Thalisa Yuwa-Amornpitak*1 and Pa-Nga Yeunyaw2
and Pa-Nga Yeunyaw2
Department of Biotechnology, Mahasarakham University, Khamrieng, Kantarawichai, Maha Sarakham, 44150, Thailand.
Corresponding Author E-mail: ythalisa@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1628
Abstract
In order to develop a procedure for production bioethanol from cassava pulp, mixed culture of Amylomyces rouxii TISTR 3667 with Zygosaccharomyces pseudorouxii TISTR 5966 or Zymomonas mobilis TISTR 550 and cellulase were evaluated. The parameters such as pH, cellulase, and cassava pulp concentration that influence on the amount of fermentable sugar were optimized by response surface methodology (RSM). Ethanol production was observed and compared to the predicted value that was calculated from the models. The models were fitted to a second-order polynomial equation. They were used to predict ethanol concentration from the use of the mixed culture of A. rouxii TISTR 3667 and Z. mobilis TISTR 550 (G2) that was higher than the amount produced using the mixed culture of A. rouxii TISTR 3667 and Zygosaccharomyces pseudorouxii TISTR 5966 (G1). The following optimum parameters were obtained: pH 6, 20% cassava pulp, and 1% cellulase for G2; and pH 4, 20% cassava pulp, and 0.55% cellulase for G1. The effect of cellulase on ethanol production, a comparative study was conducted in the fermenter by using mixed culture of A. rouxii TISTR 3667 and Z. mobilis TISTR 550. It was showed that more 15% ethanol was gained from 10% cassava pulp with 0.5% cellulase (25 g/l ethanol) compared to the system without cellulase (20 g/l). Mathematically model (equation 4) predicted the ethanol in this system near the actual value of 26.87 g/l. This study indicated that RSM is a powerful tool for optimization fermentation process by using mixed culture including cellulase. Besides these cellulase also reduced viscosity of the cassava medium and enhanced ethanol production. However this process should be more continue to study.
Keywords
Amylomyces Rouxii; Cassava Pulp; Ethanol; Mixed Culture; Response Surface Methodology (RSM); Zygosaccharomyces Pseudorouxii; Zymomonas Mobiles
Download this article as:| Copy the following to cite this article: Yuwa-Amornpitak T, Yeunyaw P. Comparative Study of Ethanol Production from Cassava Pulp by a Mixed Culture of Amylomyces Rouxii with Zygosaccharomyces Pseudorouxii and Zymomonas Mobilis. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Yuwa-Amornpitak T, Yeunyaw P. Comparative Study of Ethanol Production from Cassava Pulp by a Mixed Culture of Amylomyces Rouxii with Zygosaccharomyces Pseudorouxii and Zymomonas Mobilis. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2XJrL2S |
Introduction
The global ethanol production forecast in 2018 is approximately 30.91 billion gallons (117 billion liters).1 The United States (US) and Brazil are the world’s largest bioethanol producers. The US forecasts producing up to 16.3 billion gallons of ethanol from corn, wood chips and stalks, whereas Brazilian ethanol production was approximately 7,093 million gallons from sugar cane.1 Currently, cassava is also used as a raw material for ethanol production in various countries including Thailand. Thailand produced nearly 28.56 million tons of cassava in 2017.2 A large amount of cassava pulp (10%-15% of root weight) is generated from the starch production process.3 Approximately 2 million tons of pulp is produced annually. Because cassava pulp contains 60%-68% starch and 10%-27% fiber4,5 it was used directly as feedstuff5 and fertilizer,6 or by converting it, as biogas,7 reducing sugar,8,9 and bioethanol.10,11
Cassava pulp is a by product from starch manufacturing from cassava root (Manihot esculenta Crantz) which is widely cultivated in tropical country such as Thailand. Cassava pulp contains both starch and cellulose that can be converted to glucose by acid treatment12 and enzyme treatment.8,9 Reduced sugar derived from cassava pulp is useful for producing various products, such as glucose syrup, vinegar, ethanol, and others. Fermentable sugar from cassava pulp was prepared using several methods. Chaikaew et al., 201213 developed the method of using a water jet system (star burst system) for releasing the starch granules followed by an enzyme cocktail (cellulase and α-amylase) to hydrolyze the starch. Glucose was obtained at a rate of 1.15 g/l from 1% cassava pulp. Apiwatanapiwat et al. (2013)14 studied ethanol production from cassava pulp using Kluyveromyces marxianus TISTR 5925 in simultaneous saccharification and fermentation (SSF) and a separate hydrolysis and fermentation (SHF) process. Cassava pulp was hydrolyzed by a mixture of enzymes (cellulose, pectinase, α-amylase, and glucoamylase) after being gelatinized by boiling for 10 min. The researchers found that more ethanol was produced from SSF than the SHF process from a sample of 20% cassava pulp. Furthermore, using response surface methodology (RSM), a maximum ethanol concentration was obtained, 5% (w/v), using optimum temperature conditions of 41°C, enzyme dilution of 0.1, and a fermentation time of 27 h.
