Luay Abu-Qatouseh*1,2, Eyad Mallah1 and Kenza Mansour1
1Faculty of Pharmacy, University of Petra, Amman, Jordan.
2Jordan Company for Antibody Production (Monojo), Amman, Jordan.
Corresponding Author E-mail: labuqatouseh@uop.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/1629
Abstract
Acne vulgaris is one of the most common health problem where medical treatment is sought in adults worldwide. It has been long described the integral role of Propionibacterium acnes in the pathogenesis of this disease. In this study, a group of local herbs known for their antimicrobial effects were selected for the evaluation of potential anti-acnes effects in vitro. Phenolics and flavonoid contents of methanolic extracts of Eucalyptus globulus, Mentha rotundifolia, Inula viscosa, Utrica dioica, Malva sylvestris, Quercus calliprinos, Arum palaestinum and Achille aodorata collected from different regions in Jordan during 2016-2017 were screened for antimicrobial activity against Propionibacterium acnes by disc diffusion and by broth microdilution method. Measurement of release of interleukin 1 alpha from human skin explants by ELISA was used for the evaluation of anti-inflammatory effects of the herbal preparations and extracts. M. rotundifolia and E. globulus, showed the highest phenolic and flavonoid contents in contrast to M. sylvestris which showed the least phenolic contents. Moreover, polyphenolic fractions exhibited modest anti-acne activity of herbal extracts of E. globulus and A. palaestinum (MIC 0.125 mg/ml), U. dioica (0.25 mg/ml) and I. viscosa (0.5 mg/ml), compared to not significant antimicrobial activity for others (MIC >1mg/ml). Regarding anti-inflammatory effects of the tested fractions, E. globulus and A. palaestinum extracts showed inhibition of interleukin 1 alpha release by more than 60 % for concentrations of 0.5 mg/ml respectively. The presence of anti-inflammatory and anti-acne activities in the polyphenolic extracts of local medicinal plants would increase the potential of using these herbs in the control of Acne vulgaris.
Keywords
Acne Vulgaris; Antimicrobial and Anti-Inflammatory Effects; Polyphenolics
Download this article as:| Copy the following to cite this article: Abu-Qatouseh L, Mallah E, Mansour K. Evaluation of Anti-Propionibacterium Acnes and Anti-Inflammatory Effects of Polyphenolic Extracts of Medicinal Herbs in Jordan. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Abu-Qatouseh L, Mallah E, Mansour K. Evaluation of Anti-Propionibacterium Acnes and Anti-Inflammatory Effects of Polyphenolic Extracts of Medicinal Herbs in Jordan. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2HMKhmW |
Introduction
Acne vulgaris is considered the most frequent skin disease of sebaceous glands with characteristic inflammatory lesions called comedones in the face, back, and trunk. A growing group of evidence stated that the presence of the obligated anaerobic Gram positive bacterium Propionibacterium acnes (P. acnes) in the follicular and sebum canals is implicated in the development of inflammatory acne.1 This is generally explained by the significant capability of this microbe to interact with the components of the immune system. For instance P. acnes is remarkably capable of metabolising sebum fats into free fatty acids, leading to chemotactically attracting neutrophils and induction of monocytes to produce various pro-inflammatory mediators including tumor necrosis factor (TNF) and Interleukin -8 (IL-8).2 Routinely, major treatment options for acne vulgaris include isotretinoin, use of antibiotics like tetracyclines and hormonal drugs. However factors like the long term use of such drugs with their serious side effects in addition to the emergence of antibiotic resistance among clinical strains of P. acnes, constituted considerable limitations of optimal eradication and treatment of this disease.3,4
Medicinal herbs have been extensively investigated as a remarkable approach in encountering infections especially those caused by multi-drug resistant pathogens.5,6 As part of the Arabian folk medicine, several preparations of local herbs and oils, like olive oil and water extracts of pomegranate, were used for the treatment of skin infections.7,8 Several studies showed that phenolic extracts exhibited potent antibacterial and anti-inflammatory effects.9,10 These fractions contain high quantities of different phenolic acids including caffeic acid and ferulic acids which exhibited potent antimicrobial, antiseptic, preservative and anti-oxidant activities.11
Our previous work on polyphenolic extracts of herbs collected from Algeria showed potent anti-microbial activity against Helicobacter pylori, the causative agent of peptic ulcer in humans.12 However, their antimicrobial effects on P. acnes and anti-inflammatory effects were not well characterized. This study aims to determine the antimicrobial effects against P. acnes of polyphenolic extracts of methanolic extracts of Eucalyptus globulus, Mentha rotundifolia, Inula viscosa, Utrica dioica, Malva sylvestris, Quercus calliprinos, Arum palaestinum and Achille aodorata collected from different regions in Jordan and their potential anti-inflammatory effects which, in our opinion, might provide new therapeutic options for the management of acne vulgaris.
