
Estimation of fasting blood sugar (FBS) was done by using enzymatic colorimetric method. Centronic, Germany, according to Trinder (1969).
Plasma total cholesterol was estimated according to Richmond et al. (1973).
Liver and kidney malondialdehyde (MDA) levels were determined according to Uchiyama and Mihar (1978).
Determination of Nitric Oxide
Nitric oxide level was measured as nitrite by using Griess reagent, according to the method of Moshage et al. 1995 (Moshage et al. 1995), where nitrite, stable end product of nitric oxide radical, is mostly used as indicator for the production of No.
Determination of Urinary 8-hydroxy 2, Deoxyguanosine (8-OHdG) by HPLC
8-OHdG was estimated by HPLC system according to Hussein et al. (2016) and after modification of the method described by Kim et al. (2001). Briefly, 8-OHG standard was dissolved in ultrapure water; serial dilutions were prepared and injected onto HPLC to draw a standard curve with different concentration.
Sample Preparation
8-OHG was extracted from 1 ml urine using Strata C18-E (55 um, 70A) column. The eluents were dried under nitrogen gas stream and were reconstituted in 5 ml ultrapure water. 20 μl from each sample were injected onto HPLC.
HPLC Condition
The mobile phase consists of acetonitrile/methanol/phosphate buffer (25/10/965) v/v. Phosphate buffer was prepared by dissolving 8.8 g of potassium dihydrogen phosphate (KH2PO4) in 1000 ml ultrapure water and pH was adjusted at 3.5. The buffer was then filtered 2 times through 0.45 μm pore size sterile membrane filter before using at a flow rate of 1 ml/min through HPLC reverse phase column (250 × 4.6, particle size 5 μl) and electrochemical detector with cell potential of 600 mV. The concentration of urinary 8-OHG was calculated from the standard curve and divided by the urinary creatinine which was estimated by kinetic method described by Larsen, (1972).
Histopathological Study
The histopathologic examination was performed by light microscopy on pancreas specimens that were fixed in 10% formalin. After fixation, the samples were processed to obtain 5 μm thick paraffin sections. Pancreas sections were stained with hematoxilin and eosin (H&E) then slides were observed under a Leica photomicroscope.
Statistical Analysis
Results in this study were represented in the form of mean ± standard error; the current data were analyzed using one-way ANOVA, version 16 of SPSS. When P value is < 0.05, it was considered a significant difference.
Results and Discussion
This study aimed to evaluate the hypoglycemic and antioxidant effects of H. ghardaqensis extract on STZ-induced experimental diabetes. To our knowledge, this is the first report represents hypoglycemic activity of this extract.
The GC-MS of the n-hexane fraction led to identification of 7 major sesquiterpenoids as listed in table 1.
Table 1: the constituents of hexane fraction identified by GC-MS.
| No. | RT | Compound name | MF | % |
| 1 | 25.54 | Cis calamine | C15H20 | 7.33 |
| 2 | 26.52 | α-Muurolene, | C15H20 | 3.42 |
| 3 | 28.30 | α-Calacorene | C15H20 | 2.07 |
| 4 | 29.04 | Cyclolongfoleneoxide | C15H20O | 7.63 |
| 5 | 29.34 | Palustrol | C15H26O | 7.74 |
| 6 | 31.70 | Neoclovene oxide | C15H24O | 52.15 |
| 7 | 42.96 | Dactylol | C15H26O | 4.19 |
The previous chemical characterization of bioactive components of H. ghardaqensis afforded several compounds such as steroids, ceramides and diacylglycerols. Also, the different extracts and isolated compounds were reported to exhibit a significant anticancer, andimicrobial and protective effect of against cadmium toxicity (Elshamy et al, 2013; 2015, Abdel-Razik et al., 2016). Herein, chemical constituents of the n-hexane fractions were identified depending upon gas chromatography-mass spectroscopy technique for the 1st time. Seven sesquiterpenoids were identified as a major constitents. These seven compounds were characterized as neoclovene oxide (52.15), dactylol (4.19), palustrol (7.74), cyclolongifolene oxide (7.63), cis calamene (7.33), α-muurolene (3.42), α-calacorene (2.07).
Diabetes mellitus is known as a metabolic disorder, and described by hyperglycemia related to defect in insulin excretion and/or insulin response, in addition to the modification in intermediary carbohydrate, protein and lipids metabolism. Excessive generation of reactive oxygen species (ROS) cause oxidation of proteins, lipids, carbohydrates and nucleic acids resulting in irreversible cellular damage and thus play a pertinent role in the etiology and pathogenesis of DM and its complications (Boukhris et al., 2012).