In this current study, mixed cultures between Amylomyces rouxii TISTR 3667 or Zymomonas mobilis and yeast TY2 (Zygosaccharomyces pseudorouxii TISTR 5966) was conducted to produce ethanol from cassava pulp. Firstly, the starch granule of cassava pulp was hydrolyzed to reducing sugar by A. rouxii. After that cellulose as fiber was converted to fermentable sugar by cellulase. RSM was employed to evaluate the effect of pH, enzyme, and cassava pulp concentration on ethanol production using mixed culture. Effect of cellulase on ethanol production from cassava pulp was performed in the fermenter.
Materials and Methods
Raw Material
The cassava pulp used in all experiments was a gift from National Starch Industries, Thailand. The cassava pulp was heated at 70°C until dry, ground and sieved through a 0.5 mm sieve screen (ZM-100; Retsch GmbH; Haan, Germany) and kept in a plastic bag with zip locked until used. The chemical composition of cassava pulp was analyzed by the standard methods of the Association of Official Analytical Chemists (AOAC, 2000).15
Microorganism, maintenance and identification
Amylomyces rouxii YTH3, and yeast TY2 were the isolates at our laboratory from loog-pang and fermented sweet rice (khao mak), respectively. Yeast TY2 was identified
by D1/D2 domain sequence of the large subunit (LSU) of rDNA and the internal transcribed spacer (ITS) as Zygosaccaromyces pseudorouxii TY2. Both of them were submitted to Thailand Institute of Scientific and Technology research culture collection (TISTR) with the accession number as Amylomyces rouxii TISTR 3667, and Zygosaccaromyces pseudorouxii TISTR 5966. The sequence of Amylomyces rouxii YTH3 was submitted to GenBank with accession number KM215272). Those isolates were grown and maintained on potato dextrose agar (PDA). Zymomonas mobilis TISTR 550 was a gift from the Thailand Institute of Scientific and Technological Research, and it was cultivated and maintained on YPDA medium, yeast extract (10 g/l), peptone (10 g/l), glucose (20 g/l), and agar (16 g/l).
Genomic DNA was isolated from yeast TISTR 5966 cells that were collected from YM broth using a nucleic acid extraction kit (GF-1 plant DNA extraction kit, Vivantis). The polymerase chain reaction (PCR) products at the D1/D2 domain sequence of the large subunit (LSU) of rDNA and the internal transcribed spacer (ITS) were amplified using the forward primer NL-1 (5’-GCA TAT CAA TAA GCG GAG GAA AAG-3’) and the reverse primer NL-4 (5’-GGT CCG TGT TTC AAG ACG G-3’). The PCR mixtures contained approximately 10-20 ng of genomic DNA, 1 µl of 20 pmol of each primer, 12.5 µl of master mix Taq polymerase in 25 µl of total reaction volume. PCR products were amplified in a thermal cycler machine. The reaction cycle was as follows: initial denaturation at 94°C for 5 min; followed by 30 cycles of 40 s at 94°C for DNA denaturation; 30 s at 50°C for primer annealing; and 40 s at 72°C for primer extension; with a final extension of 7 min at 72°C, followed by storage at 4°C.
The PCR product was purified with a purification kit. The sequencing reactions of each primer were performed in the DNA Engine Tetrad 2 Peltier Thermal Cycle (BIO-RAD) using ABI BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems Co.,). Chromas version 2.6.4 (www.technelysium.com), Blastn (https://www.ncbi.nlm.nih.gov) and CLUSTAL Omega multiple sequence alignment tool16 were used for retrieving similar sequences and sequence alignment, respectively. A phylogenetic tree was reconstructed by MEGA6.17
Inoculum and Medium Preparation
A koji starter of A. rouxii TISTR 3667 was prepared as inoculum in a solid-state medium using the following methods: 20 g of sticky rice was combined with 30 ml of water and sterilized at 121°C for 15 min. Subsequently, one colony (1 cm diameter) of the Amylomyces strain that was well growth on YM agar plate was transferred into the sterilized sticky rice and then incubated for two days at 30°C. Yeast TISTR 5966 and Zymomonas mobilis TISTR 550 inoculum were prepared in a liquid medium of YPD (yeast extract 10 g/l, peptone 20 g/l, glucose 20 g/l) by transferring a loopful of cells from a stocked slant and incubating the liquid medium at 30°C on a rotary shaker at 150 rpm for 24 h.
Hydrolysis of Cassava Pulp by Amylolytic Fungi
Cassava pulp (CP) at various concentrations (7%-20%) was prepared in a flask as a solid medium by adding water at 3.5 ml/g cassava pulp before autoclaving at 121°C for 15 min. Saccharification was carried out by adding koji starter of A. rouxii TISTR 3667 followed by incubation at 30°C for 24 h, 36 h, and 48 h. After that, the remaining volume was adjusted with sterilized distilled water to the setting CP concentration volume of 100 ml. Then, the mixture was mixed by shaking at 200 rpm at 30°C for 1 h. The supernatant was separated from each concentration by centrifugation at 10,000 rpm for 10 min at 4°C and stored at -20°C. Starch and reducing sugar content were measured by the iodine method18 and Miller’s method19, respectively.