Materials and Methods
Plants and Extraction of Polyphenolic Fractions
During 2016-2017, leaves of M. rotundifolia, I. viscose, U. dioica, E. globules, A. odorata, Q. calliprinos, A. palaestinum and M. sylvestris were collected from different regions of Jordan. The plant material was stored at room temperature in a dry place until subsequent use. Leaves were firstly air dried, ground by an electric grinder until becoming a fine powder.13 Next, stitches 50 μm in diameter were used for sifting purposes. The plant powder was then kept in small bottles of tinted glass to avoid the oxidization of their contents. Polyphenolic extraction was performed by maceration in methanol-water 80 / 20 (v / v) at a solid-liquid ratio 1 / 10 (w / v) with continuous stirring at ambient temperature for 48 h followed by filtration with filter papers. Defatting with hexane of the aqueous phase was carried out as described by Yu et al. 2005.14 Subsequently, the obtained methanol phase was concentrated using a rotary evaporator with a vacuum controller, at a temperature of 40°C.
Total Phenolic and Flavonoid Content Measurements
Determination of the total phenols content was performed according to Folin–Ciocalteu’s method.15 Briefly, a total of 0.3 mL of sample was dissolved in 1.7 mL of 1:10 Folin–Ciocalteu reagent. The solutions were then mixed and incubated at room temperature for 10 min. Two mL of 7.5% sodium carbonate (Na2CO3) solution were then added and incubation for 90 min at room temperature was performed. Finally, measurement of the optical density of the solutions at 725 nm was carried out in relation to gallic acid calibration curves where the results were expressed as Gallic acid equivalents (GAE). Total flavonoid content was measured spectrophotometrically at 430 nm. 1:5 diluted samples were equally mixed with 2% aluminium chloride solution and incubated at room temperature for 30 min.16 Quercetin was used to construct the calibration curves and the flavonoids content was expressed in mg of quercetin equivalent (QE) per gram of crude extract. All experiments were carried out in at least three replicates.
Screening for Antibacterial Effect Against P. Acnes by Disc Diffusion Method
The extracts were tested for their potential anti-bacterial effect on P. acnes by the method of Hayes and Markovic17 with some modifications. In brief, standard aliquots with 1.0 × 108 CFU / mL of a control strain of P. acnes (NCTC 747) provided by the Jordan Company for Antibody Production (Monojo) was incubated in tryptic soy broth supplemented with 1% glucose for 48 h under anaerobic conditions using CampyGen atmosphere generating system (Oxoid, UK). The agar base was constituted of molten TSA with glucose. The prepared bacterium inoculum was then added to the molten agar, mixed and evenly poured over the surface of the agar base until it was completely solidified. A sterile paper disc was impregnated with 25 μl of 50 mg / mL of each extract and fraction and then placed on the surface of the agar plates. Plates were then incubated at 37°C for 48 h under anaerobic conditions in an anaerobic jar (Oxoid, UK) under aerobic conditions. Antibacterial activity was determined by the production of an inhibition zone around the impregnated disc with the extracts.18 Positive control discs were made of Clindamycin (10 µg / ml) while discs impregnated with solvents served as negative controls. Growth of P. acnes was confirmed according to Gram staining, biochemical reactions and subsequently by standard PCR of 16S rDNA test.19 P. acnes cultures were stored at -70°C in TSB containing 1% glucose and 15% glycerol.