STZ is known as a toxic agent to the insulin producing β cells of pancreatic islets. It is generally supposed that STZ is penetrates the cell membrane through glucose transporter-2 (GLUT2) causing alkylation of DNA followed by definitively β cell damage (Szkudelski, 2001).
On the other hand, diabetes is frequently associated with lipid alterations including hypertriacylglycerolemia, increased levels of VLDL cholesterol, and decreased HDL cholesterol (El-Bana et al., 2017).
In this study, STZ administration causes a significant increase in FBS (table2), which may be related to destruction of pancreatic cells. Also, it was observed that in diabetic group there was an elevation in cholesterol level (table 2), NO and MDA levels in both kidney and liver tissues (table 3, 4) concomitant with an elevation of urinary 8-OHdG (table 5).
Table 2: Fasting blood sugar and serum cholesterol in different studied groups.
| Parameters / Groups | FBS (mg/ dl) | Cholesterol (mg/ dl) |
| Control group | 79±0 .4 | 98 ± 0.2 |
| Diabetic group | 274±0.17a | 200±0 .5a |
| Treated I | 125± 0.12a,b | 114±0 .5a.b |
| Treated II | 85± 0.3b | 102±0 .3 b |
Significant P value < 0.05, a = significant difference compared to control group, b = significant difference compared to diabetic group, number of animals in each group =10.
Table 3: liver No, MDA levels in different studied groups.
| Parameters / Groups | No (μmol/g tissue) | MDA (nmol/g tissue) |
| Control group | 8.2 ± 0.8 | 11.7 ± 1.0 |
| Diabetic group | 10.9 ± 1.0 | 31± 0.89a |
| Treatment I | 10.5 ± 1.1 | 19.8±1.0a,b |
| Treatment II | 9.8 ± 0.4 | 14.8 ± 0.6b |
Significant P value < 0.05, a = significant difference compared to control group, b = significant difference compared to diabetic group, number of animals in each group =10.
Table 4: Kidney No and MDA levels in different studied groups.
| Parameters / Groups | MDA (nmol/g tissue) | No (μmol/g tissue) |
| Control group | 17.1 ± 0.8 | 5.5 ± 0.8 |
| Diabetic group | 32.8± 1.2a | 11± 0.9a |
| Treated I | 26.5 ± 1.0a,b | 10.5± 0.7a |
| Treated II | 21.5± 0.28b | 7.7 ± 0.5a ,b |
Significant P value < 0.05, a = significant difference compared to control group, b = significant difference compared to diabetic group, number of animals in each group = 10.
Table 5: Urinary 8-Hydroxyguanosine in different studied groups.
| Parameter / Groups | Urinary 8-OH-dG (ng/mg creatinine) |
| Control group | 3.8 ± 0.17 |
| Diabetic group | 13.4±0.25a |
| Treated I | 8.07±0.24 a,b |
| Treated II | 6.7±0.23 a,b |
Significant P value < 0.05, a = significant difference compared to control group, b = significant difference compared to diabetic group, number of animals in each group =10.
Pancreatic biopsies, white blood cells, plasma, and serum of type 2 diabetic patients showed high levels of pro-oxidants, peroxides and oxidative stress-induced tissue damage biomarkers including oxidation of DNA bases, 4-hydroxy-2nonenal [HNE] proteins, hydroperoxides, 8-hydroxy-deoxyguanine, and 8-epi-prostaglandin F2a (Rehman et al., 2017) accompanied by depletion in both enzymatic anti-oxidant (glutathione peroxidase, catalase, and superoxide dismutase) and non-enzymatic anti-oxidant (vitamins C and E) (Demircan et al., 2008).
Pancreatic β-cell comprise massive amount of mitochondria, redundant subjection to ROS results in β-cells impairment and eventually diabetes. Hyperglycemia-induced functional modifications, release of hydrogen peroxide, superoxide, mitochondrial membrane polarization, and gene expression fingerprints of associated enzymes in endothelial cells indicated that hyperglycemia decline antioxidants gene expression (Patel et al., 2013). In addition, β-cells are more sensitive to damage by nitric oxide and ROS due to their low levels of enzymes that scavenge free radicals. In pancreatic cells, STZ has the ability to act as NO donor (Som et al., 2001), which prevents mitochondrial matrix aconitase activity, leading to DNA alkylation and damage increases the activity of guanylyl cyclase and the formation of cGMP, which are characteristic actions of No.