Ethanol Production from Mixed Culture by Response Surface Methodology
An experimental design was used to determine the variables that affect cellulose hydrolysis and directly influence ethanol yield. The parameters used in the experimental design were pH (4-8, A), cassava pulp percentage (13% – 20%, B), and cellulase enzyme concentration (0.1%-1% w/v, C) with a Box-Behnken design for three factors that enabled the construction of a second-order polynomial model to be used with Design Expert software version 7.15 (Stat-Ease, Inc.). The mathematical model derived from the experimental design is according to equation (1):
![]()
where:
Yi = response function (ethanol, %);
A, B, C = the independent variable value pH, % CP and cellulase concentration, respectively;
A, B, C = the independent variable value pH, % CP and cellulase concentration, respectively;
βo = coefficient relating the interception of the plane with the axis of response;
β1, β2, β3 = linear coefficients estimated by the method of least squares;
Β11, β22, β33 = coefficients of the quadratic variables;
Β12, β13, β23 = coefficients of the interaction between the independent variables,
All experiments were performed in a shake flask in triplicate. Solidified cassava pulp at various concentrations was hydrolyzed by A. rouxii TISTR 3667 for 2 days. Fermentation was started by adding 10% yeast TISTR 5966 inoculum for the G1 experiment and Z. mobilis TISTR 550 for the G2 experiment and incubating the mixtures at 30°C with shaking at 150 rpm. The ethanol concentration (observed value) was measured after harvesting the fermentation broth at 48 h by centrifugation at 10,732 g for 10 min (Beckman, S0410).
Ethanol Production in Batch Bioreactor
A solid-state medium of 130 g (10%) cassava pulp powder was prepared by adding water at 3.5 ml/g cassava pulp before autoclaving at 121°C for 15 min. A koji starter of 20 g of A. rouxii TISTR 3667 (prepared as above) was inoculated and incubated at 30°C for 2 days. Then, the hydrolyzing cassava medium was transferred aseptically into the fermenter (Biostat B, Sartorious, Germany) and adding 715 ml of sterilized water and 6.5 g (0.5%) of cellulase. Subsequently, the medium was mixed by continuous agitation at 400 rpm (because it was very viscous) at the same temperature for 2 h. Ethanol fermentation was then started by adding 10% v/v (130 ml) of yeast TISTR 5966 inoculum (G1 experiment) or Z. mobilis TISTS 550 inoculum (G2 experiment). The fermentation process was controlled at 30°C with a continuous agitation speed of 200 rpm. Sample was collected at different time points, and the supernatant was stored at -20°C before being analyzed.
Analytic Methods
The starch was analyzed by iodine solution.18 Starch samples 100 µL were mixed with 2.5 ml of iodine solution (0.2 g I2 and 2 g KI was dissolved and make the volume 100 ml) and measured at 600 nm after well mixing by spectrophotometer (UV-160A Shimadzu, Japan). The reducing sugar was determined following the method proposed by Miller (1959)19. Briefly, diluted 200 µL samples were mixed with 300 µL of 0.044 M 3,5-dinitrosalicylic acid solution (1g 3,5-dinitrosalicylic acid dissolved with 20 ml 2N NaOH.
And adjusted volume 100 ml) and boiled in water bath for 10 min. After the mixture was cooled down, 2.5 ml distilled water was added. The optical density was measure at 520 nm by spectrophotometer. The reducing sugar concentration was compared with the standard glucose curve. Ethanol was measured by gas chromatography (Shimadzu GC-14A, Japan) using a Porapak Q column 80/100 (6 mm × 1/8 mm) with a flame ionization detector (FID). The column temperature, injection temperature, and detector temperature were 180, 200 and 220°C, respectively. The carrier gas was nitrogen, and the sample injection was 1 µl. Isopropanol was used as the internal standard.
Proximate analysis of cassava pulp was analyzed as described by the method AOAC, 2000. Moisture content was measured by drying the cassava pulp in an oven at 105°C for 3 h. Protein was analyzed by Kjeldahl’s method. Lipid content was determined by a Soxhlet apparatus. Impurities were burned in a furnace at 550°C for ash measurement. Fiber content was measured as the weight of ash after hydrolyzing the sample with an acid and base. Carbohydrate content was determined by the following equation (2):
Carbohydrate ate, % = 100 – (% moisture + % ash + %protein + %lipids + % fiber) (2)
Results and Discussions
Chemical Compositions of Cassava Pulp
Due to differences between species, climatic differences, soil fertility, age of the harvested plants, processing methods and instrument type, etc.,20 the chemical compositions of cassava pulp were different, as shown in Table 1.
Table 1: The chemical composition of cassava pulp.
| Chemical composition | Percentage, % (This study) | Percentage, % Ref. 9 | Percentage,% Ref. 4 | Percentage,% Ref. 11 |
| Carbohydrate
-starch -cellulose -hemicellulose |
63.36±0.14
– – – |
66.22
– – – |
69.89
– – – |
–
40.12 11.4 8.29 |
| Protein | 3.46±0.03 | 3.39 | 1.55 | 4.43 |
| Lipid | 0.99±0.004 | 0.24 | 0.12 | 3.45 |
| Fiber | 19.14±0.06 | 15.25 | 27.75 | – |
| Ash | 3.24±0.013 | – | 1.7 | 2.84 |
| Moisture content | 9.80±0.03 | – | – | – |
*Each value represents the mean ± SD of triplicates.