Determination of Minimal Inhibitory Concentrations (MICs) and Minimal Bactericidal Concentrations (MBCs)
Broth Microdilution method was used to measure MIC values of the extracts and fractions against P. acnes. Cultures of P. acnes containing 1 x 105 cells / mL were prepared in Nutrient yeast glucose broth (NYG). A total of 20 µl of the bacterial suspension was added to 200 µl of two fold serially diluted plant extracts dissolved in NYG broth with a concentration range of 0.06 to 8 mg / mL. subsequently, plates were then incubated for 48 h at 37°C under anaerobic conditions. The growth of P. acnes was measured as a function of turbidity at 600 nm. The MIC was defined as the lowest concentration of the compound to inhibit the visible growth of microorganisms. Triplicates of each extract were performed and the average MIC values were taken.
Evaluation of Anti-Inflammatory Effects by Interleukin Release Assay
Measurement of the interleukin-1a secretion from UVB irradiated human skin cultures (epiCS® skin model, (CellSystems, Germany) was carried out using Human IL-1a immunoassay ELISA Kit (R&D Systems GmbH, Germany) as an indicator for inflammatory process.20 First, human skin explants were exposed for UVB 2 J / cm2 UVB irradiation maximum at 312 nm for 20 min. Then, immediately after irradiation, polyphenolic extracts of each tested herb (two different concentrations; 0.25 and 0.5 mg / mL) were applied to the irradiated skin explants for 5 min. After 24 h, IL-1a secretion was measured in pg / mL. The baseline level of IL-1a was set to the amount of IL-1a in the non-treated non-exposed skin while the maximal IL-1a production as considered as the level in the non-treated UVB-exposed skin. Dexamethazone in three different concentrations (1 uM, 10 uM and 50 uM) was used as anti-inflammatory positive controls and the inhibitory effects of the tested extracts were statistically compared with the non-treated UVB-exposed control according to a one-way anova followed by Dunnett’s t-test (P < 0,05).
Results
Total Phenols and Flavonoids Content
The total phenolic and flavonoid contents in the methanloic extracts of each studied plant material, was calculated as gallic acid and quercetine equivalents, respectively. The reference standard curve of y = 0.0024x + 0.0886, r2= 0.9642 was used for this purpose. The phenolic contents of extracts from M. rotundifolia and E. globulus showed the highest concentrations of 720.56±1.27 and 670.66±1.55 mg GAE / g extract respectively. In contrast, A. palaestinum showed the least total phenolic contents (48.67±0.65 mg GAE/g extract) (Table 1). The flavonoid contents were highest in E.globulus and A. odorata with concentrations of 110.56±0.82 and 101.56±1.27 mg QE / g extract respectively. The percentage of flavonoids in the phenolic fraction was highest in M. sylvestris (37.6%).
Table 1: Concentrations of phenolic and flavonoid contents of methanolic extracts of plants materials used in this study.
| Plant | Total phenols content (mg GAE/g extract) | Total flavonoids content (mg QE/g extract) | % of flavonoids in the phenol fraction |
| E. globulus | 670.66±1.55 | 110.56±0.82 | 16.4 |
| A. odorata | 420.88±1.04 | 101.56±1.27 | 24.0 |
| M. sylvestris | 165.32±0.87 | 62. 40±0.37 | 37.6 |
| Q. calliprinos, | 80.36±0.24 | 18.45±0.19 | 22.5 |
| A. palaestinum | 48.67±0.65 | 12.36±0.84 | 25.0 |
| I. viscosa | 360.45±0.75 | 51.34±0.56 | 14.1 |
| U. dioica | 410.99±1.66 | 41.25±1.51 | 10.0 |
| M. rotundifolia | 720.56±1.27 | 114.17±0.63 | 15.8 |
Growth Inhibition of P. Acnes by Herbal Extracts
In this study, the antimicrobial effect of eight herbal extracts was evaluated (table 2). Notable growth inhibitory effects against P. acnes of polyphenolic extracts of E. globulus, A. palaestinum, U. dioica and I. viscosa were reported. Both extracts from E. globulus, and A. palaestinum presented the highest antimicrobial activity with MIC values of 0.125 mg / mL. Moderate antimicrobial activity of I. visocosa was observed with MIC values of 0.5 mg / mL.