8-OHdG is known as an ROS-induced alteration in DNA purine bases residue, is a sensitive index of oxidative DNA damage. Plasma 8-OHdG increases with age (Kanek et al., 1996), cigarette smoking (Loft et al., 1996), diabetes (Hussein et al., 2013), hepatitis (Hussein et al., 2016) and during tumorgenesis. Recently, the urinary level of 8-OHdG is a biomarker for the total systemic oxidative stress in vivo (Hussein et al., 2016).
Moreover STZ toxicity not only affects β cells but also have the ability to damage many other tissues including liver and kidney (Imaeda et al., 2002) as was observed in our results.
Concomitant with our results the histological investigation of pancreatic tissues showed a normal structure in case of normal control group. The exocrine components that include closely packed acini that appeared in a well organized and with normal morphology. The interlobular duct is surrounded with the supporting tissue. While, the endocrine portions of pancreas or islets of Langerhans are scattered throughout the exocrine tissue of the pancreas and featured circular shapes with normal cell lining (Figure 1A). Examination of sections of pancreatic endocrine region of diabetic rats revealed a significant islet cells size reduction, pancreatic architecture deformation and sinusoidal spaces when compared to control rats. Significant reduction in the number of islet cells was detected when compared to that of normal group. Reduction in pancreatic islet number and size, atrophy and vacuolation, and connective tissue invasion in the parenchyma of pancreas islet were detected (Figure 1B).
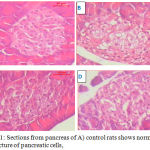 |
Figure 1: Sections from pancreas of A) control rats shows normal architecture of pancreatic cells,
|
Dense-staining acinar cells and a light-staining islet of Langerhans, B) diabetic rat shows distorted pancreatic architecture, C) diabetic rat treated with H. ghardaqensis extract shows restoration of size of the islets along with β–cells repair. Sinusoidal spaces with few scattered areas of necrotic Islet cells were shown, and D) a diabetic rat treated with H. ghardaqensis extract shows more evident recovery of the β-cell (H & E, Sca).
Our data represents that levels of both FBS and serum cholesterol were still significantly increased in treated group compared to control group, where as in treated II group theses values were not significantly different from the control group. Treatment with H. ghardaqensis extract in two different doses slightly decreased this level, but these changes were insignificant. In addition liver and kidney MDA was significantly decreased in the treated groups compared to diabetic groups, the reduction of liver and kidney MDA in treated I group was still significantly increased compared to control group however in treated II group, MDA level decreased to become more or less near the control group.
Treatment with H. ghardaqensis extract in two different doses slightly decreased liver NO, but these changes were insignificant. Moreover, H. ghardaqensis extract in a dose of 30 mg/kg decreased level of kidney NO insignificantly compared to diabetic group. Contrarily, H. ghardaqensis extract in a dose of 60 mg/kg significantly decreased NO compared to the diabetic group.
One of the important results in our study is the elevation of urinary 8-OHdG, which was significantly increased in diabetic group compared to the control group while treatment with H. ghardaqensis extract in two different doses significantly decreased urinary 8-OHdG level compared to the diabetic group. However, these values were still significantly increased in the two treated groups compared to control group.
Also, the administration of the H. ghardaqensis extract (30 mg/kg body weight/day) orally for four weeks results in reformation of architecture and size of pancreatic beta cells concomitant with β–cells repair. Figure (1C) shows sinusoidal spaces with less scattered areas of necrotic Islet cells. β-cell recovery was more evident at the administration of higher dose level (60 mg/kg body weight/day) of the H. ghardaqensis (figure 1D).
Sesquiterpenoids and diterpenes (including the retinoids), and tetraterpenes (carotenoids such as α and β carotene, lutein, lycopene, zeaxanthin and cryptoxanthin) are of the ingredients of H. ghardaqensis that were known to have prophylactic effects from cardiovascular diseases and cancer (Nagarajan, and Brindha, 2012).
The anti-diabetic effect of Diterpenes is caused by reduction of α-glucosidase (Ayinampudi et al., 2012) and activation of nuclear receptor PPAR gamma (Rau et al., 2006). In addition Jung et al. (2009) suggested that phosphorylation of tyrosine in insulin receptor (IR) beta-subunit and in 3T3-L1 adipocytes could affect GLUT- 4 translocation in the presence of insulin, thus these diterpenes were reported as particular activators for insulin receptor.