However, the primary compositions were 62%-69% carbohydrate and 15%-28% fiber that can be converted to fermentable sugar. Generally, acid or enzymatic reactions were used to convert starch and fibrous components into fermentable sugars. Furfural (F) and 5-hydroxymethylfurfural (HMF) are byproducts from acid hydrolysis that are highly toxic to microbial growth and metabolism. It was found that 0.2% furfural is a strong inhibitor of both functions. HMF was a weaker inhibitor than furfural.21,22,23 Glucose and xylose were liberated from cassava pulp by an enzyme cocktail (α-amylase, β-amylase, glucoamylase, and cellulase) and not inhibition by byproducts such as furfural or HMF.11 In this study, high-efficiency fungi such as A. rouxii TISTR 3667 supplemented with cellulase were used to convert carbohydrates into fermentable sugars.
Identification of Yeast Species
Yeast TISTR 5966 was identified by D1/D2 domain sequence of the large subunit (LSU) of rDNA and the internal transcribed spacer (ITS). The complete sequence of yeast TISTR 5966 was derived from the complementary sequence from the forward and reverse primers NL-1 and NL-4, respectively, using Chromas version 2.6.4 (www.technelysium.com). The nucleotide sequence of yeast TISTR 5966 was compared with the GenBank database using the blastn tool. The results showed various strains of Zygosaccharomyces rouxii and Zygosaccharomyces pseudorouxii with the most similarity at 96-99%. Subsequently, similar sequences of those various strains were selected and used to construct a phylogenetic tree by MEGA6. A maximum likelihood tree with 1000 replicates is shown in Figure 1. S. cerevisiae and Torulaspora quercuum were used as the outgroup. Yeast TISTR 5966 was placed in the same branch as Zygosaccharomyces pseudorouxii AM947680. Then, it was named Zygosaccharomyces pseudorouxii TISTR 5966.
The genus Zygosaccharomyces was first placed in the genus Saccharomyces. In 1983, it was reclassified to its current name by Barnett et al., 1983.24 This genus now comprises more than 10 species. Z. pseudorouxii is an unofficial name and is closely related to the species Z. sapae.25 Zygosaccharomyces is tolerant to high sugar, high ethanol, high acetic acid, and high salt and is also abundant in common food preservatives such as ascorbic and benzoic acid. In most food and beverage products, it is considered a spoilage yeast. However, some species, such as Z. bailii, produce high esters. The mixed culture of Z. bailii with S. cerevisiae was used to improve the production of ethyl esters in wine.26,27
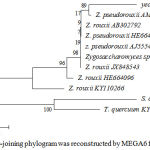 |
Figure 1: Neighbour-joining phylogram was reconstructed by MEGA6 from D1/D2 domain.
|
Sequence of the large subunit (LSU) of rDNA and the internal transcribed spacer (ITS) of the sequence of yeast TISTR 5966 with similar sequences from the Gen Bank data base.
The number at nodes indicate percentage of 1000 bootstraps replicates. The bar scale indicates nucleotide substitutions/ site. The symbol T. and Z. represent Torulaspora and Zygosaccharomyces, repectively.
Effect of Solid State Cassava Pulp Concentration on Fungal Hydrolysis
Solid cassava pulp (CP) at various concentrations (7% — 20%) was hydrolyzed by A. rouxii TISTR 3667 for various time courses before addition of distilled water to those concentrations and determination of the reducing sugar and starch content. The results are shown in Figure 2. It was found that A. rouxii TISTR 3667 in the solid-state can hydrolyze highly concentrated CP. However, at a higher CP concentration, a higher concentration of sugar was released. Furthermore, the more time used for hydrolyzing, the more reducing sugar was rapidly consumed in 48 h. However, the mycelium of the fungi could not penetrate the solidified starch at 20% CP, and a little setting CP remained. The highest reducing sugar was approximately 38 g/l from 20% CP at 48 h of hydrolysis. However, the sugar remaining in the 10% CP concentration was nearly half that of 20% CP concentration. Zhu et al., 201211 reported that 52.70 g/l and 6.01 g/l of glucose and xylose, respectively, were produced from 20% CP using an enzyme cocktail (amylase, cellulase, glucoamylase, and glucosidase) at 50°C for 96 h. These results indicated that A. rouxii TISTR 3667 was an efficient microbe for hydrolyzing starch at low temperature and obtaining high glucose content. However, the problem of solidification of gelatinized cassava pulp at high concentration must be solved by milling the pulp into small pieces to enable easy penetration by the fungal mycelium, thus obtaining higher glucose content and decreasing viscosity of the CP medium. The advantage of glucose preparation by fungal mycelium treatment is that there is no furfural or HMF formation.
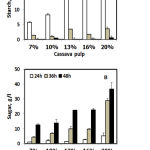 |
Figure 2: Starch hydrolysis and sugar released by A. rouxii TISTR 3667 on solid state from various concentration of cassava pulp at various hydrolysing time.
|
Effect of Parameters on Ethanol Production from Cassava Pulp by Response Surface Methodology
Due to the fiber content of cassava, cassava pulp can be converted to fermentable sugar by cellulase enzyme activity. To produce ethanol, three parameters, pH (4-8), cellulase concentration (0.1%-1%), and CP concentration (13%-20%) were studied by response surface methodology using two groups of mixed culture. The first group was A. rouxii TISTR 3667 and yeast TISTR 5966, and the second group was A. rouxii TISTR 3667 and Zymomonas mobilis TISTR 550. Seventeen runs were introduced by the Design Expert program (Version 7.15), as shown in Table 2. Ethanol production was calculated from the model (equations 3 and 4) that was established for ethanol prediction under various conditions after two days of fermentation. These results are also shown in Table 2. Equations 3 and 4 were used with the mixed culture of groups 1 and 2, respectively.
