Jameelah Kadhim Taher Al-Isawi*1 and Essam Fadel Al-Jumaily2
1Department of Chemistry, College of Education for Pure Science (Ibn Al-Haitham), University of Baghdad, Baghdad, Iraq.
2Department of Biotechnology, Genetic Engineering and Biotechnology Institute for Postgraduate Studies, University of Baghdad, Baghdad, Iraq.
Corresponding Author E-mail: saif.jawadk@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1655
Abstract
This study was designed to investigate the hepatoprotective activity and antioxidant enzymes of purified Bauhinia variegate leaves extract and purified flowers extract were administered (200 mg/kg, orally once daily) to reduce the effect of carbon tetrachloride-damage in rat's liver for three weeks. Thereafter, the levels of some serum biochemical factors such as alanine amino transferase, aspartate amino transferase, alkaline phosphatase, and the activity of three different antioxidant enzymes (glutathione, superoxide dismutase, and catalase) were investigated. Liver homogenate can used to estimated antioxidant parameters: glutathione, superoxide dismutase and catalase. The purified Bauhinia variegate leaves and purified flowers significantly (p < 0.05) inhibited the carbon tetrachloride-induced increase in alanine aminotransferase (149.0± 4.40 and 133.08±6.84) unit/L, aspartate aminotransferase (114±9.28 and 117.93±1.96) unit/L, alkaline phosphatase (3.60±0.28 and 2.43±0.11) unit/100 ml, levels at the tested doses, respectively after treatment. However, purified Bauhinia variegate leaves and purified flowers treatment noticeably improved the activity of antioxidant limitations: glutathione, superoxide dismutase, and catalase. The hepatoprotective activity can confirmed by histological finding. From these results it can be concluded that the Bauhinia variegate leaves and flowers extracts contain remarkable of flavonoid can be used as reducing oxidative stress.
Keywords
Antioxidant; Bauhinia Variegate; Hepatoprotective Activity
Download this article as:| Copy the following to cite this article: Al-Isawi J. K. T, Al-Jumaily E. F. Antioxidants and Hepatoprotective Study of a Purified Bauhinia Variegate Leaves and Flowers Against Carbon Tetrachloride-Induced Toxicity in Experimental Rats. Biomed Pharmacol J 2019;12(1). |
| Copy the following to cite this URL: Al-Isawi J. K. T, Al-Jumaily E. F. Antioxidants and Hepatoprotective Study of a Purified Bauhinia Variegate Leaves and Flowers Against Carbon Tetrachloride-Induced Toxicity in Experimental Rats. Biomed Pharmacol J 2019;12(1). Available from: https://bit.ly/2NLwr3D |
Introduction
Liver is the most important organ in the body. Its role in the regulation of various physiological processes, and its activity is associated with its vigorous functions, such as metabolism, secretion, and storage. Its capacity to detoxify waste products of metabolites and toxic substances as well as for create good compounds. The Bauhinia variegate contains health promoting phenolic compounds, proteins, vitamin C and flavonoids, which have been shown good in vitro as anti-oxidative properties and anti-inflammatory which are able to scavenge free radicals and protect the liver from carbon tetrachloride (CCL4)- injury.1
In recent years, however, several studies have attempted to study the connection between antioxidant and anti-inflammatory mechanisms such as phytochemicals. The phytochemical constructions from plant sources which are able to capably moderate oxidative and inflammatory stress to prevent diet-related diseases.2,3
Dealing with natural produces has less their lateral properties than when equated to manufacturer medications.4
A previous report demonstrated that plants are deliberated as prospective hepatoprotective mediators since they comprise a mixture of diverse phytomedicine that are synergistic in their action.5
Many plant nutrients like, vegetables, fruits, and legumes has abundant poly-phenolic compound and of their beneficial health effects. Mani and co-workers illustrated that nutritional poly-phenolic complexes aid to reinstate the equilibrium between the natural antioxidants and free radicals by improving the activity of regular antioxidant defenses for example, superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione-S-transferase (GST) and by direct scavenging of free radicals.2,6
The genus Bauhinia (Fabaceae) comprises three hundred species and famous cow’s paw plant, because of the character of their leaves.7 There are many pharmacological actions have been stated for Bauhinia species, comprising anti-inflammatory, antioxidant, and anti-hyperlipidemic, and hepatoprotective properties. Bodakhe and Ram., reported that alcoholic extract of the stem bark of B. variegata indicates hepatoprotective activity against CCl4 induced hepatotoxicity in rats at the dose of 100 and 200 mg/kg. Oral administration of ethanol extract reduce the level of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyltransferase (GGT), total lipids, and raise the level of total protein through the hepatotoxicity.8
The aims of the study are to carry out biochemical and histological analyses and comparative study of purified B. variegate leaves and flowers extractions on CCL4–induced injury in the liver’s experimental rats.