Diterpenes main targets include, protein tyrosine phosphatase 1B (PTP 1B), AMP-activated protein kinase (AMPK), tumor necrosis factor alpha (TNF-α), glucose transporter-4 (GLUT-4), nuclear receptors (PPAR α, PPAR ɤ, α glucosidase, α amylase inhibitory activity, insulin secretion. Since these targets have special mechanisms for insulin receptor ligand interaction, an understanding of the target structure could be useful to characterize the main reactions and then suggest changes of the ligand composition for more active interaction. Structural features of the diterpenes (contain COOH groups) founded to have activity against diabetes, lactone rings and steroid type structures (Nagarajan, and Brindha, 2012).
ghardaqensis are rich in sterols, which chemically resemble cholesterol; sterols inhibit the intake of dietary and endogenously derived cholesterol from the intestine. Naturally produced sterols cannot synthesize by the human body and are absorbed by the human intestine. Lichtenstein and Deckelbaum (2001) suggested that daily consuming of 0.7–3.2 g of plant sterols have the ability to decrease plasma triglycerides by 5.0–13.0%, and LDL cholesterol by 5.6–24.4% in both normo and hypercholesterolemic cases. Thus sterols play a key role as a food supplementary that control hypercholesterolemia (Lau et al., 2005).
We concluded that, H. ghardaqensis is a promising agent that could be used safely in diabetes mellitus to attenuate elevation of hyperglycemia and DNA damage and we referred these results to the constituents of this organism from antioxidant compounds that appeared in our the analysis of this extract in this study.
Conflict of Interest
There is no conflict of interest.
Acknowledgments
This work was supported by the research project of the National Research Centre (Project ID: 10010303).
References
- Abdel-Razik A. F., Nassar M. I., Elshamy A. I., Kubacy T. M., Hegazy M. E., Ibrahim N., Le Lamer A., Farrag A. H. A new cytotoxic ceramide from Heteroxenia ghardaqensis and protective effect of chloroform extract against cadmium toxicity in rats. Arab. J. Chem. 2016;9(5):649–655.
CrossRef - Arumugam G., Manjula P., Paari N. A review: Anti diabetic medicinal plants used for diabetes mellitus. Journal of Acute Disease. 2013;12:196-200.
CrossRef - Ayinampudi S. R., Domala R., Merugu R., Bathula S., Janaswamy M. R. New icetexane diterpenes with intestinal a-glucosidase inhibitory and free-radical scavenging activity isolated from Premna tomentosa roots. Fitoterapia. 2012;83(1):88-92.
CrossRef - Boukhris M., Bouaziz M., Feki I., Jemai V., El Feki A and Sayadi S. Hypoglycemic and antioxidant effects of leaf essential oil of Pelargonium graveolens L’Hér. in alloxan induced diabetic rats. Health and Disease. 2012;11-81.
- Cragg G. M and Newman D. J. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830(6):3670–3695.
CrossRef - Demircan N., Gurel A., Armutcu F., Unalacak M., Aktunc E., Atmaca H. The evaluation of serum cystatin C, malondialdehyde and total antioxidant status in patients with metabolic syndrome. Med Sci Monit. 2008;14:97–101.
- El-Bana M. A., Medhat D., Ashour M. N., Diab Y., Hussein J. Myrtus communis extract attenuates atherosclerosis in streptozotocin – induced diabetic rats. Bioscience Research. 2017;14(2):257-264.
- Elshamy A. I., Nassar M. I., Mohamed T. K., Madkour H. A new hydroxymethyl diacylglycerol from methanol extract of Egyptian soft coral Heteroxenia ghardaqensis. J of biologically active prod. From Nat. 2015;5(3):172–177.
- Evans M. D., Dizdaroglu M., Cooke M. S. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61.
CrossRef - Greenman I. C., Gomez E., Moore C. E., Herbert T. P. Distinct glucose-dependent stress responses revealed by translational profiling in pancreatic beta-cells. J Endocrinol. 2007;192:179–187.
CrossRef - Hussein J., Abo Elmatty D., Medhat D., Mesbah N., Farrag A. R and Fahmy H. Flaxseed oil attenuates experimental liver hepatitis. Der Pharmacia Lettre. 2016;8(8):142-150.
- Hussein J., Mostafa E., El-Waseef M., El-Khayat Z., Badawy E., Medhat D. Effect of Omega-3 Fatty Acids on Erythrocyte Membrane in Diabetic Rats. Macedonian Journal of Medical Sciences. 2011; 30;4(3):234-239.