Table 2: Antimicrobial activity of methanolic extracts against P. acnes.
| Plant | Stock concentration (mg / ml) | Antimicrobial effect Zone of inhibition MIC
(mm) (mg / ml) |
|
| E. globulus | 50 | 28 | 0.125 |
| A. palaestinum | 50 | 26 | 0.125 |
| U. dioica | 50 | 22 | 0.25 |
| I. viscosa | 50 | 19 | 0.5 |
| M. rotundifolia | 50 | 9 | 2 |
| M. sylvestris | 50 | None | > 8 |
| Q. calliprinos | 50 | None | > 8 |
| A. odorata | 50 | None | > 8 |
Anti-Inflammatory Effects of the Herbal Extracts
The anti-inflammatory effects of the herbal extracts were summarized in figure 1. The IL-1a release was remarkably increased by 10 folds in the non-treated UVB exposed skin explants, compared with the non-irradiated skin. The UVB exposed skin explants treated with 0.5 mg / ml extracts of E. globulus and A. palaestinum showed marked inhibition of release of IL-1a with figures reading 60% and 75% respectively. Less inhibition of IL-1a release with figures ranged from 5-30% for 0.25 and 0.5 mg / mL was observed for extracts of other herbs.
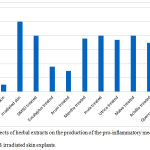 |
Figure 1: Effects of herbal extracts on the production of the pro-inflammatory mediator such IL-1a in UVB irradiated skin explants.
|
Data are expressed as the mean ±SD. p < 0.05 compared to the DMSO solvent was set as significant. Control was made of non irradiated skin explants.
Discussion
Recent trends toward natural products have increased as sources of innovative therapeutic agents which, would in principle constitute adjunctive and alternatives to the currently used antimicrobial agents.21 In Jordan, there is a wide variety of plants that have been widely used in traditional medicine for different types of diseases including skin infections.22,23 However, little is known regarding the potential antimicrobial and anti-inflammatory effects of these plants as a model for the prevention and control of Acne vulgaris.
The present study, investigated the antimicrobial activity of polyphenolic extracts of eight local herbs including against P. acnes. In addition, and for the first time, their anti-inflammatory effects were evaluated in vitro using skin explants. Our preliminary screening revealed that the polyphenolic fractions of some of the selected herbs possess considerable anti-P. acnes activity with a marked anti-inflammatory effects. The results also showed significant variations in the total phenolic and flavonoid contents among the extracts of the tested plants. With particular concern, E.globulus showed both strong antimicrobial (MIC 0.125 mg / mL) and inhibition of IL-1a release (more than 60% at concentrations 0.5 mg / mL). Both A. palaestinum and U. dioica showed modest antimicrobial activities in comparison to other herbs which insignificantly affected the growth of P. acnes. Our results are in agreement with most of the studies conducted on the evaluation of biological activities of Eucalyptus extracts. Oils of Eucalyptus showed potent antimicrobial activity against macrolide resistant P. acnes with a notable decrease of sebum production.24 In contrast to our results, a stronger effect of Achillea against P. acnes with MIC of 0.83 mg / mL was reported25 however, the activity was related to the petroleum ether extract which is known to contain Tannins and alkaloids rather than flavonoids as in our study.