Table 2: Predicted and observed value of ethanol production under Box-Behken experimental design from various parameters of pH, cassava pulp and cellulase concentration.
| Treatment | pH (A) | CP,% (B) | Cellulase, (C) | Ethanol, % (v/v)* | Ethanol, % (v/v)** | ||
| Observed | Predicted | Observed | Predicted | ||||
| 1 | 6 | 13 | 0.1 | 1.81 | 1.712 | 3.11 | 3.446 |
| 2 | 4 | 20 | 0.55 | 3.09 | 3.008 | 4.48 | 4.93 |
| 3 | 8 | 16.5 | 0.1 | 2.8 | 2.816 | 3.6 | 3.70 |
| 4 | 6 | 20 | 1.0 | 2.43 | 2.527 | 6.09 | 6.09 |
| 5 | 4 | 16.5 | 0.1 | 2.53 | 2.366 | 3.5 | 3.42 |
| 6 | 4 | 16.5 | 1.0 | 2.74 | 2.723 | 3.86 | 4.09 |
| 7 | 8 | 16.5 | 1.0 | 2.65 | 2.813 | 4.35 | 4.67 |
| 8 | 8 | 20 | 0.55 | 2.77 | 2.508 | 5.1 | 5.46 |
| 9 | 8 | 13 | 0.55 | 2.56 | 2.641 | 3.64 | 3.44 |
| 10 | 6 | 16.5 | 0.55 | 2.89 | 2.722 | 4.36 | 4.36 |
| 11 | 4 | 13 | 0.55 | 1.34 | 1.601 | 3.33 | 3.22 |
| 12 | 6 | 13 | 1.0 | 2.44 | 2.195 | 3.35 | 3.92 |
| 13 | 6 | 20 | 0.1 | 2.41 | 2.655 | 5.25 | 5.02 |
| 14 | 6 | 16.5 | 0.55 | 2.78 | 2.722 | 3.9 | 4.36 |
| 15 | 6 | 16.5 | 0.55 | 2.78 | 2.722 | 4.45 | 4.36 |
| 16 | 6 | 16.5 | 0.55 | 2.7 | 2.722 | 3.89 | 4.36 |
| 17 | 6 | 16.5 | 0.55 | 2.46 | 2.722 | 4.57 | 4.35 |
* Mixed culture between A. rouxii TISTR 3667 and yeast TISTR 5966 (or Group 1).
** Mixed culture between A. rouxii TISTR 3667 and Zymomonas mobilis TISTR 550 (or group 2)
Yi = –13.2015 + 0.84175 A + 1.40304 B + 2.963854 C – 0.055 AB – 0.1 AC – 0.09683 BC + 0.015688 A2 – 0.02814 B2 – 0.51728 C2 (3)
Yii = 3.7278 + 0.9312 A – 0.5598 B – 1.3577 C + 0.011 AB + 0.1083 AC + 0.0952 BC – 0.0898 A2 + 0.0214 B2 – 0.2321 C2 (4)
where Y is the predicted response value (% ethanol). A, B, C are the levels of pH, CP and cellulose concentrations, respectively.
Equation 3 and 4 represent the model of ethanol production from the mixed culture of G1 (A. rouxii TISTR 3667 and yeast TISTR 5966) and G2 (A. rouxii TISTR 3667 and Z. mobilis TISTR 550) with R2 values of 0.8379 and 0.8928, respectively. The models were used to predict ethanol production, and they correspond to the observed data, as shown in Tables 3 and 4.
Table 3: Analysis of variance for the fitted quadratic polynomial model of mixed culture between A. rouxii TISTR 3667 and Zygosaccharomyces pseudorouxii TISTR 5966 (G1).
| Source Model | Coefficient Estimate | Sum of Squares | df | Standard error | F Value | p-Value Prob >F |
| Intercept | 2.72 | – | 1 | 0.11 | – | – |
| A-pH | 0.14 | 0.15 | 1 | 0.089 | 2.28 | 0.1745 |
| B-cassava pulp | 0.32 | 0.81 | 1 | 0.089 | 12.73 | 0.0091* |
| C-cellulase | 0.089 | 0.063 | 1 | 00.089 | 0.99 | 0.3536 |
| AB | -0.39 | 0.59 | 1 | 0.13 | 9.28 | 0.0187* |
| AC | -0.09 | 0.032 | 1 | 0.13 | 0.51 | 0.4993 |
| BC | -0.15 | 0.093 | 1 | 0.13 | 1.46 | 0.2666 |
| A2 | 0.063 | 0.017 | 1 | 0.12 | 0.26 | 0.6260 |
| B2 | -0.34 | 0.50 | 1 | 0.12 | 7.84 | 0.0265* |
| C2 | -0.10 | 0.046 | 1 | 0.12 | 0.72 | 0.4231 |
| Model | 0.26 | 9 | – | 4.02 | 0.0401* | |
| Pure Error | 0.1 | 4 | ||||
| Total | 2.76 | 16 |
df; degree of freedom, *significant, R2 = 0.8379.