- Hussein J., EL khayat Z., Taha M., Morsy S., Drees E and Khateeb S. Dietary omega-3 fatty acids prevent erythrocyte membrane atpase reduction in streptozotocin induced diabetic rats. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;5(4):211-216.
- Imaeda A., Kaneko T., Aoki T., Kondo Y., Nagase H. DNA damage and the effect of antioxidants in streptozotocin-treated mice. Food Chem Toxicol. 2002;40(7):979–87.
CrossRef - Jung S. H., Seol H. J., Jeon S. J., Son K. H., Lee Y. R. Insulin-sensitizing activities of tanshinones, diterpene compounds of the root of Salvia miltiorrhiza Bunge. Phytomedicine. 2009;6(4):327-35.
CrossRef - Kanek T., Tahara S., Matsuo M. Non-linear accumulation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidized DNA damage during aging. Mutat Res. 1996;316:277–285.
CrossRef - Kim M., Moon H., Hong S. Determination of urinary 8 hydroxyeoxy guanosine as a DNA damage marker. Am. Clin. Lab. 2001;42-45.
- Larsen K. Creatinine assay by a reaction-kinetic principle. Chim.Acta. 1972;41:209-217.
CrossRef - Lau V. W., Journoud M and Jones J. H. Plant sterols are efficacious in lowering plasma LDL and non-HDL cholesterol in hypercholesterolemic type 2 diabetic and nondiabetic persons American Society for. Clinical Nutrition. 2005;81:1351-1357.
- Lichtenstein A. H.,Deckelbaum R. J. Stanol/sterol ester-containing foods and blood cholesterol levels. Circulation. 2001;103:1177–9.
CrossRef - Loft S., Vistisen K., Ewertz M., Tjonneland A., Overvad K and Poulsen H. E. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–2247.
CrossRef - Mohamed R., Seliem M. A., Mohamed T. A., Abed-Elfatah A., Abo- Youssef A. M., Thabet M. Bioactive secondary metabolites from the Red Sea soft coral Heteroxenia fuscescens. Int. J. Appl. Res. Nat. Prod. 2012;4(4):15–27.
- Moshage H., Kok B., Huizenga J. R. Nitrite and nitrate determinations in plasma a critical evaluation. Clin. Chem. 1995;41:892-896.
- Mujić A., Grdović N., Mujić I., Mihailović M., Živković J., Poznanović G., Vidaković M. Antioxidative effects of phenolic extracts from chestnut leaves, catkins and spiny burs in streptozotocin-treated rat pancreatic β-cells. Food Chem. 2011;125:841-849.
CrossRef - Nagarajan A and Brindha P. Diterpenes-A Review on Therapeutic uses with special emphasis on Antidiabetic. Activity Journal of Pharmacy Research. 2012;5(8):4530-4540.
- Rachek L. I., Musiyenko S. I., LeDoux S. P., Wilson G. L. Palmitate induced mitochondrial deoxyribonucleic acid damage and apoptosis in L6 rat skeletal muscle cells. Endocrinology. 2007;148:293–299.
CrossRef - Rau O., Wurglics M., Dingermann T. H., Abdel-Tawab M., Zsilavecz M. S. Screening of herbal extracts for activation of the human peroxisome proliferator-activated receptor. Pharmazie. 2006;61(11):952-56.
- Rehman K and Akash M. S. H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? 2017. Journal of Cellular Biochemistry. 118:3577–3585.
CrossRef - Richmond W. Preparation and Properties of a Cholesterol Oxidase from Nocardia sp. and Its Application to the Enzymatic Assay of Total Cholestero in Serum. Clinical chemistry. 1973;19(12):1350-1356.
- Som N. S., Praveen V., Shoba S., Radhey S., Kumria M. M. L., Ranganathan S., Sridharan K. Effect of an anti-diabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmacol. 2001;12:269–277.
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological research / Academia Scientiarum Bohemoslovaca. 2001;50(6):537–46.
- Trinder P. Determination of glucose in blood using glucose oxidase with alternative oxygen acceptor. Annals Clinical Biochemistry. 1969;6:24.
CrossRef - Uchiyama M and Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal.Biochem. 1978;86:271-278.
CrossRef - Uchiyama S., Yamaguchi M. Alteration in serum and bone component findings induced in streptozotocin-diabetic rats is restored by zinc acexamate. Int J Mol Med. 2003;12(6):949-54.
CrossRef