In regards to the anti-inflammatory effects of the selected herbs, Arum exhibited a unique potent inhibitory effects on the release of IL-1a pro-inflammatory cytokine. The inhibition was measured to be more than 70% on the concentration of 0.5 mg / mL of the polyphenolic extract of Arum. This is the first study to report the anti-inflammatory effect of Arum leaves as a raw model for the management of Acne. Our results are however in contrast with a study conducted by Khalaf et al., 2015 in which no significant anti-inflammatory effect of Arum was reported compared to extracts from leaves of Rosmarinus officinalis.26
Based on the results of this study, the presence of anti- acne specific phenolic compound / compounds among the examined plants is not the same. The total polyphenolic and the flavonoid contents have not been shown to be solely linked to the antimicrobial and anti-inflammatory effects of the tested herbs. Variation of the chemical compositions of numerous natural phenolic compounds like rosmarinic acid, ferruginol and kaempferol would significantly affect the results of the antimicrobial activity of the fractions.27,28 Moreover, synergistic effects of the extracts with various phenolic compounds could not be excluded in the antimicrobial activity of the herbs. As previously shown, the presence of anti-oxidants including ascorbic acid and ferulic acid, alone or in combination with other phenols play a key role in the antimicrobial effects of many medicinal herbs.6,12,29 This can also be postulated for the anti-inflammatory effects of the tested plants in this study, since the activity was shown to be independent and not linked to the antimicrobial activity.
In conclusion, the presented work demonstrated that polyphenolic fractions of extracts from local plants in Jordan have considerable in vitro activities against P. acnes and its associated inflammatory potential. further examination of these herbs as adjunct to or as an alternative treatment of acne vulgaris is required.
Acknowledgments
The authors would like to express their sincere gratitude to the Deanship of Scientific Research-University of Petra, the technical support from research team of the University of Petra pharmaceutical center (UPPC), Jordan company for Antibody Production (Monojo) and all whose contributed to finish this research, highly appreciated.
Conflict of Interest
There is no conflict of interest.
References
- Leyden J. A. Review of the use of combination therapies for the treatment of acne vulgaris. J. Am. Acad. Dermatol. 2003;49:S200–210.
CrossRef - Kim J. Review of the innate immune response in acne vulgaris: Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatol. 2005;211:193–198.
CrossRef - Dessinioti C and Katsambas A. Propionibacterium acnes and antimicrobial resistance in acne. Clin. dermatol. 2017;35:163-167.
CrossRef - Walsh T. R., Efthimiou J and Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Infect. Dis. 2016;16:e23-e33.
CrossRef - Sokmen A., Jones B. M and Erturk M. Antimicrobial activity of extracts from the cell cultures of some Turkish medicinal plants. Phytother Res. 1999;13:355-357.
CrossRef - Funatogawa K., Hayashi S and Shimomura H., et al. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microbiol. Immunol. 2004;48:251-261.
CrossRef - Palici I. F., Liktor-Busa E.,Zupkó I.,Touzard B.,Chaieb M., Urbán E and Hohmann J. Study of in vitro antimicrobial and antiproliferative activities of selected Saharan plants. Acta. Biol. Hungar. 2015;66:385-394.
CrossRef - Al-Snafi A. E. Anticancer effects of Arabian medicinal plants [part 1]-A review. IOSR Pharm. 2017; 7:63-102.
CrossRef - Al-Sokari S. S and El Sheikha A. F. In vitro antimicrobial activity of crude extracts of some medicinal plants from Al-Baha region in Saudi Arabia. J. Food. Nut. Sci. 2015;3:74-78.
- Rayyan W. A., Alshammari S. A., ALSammary A. M., AL-Shammari M. S., Seder N and Abu-Qatouseh L. F. The Phytochemical Analysis and Antimicrobial Activity of Pergularia Tomentosa in North East Kingdome of Saudi Arabia KSA. Biomed. Pharmacol. J. 2018;11:4.