Table 4: Analysis of variance for the fitted quadratic polynomial model of mixed culture between A. rouxii TISTR 3667 and Z. mobilis TISTR 550 (G2).
| Source Model | Coefficient Estimate | Sum of Squares | Df | Standard error | F Value | p-Value Prob >F |
| Intercept | 3.772 | – | 1 | 0.17 | – | – |
| A-pH | 0.19 | 0.29 | 1 | 0.14 | 1.9 | 0.2105 |
| B-cassava pulp | 0.94 | 7.01 | 1 | 0.14 | 46.14 | 0.0003* |
| C-cellulase | 0.27 | 0.60 | 1 | 0.14 | 3.94 | 0.0874* |
| AB | 0.077 | 0.024 | 1 | 0.19 | 0.16 | 0.7028 |
| AC | 0.097 | 0.038 | 1 | 0.19 | 0.25 | 0.6323 |
| BC | 0.15 | 0.090 | 1 | 0.19 | 0.59 | 0.4668 |
| A2 | -0.36 | 0.54 | 1 | 0.19 | 3.58 | 0.1004 |
| B2 | 0.26 | 0.29 | 1 | 0.19 | 1.92 | 0.2088 |
| C2 | -0.047 | 0.0009301 | 1 | 0.19 | 0.061 | 0.8117 |
| Model | – | 8.86 | 9 | – | 6.48 | 0.0111* |
| Pure Error | – | 0.41 | 4 | – | – | – |
| Total | – | 11.03 | 16 | – | – | – |
df; degree of freedom, *significant, R2 = 0.8928.
Ethanol production by G2 was higher than G1 at each level of experimentation. The models from G1 and G2 were fitted to the observed data with p-values of 0.0401 and 0.0111 (Table 3 and Table 4), respectively. Both groups of data were significantly fitted to the models. The optimum pH, CP and cellulase of G1 were 4, 20% and 0.55%, respectively, and 3.08% ethanol was produced. While pH 6, 20% CP, and 1% cellulase were the optimum parameters for G2, for which ethanol production was 6.09%. The fitted responses for the regression models are plotted in Figures 3 and 4 for G1 and G2, respectively.
 |
Figure 3: Surface and contour plots show the relative effects of parameter pairs on ethanol production in a mixed culture of A. rouxii TISTR 3667 and Zygosaccharomyces pseudorouxii TISTR 5966 (G1): (a) % cassava pulp and cellulase, (b) pH and % cellulase, (c) cassava pulp and pH.
|
 |
Figure 4: Surface and contour plots show in the relative effects of parameter pairs on ethanol production in mixed culture of A. rouxii TISTR 3667 and Z. mobilis TISTR 550 (G2): (a) % cassava pulp and cellulase, (b) pH and % cellulase, (c) cassava pulp and pH.
|
Sivasakthivelan et al., 201428 reported that the optimum pH for ethanol production from yeast and Z. mobilis were 5.5 and 6.5, respectively. In this study, the contour plots from G1 showed that pH and CP concentration had a significant interactive effect on ethanol production (Figure 3 c). Alternatively, contour plots from G2 showed the interactive effect on ethanol production between pH and cellulase concentrations (Figure 4 b). However, cellulase concentration showed no significant interaction with the other variable parameters of CP and pH in G1. In G2, pH showed an insignificant interaction with CP and cellulase concentration. However, glucose was the main C source that was derived from CP hydrolysis by Amylomyces rouxii TISTR 3667. Then, CP was the main parameter for ethanol production. In this study, cellulase was used to hydrolyze cellulose from cassava pulp to glucose and xylose. Both yeast and Z. mobilis cannot utilize pentose sugars such as xylose and arabinose.
Ethanol Production in the Fermenter
Comparative studies of ethanol production from cassava pulp using the mixed culture of A. rouxii TISTR 3667 and Z. mobilis TISTR 550 in the presence and absence of cellulase were carried out in a 1.3 l working volume. The result is shown in Figure 5. A small portion of starch remained from the cassava pulp after two days of hydrolyzing by A. rouxii TISTR 3667 (in solid-state), and it was all used within 24 h of the fermentation process. It was clear that in the presence of cellulase, more reducing sugar was released than in the absence of cellulase. The more reducing sugar in the system, the more ethanol production was gained. In this study, 20 and 25 g/l of ethanol were produced without and with cellulase added to the fermentation process, respectively. The result from equation (4) that predicted ethanol at 26.87 g/l (3.402%) corresponded with the actual value. This research showed that higher glucose was gained from the addition of cellulase to the system while the viscosity of the fermentation broth was decreased.
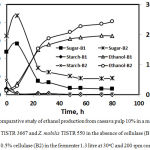 |
Figure 5: Comparative study of ethanol production from cassava pulp 10% in a mixed culture of A. rouxii TISTR 3667 and Z. mobilis TISTR 550 in the absence of cellulase (B1), and the presence of 0.5% cellulase (B2) in the fermenter 1.3 litre at 30°C and 200 rpm continuously.
|
Zhu el at., 201211 studied ethanol production from cassava pulp using an enzyme cocktail in simultaneous saccharification and fermentation (SSF) and separate hydrolysis and fermentation (SHF) processes. They found that the optimum conditions for SSF were a temperature of 37°C, a pH of 5.0, and an inoculum size of 6%. The fed-batch process resulted in more ethanol than the batch process, and SSF provided a more efficient method than SHF for utilization of cassava pulp. Ethanol production in the batch process from SHF and SSF was 23.51 and 34.67 g/l from 20% cassava pulp, respectively. As in the fed-batch process, the levels of ethanol production from SHF and SSF increased to 29.39 and 43.25 g/l, respectively.