CrossRef - Miyague L., Macedo R. E., Meca G., Holley R. A and Luciano F. B. Combination of phenolic acids and essential oils against Listeria monocytogenes. LWT-Food. Sci Tech. 2015;64:333-336.
- Abu-Qatouseh L. F., Boutennone H., Boussouf L., Madani K., Shihab P and Al-Qaoud K. In Vitro anti-Helicobacter pylori and urease inhibitory effects of polyphenolic extracts of local herbs from Algeria. IAJAA. 2014;3.
- Awika J. M., Rooney L.W and Waniska R. D. Anthocyanins from black sorghum and their antioxidant properties. Food Chemistry. 2005;90:293-301.
CrossRef - Yu J., Ahmedna M., Goktepe I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005;90:199-206.
CrossRef - Othman A., Ismail A., Abdul Ghani N., Adenan I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007;100:1523-1530.
CrossRef - Djeridane A., Yousfi M., Nadjemi B., Boutassouna D., Stocker P., Vidal N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97: 654-660.
CrossRef - Hayes J and Markovic B. Toxicity of Australian essential oil Backhousia citriodora (Lemon Myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food. Chem. Toxicol. 2002;40:535-
CrossRef - Valgas C., Souza S. M., Smoónia E. A and Smoónia J. Screening methods to determine antibacterial activity of natural products. B. J. Microbiol. 2007;38:369-80.
CrossRef - Harris K. A., Yam T., Jalili S., Williams O. M., Alshafi K., Gouliouris T and Hartley J. C. Service evaluation to establish the sensitivity, specificity and additional value of broad-range 16S rDNA PCR for the diagnosis of infective endocarditis from resected endocardial material in patients from eight UK and Ireland hospitals. E. J. Clin. Microbio. Infect. Dis. 2014;33:2061-2066.
CrossRef - Pavicic T., Wollenweber U., Farwick M and Korting H. C. Anti‐microbial and‐inflammatory activity and efficacy of phytosphingosine: an in vitro and in vivo study addressing acne vulgaris. I. J. Cosm. Sci. 2007;29:181-190.
CrossRef - Cowan M. M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564-582.
CrossRef - Mahasneh A. M. Screening of some indigenous Qatari medicinal plants for antimicrobial activity. Phytother. Res. 2002;16:751-753.
CrossRef - Abu-Darwish M. S., Al-Ramamneh E. A., Kyslychenko V. S and Karpiuk U. V. The antimicrobial activity of essential oils and extracts of some medicinal plants grown in Ash-shoubak region-South of Jordan. Pak. J. Pharm. Sci. 2012;25:239-246.
- Bhatt D., Sachan A. K., Jain S and Barik R. Studies on inhibitory effect of Eucalyptus oil on sebaceous glands for the management of acne. Ind. J. Nat. Prod. Res. 2015;2:345–349.
- Shah R., Patel A., Shah M and Peethambaran B. Anti-acne activity of Achillea Moonshine petroleum ether extract. J. Medic. Plant. Res. 2015;9:755-763.
CrossRef - Khalaf N. A., Naik R. R., Shakya A. K., Shalan N and Al-Othman A. Antioxidant, anti-inflammatory and anti-diarrheal activity of ethanolic extract of medicinal plants grown in Jordan and Palestine. O. J. Chem. 2015;31:1923-1928.
CrossRef - Klancnik A., Guzej B., Kolar M. H., Abramovic H and Mozina S. S. In vitro antimicrobial and antioxidant activity of commercial rosemary extract formulations. J. Food. Prot. 2009;72:1744-52.
CrossRef - Matkowski A. Plant in vitro culture for the production of antioxidants–a review. Biotechnol. Adv. 2008;26:548-60.
CrossRef - Wu N., Fu K., Fu Y., Zu Y., Chang F., Chen Y., Liu X., Kong Y., Liu W and Gu C. Antioxidant Activities of Extracts and Main Components of Pigeonpea [Cajanus cajan (L.) Millsp.] Leaves. Molecules. 2009;14:1032-1043.
CrossRef