In order to produce high reducing sugar from cassava pulp, various enzymes dosage (cellulase, pectinase, α-amylase, and glucoamylase) fermentation times, and temperatures were designed using RSM for ethanol production by Kluyveromyces marxianus TISTR 5925.29 The optimum parameters were enzyme dosage 0.1 (cellulose 1 U, pectinase 10 U, amylase 5.5 U, and glucoamylase 2 U/g dry pulp), temperature 41oC and fermentation time 27 h. The maximum ethanol production was 5.0% (w/v) from cassava pulp 20% that was very close to the prediction value at 5.3% (w/v). Cassava pulp is a C-source containing starch and cellulose. Then many types of enzymes should be used for hydrolyzing both of starch and cellulose to glucose. Molecular technique for yeast strain that was containing amylolytic, cellulolytic, and β-glucosidase enzymes was constructed.30 However ethanol production was very low. Enzyme regulation, coordinated action, and gene expression are needed to study more.
Conclusion
This study demonstrated a mild condition to hydrolyze starch and cellulose from cassava pulp by using A. rouxii TISTR 3667 and cellulase. RSM was performed to optimize the parameters for gaining the highest fermentable sugar. It was found that the optimum pH was different in a mixed culture of G1 and G2. The highest cassava pulp concentration of 20% was preferred for the highest reducing sugar release by fungal hydrolysis using A. rouxii TISTR 3667. However, this concentration was highly viscous and difficult to handle by agitation. This problem can be solved by using cellulase. The results of RSM showed that the cellulase contents of the two mixed cultures were different. Optimum cellulase concentration of the mixture of A. rouxii TISTR 3667 and yeast TISTR 5966 was 0.55%, while 1% cellulase was optimum for the co-culture of A. rouxii TISTR 3667 and Z. mobilis 550.
The comparative study for ethanol production in the fermenter was performed with 10% cassava pulp using mixed culture of A. rouxii TISTR 3667 and Z. mobilis 550 with and without added cellulase. The results showed that the viscosity of the medium was decreased and ethanol production was increased by adding cellulase. Equation (4) of the model fit the actual value of ethanol produced using fermentation parameters of 10% cassava pulp, pH 6 and 0.5% cellulase.
This promising results reveal that the development procedure by using A. rouxii TISTR 3667 with enzyme cellulase is an efficient process for hydrolyzing cassava pulp. Response surface methodology is a powerful tool for optimizing parameters and the mathematical model can predict the accurately results.
Conflicts of Interest
There is no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge Mahasarakham University for the financial support for this project with grant no 6005044.
References
- Nguyen L. World ethanol production growth may accelerate in 2018. KNect365 Energy. https://knect365.com/energy/article/bb855c8d-460e-4185-a2a1- 14ce8800f56b/world-ethanol-production-growth-may-accelerate-in-2018. [December 2, 2018].
- Tapioca Production, Tapioca Production and Trading Survey Committee, Thai Tapioca Development Institute. http://www.tapiocathai.org/L1.html [December 2, 2018].
- Edama N. A., Sulaiman A and Rahim S. N. Enzymatic saccharification of tapioca processing wastes into biosugars through immobilization technology. Biof Res J. 2014;1:2-6.
CrossRef - Sriroth K., Chollakup R., Chotineeranat S., Piyachomkwan K and Oates C. G. Processing of cassava waste for improved biomass utilization. Biores Technol. 2000;71(1):63-69.
CrossRef - Khempaka S., Molee W., Guillaume M. Dried cassava pulp as alternative feedstuff for broilers: effect on growth performance, carcass traits, digestive organs, and nutrient digestibility. J App. Poul. Res. 2009;18:487-493.
CrossRef - Kamolmanit N and Reungsang A. Effect of carbon to nitrogen ratio on the composting of cassava pulp with swine manure. J Wat Envi Technol. 2006;4(1):33-50.
CrossRef - Piyachomkwan K and Tanticharoen M. Cassava Industry in Thailand: Prospects. J. of the Royal Insti Thailand. 2011;3:160-170.
- Souto L. R. F., Caliari M., Soares Junior M. S., Fiorda F. A and Garcia M. C. Utilization of residue from cassava starch processing for production of fermentable sugar by enzymatic hydrolysis. Food Sci. Techno. 2017;37(1):19-24.
CrossRef - Chotineeranat S., Pradistsuwana C., Siritheerasas P and Tantratian S. Reducing sugar production from cassava pulp using enzymes and ultrafiltration I: Enzymatic hydrolyzation. J. Scient. Res. Chulalongkorn Univ. 2004;29(2):119-128.
- Archibong E. J., Obika I. E., Okafor O. I., Okafor U. I., Ezewuzie C. S., Ezemba C. C., Awah N. C., Okeke B. C., Anaukwu G. C and Anakwenzw V. N. Ethanol production from cassava wastes (Pulp and peel) using alcohol tolerant yeast isolated from palm wine. Amer. J. Lif. Sci. Res. 2016;4:92-97. DOI: 10.21859/ajlsr-040305.
CrossRef - Zhu M., Li P., Gong Z and Wang J. A comparison of the production of ethanol between simultaneous saccarification and fermentation and separate hydrolysis and fermentation using unpretreated cassava pulp and enzyme cocktail. J Biosci Biotechnol Biochem. 2012;76(4):671-678. https://doi.org/10.1271/bbb.110750.
CrossRef - Hermiati E., Tsubaki S and Azuma J. Cassava pulp hydrolysis under microwave irradiation with oxalic acid catalyst for ethanol production. J Math Fund Sci. 2014;46(2):125-139.
CrossRef - Chaikaew S., Maeno Y., Visessanguan W., Ogura K., Sugino G., Lee S. H and Ishikawa K. Application of thermophilic enzymes and water jet system to cassava pulp. Biores. Technol. 2012;126:87-91.
CrossRef - Apiwatanapiwat W., Rugthaworn P., Vaithanomsat P., Thanapase W., Kosugi A., Arai T., Mori Y., and Murata Y. Ethanol production at hightemperature from cassava pulp by a newly isolated Kluyveromeces marxianus strain, TISTR 5925. AIMS Ener. 2013;1:3-16.
CrossRef - Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC. International, 17th edition; AOAC International: Gaithersburg, MD, USA Association of Analytical Communities. 2000.
- Sievers F., Wilm A., Dineen D., Gibson T. J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., Thompson J. D and Higgins D. G. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539.
CrossRef - Stecher G., Liu L., Sanderford M., Peterson D., Tamura K and Kumar S. MEGA-MD: molecular evolutionary genetics analysis software with mutational diagnosis of amino acid variation. Bioinfor. 2014;30(9):1305-1307. doi:10.1093/bioinformatics/btu018.
CrossRef - Hoover R and Ratnayake W. S. Determination of Total Amylose Content of starch. Current Protocals in Food Analytical Chemistry. 2001;E2.3.1-E2.3.5. Copyright by John Wiley & Sons, Inc.
- Miller G. L. Use of dinitrosalicylic acid reagent for the determination of reducing sugars. Analytical Chemistry. 1959;31(3):426-428.
CrossRef - Oliveira M. A and Moraes P. S. B. Technological and postharvest characteristic and productivity of cassava. Ciência e Agrotecnologia. 2009;33(3):837-843.
CrossRef - Sanchez B and Bautista J. Effects of furfural and 5-hydroxymethylfurfural on the fermentation of Saccharomyces cerevisiae and biomass production from Candida guilliermondii. Enz Micro Technol. 1988;10(5):315-318.
CrossRef - Michael T., Guarnieri M. A., Franden C., JohnsonGregg W and Beckham T. Conversion and assimilation of furfural and 5-(hydroxymethyl) furfural by Pseudomonas putida KT2440. Met Engine Commun. 2017;4:22–28.
CrossRef - Boyer L. J., Vega J. L., Kjell T. K., Clausen E. C and Gaddy J. L. The effects of furfural on ethanol production by Saccharomyces cerevisiae in batch culture. Biomass Bioen. 1992;3(1):41–48.
CrossRef - Barnett J. A., Payne R. W and Yarrow D. Yeasts: Characteristics and Identification. Cambridge University Press, Cambridge. 1983.
- Hulin M and Wheals A. Rapid identification of Zygosaccharomyces with genus specific primers. Inter. J. Food Micro. 2014;173(3):9-13.
CrossRef - Garavaglia J., de Schneider R. D. S., Mendes S. D., Welke J. E., Zini C. A., Caramão E. B and Valente P. Evaluation of Zygosaccharomyces bailii BCV 08 as a co-starter in wine fermentation for the improvement of ethyl esters production. Microbiological Research. 2015;173:59–65.
CrossRef - Xu Y., Zhi Y., Wu Q., Du R and Xu Y. Zygosaccharomyces bailii is a potential producer of various flavor compounda in Chiness Maotai-flavor liquor fermentation. Front. Microbio. 2017;8:1-9.
CrossRef - Sivasakthivelan P., Saranraj P and Sivassakthi S. Production of ethanol by Zymomonas mobilis and Saccharomyces cerevisiae using sunflower head waste – A comparative study. International Inter. J. Microbio. Res. 2014;5(3):208-216.
- Apiwatanapiwat W., Rugthaworn P., Vaithanomsat P., Thanapase W., Kosugi A., Arai T., Mori Y., and Murata Y. Ethanol production at high temperature from cassava pulp by a newly isolated Kluyveromyces marxianus strain, TISTR 5925. AIMS Energy. 2013;1:3-16.
CrossRef - Apiwatanapiwat W., Murata Y., Kosugi A., Yamada R., Kondo A., Arai T., Rugthaworn P and Mori Y. Direct ethanol production from cassava pulp using a surface-engineered yeast strain-co-displaying two amylases, two cellulases, and β-glucosidase. Appl. Microbiol. Biotechnol. 2011;90:377-384.
CrossRef