Materials and Methods
Extraction and Purification of Flavonoids
Bauhinia leaves and flowers were collected from Garden University of Baghdad in November 2016 and flowers during May 2017. The leaves and flowers were wash and dry then crushed by electric grinder to powder then stored in an air tight container until used. The extracts of flavonoids compounds from leaves purified Bauhinia variegate leaves (PBVL) extract and purified flowers (PBVF) extract were papered according to 9,10 by using methanol solvent with ratio (7:1) by using soxhlet extractor the filtered solvent dried by rotary at 40°C.
Column Chromatography
A purification of the flavonoids is preceding using open glass column (2.5 x 16) cm was filled with lica gel G60 special for column chromatography. Five milliliters of methanol of leaves extract by flowers of Bauhinia by methanol was exposed to column and eluted with methanol solution, and the rate of flow regulated to be 60 ml /min 11. The ferric chloride (FeCL3) 1% solution were tested in all fractions as a colorimetric method for polyphenols identification 12.
General Chemical Detection Methods
All extracts of leaves and flowers were tested by lead acetate 13 and flavonoids test by Liebermann reaction and ferric chloride test.11
Experimental Animals
Rats (n= 25); their average weight (250-300 gm) were used in this experiment. Rats were individually housed in plastic cages at controlled rooms in temperature and humidity. The light/dark cycle was 12l/12d- hours. Rats were divided into 5 groups of five rats per group each as following:
Group I: Control rats received normal diet and water.14
Group II: (CCl4 group). Rat (n= 5) in this group treated by CCL4 (3.2 mg/ kg) at the 1st day and the 8th day.
Group III: (Vitamin C group). Rats (n= 5) have been treated with oral daily ingestion of vitamin C in dose 180 mg/kg for 3 weeks and treated with CCL4 at 1st day and the 8th day.
Group IV: (Flowers purified group). Rats (n= 5) have been treated with oral daily ingestion of flowers purified extract 200 mg/kg for 3 weeks and treated with CCL4 at 1st day and the 8th day.
Group V: (Leaves purified group). Rats (n= 5) have been treated with oral daily ingestion with leaves purified extract 200 mg/kg for 3 weeks and treated with CCL4 at 1st day and the 8th day.
Methods of Biochemical Analysis of Blood Samples
Samples Collection
After sacrifice the animals by anesthetic ether, previous to partition either perfused or rinse tissue with a phosphate buffer saline (PBS) solution, pH 7.4, to eliminate any red blood cells and clots. Homogenize the tissue in 5 ml of cold buffer (50 mM phosphate, pH 6-7, comprising 1 mM EDTA) per gram tissue, then centrifuge at 10,000ˣ g for 15 minutes at 4ºC, eradicate the supernatant and store on ice the supernatant will have to be deprotonated before assaying.
Estimation of Serum Glutathione
Glutathione (GSH) was estimated rendering to the method of Jollow et al.,15 using Kit depending on {5,5dithio-bis(2-nitrobenzoic acid)} which reduced by sulfhydryl (SH group) to yellow composite. The absorbance of chromogen was identified at 412 nm for serum GSH concentration.
Determination of Serum Catalase
Catalase (CAT) is a significant cellular antioxidant enzyme that protects against oxidative stress using ready-made kit.16
Determination of Serum Superoxide Dismutase
Superoxide dismutase was determined using ready-made kit.17
Determination of Serum Liver Functions Tests
The serum was used for the assessment of serum ALT, AST, and ALP as limitations of liver function tests. Serum ALT and AST were estimated using ready-made kit from Linear® chemicals.18 While Serum ALP activity have been estimated using ready-made kit.19
Histopathological Examinations
Tissue samples from liver and kidney were prepared for histopathological studies according to20: Using fixation and paraffin embedding technique. Liver and kidney samples were de-waxed by xylene first, dry and then washed shortly in 3 changes of absolute alcohol, then 95% alcohol and 70% alcohol. Washed in water for 5 minutes. Stain with haemotoxylin for 5-10 minutes then washed again in water for five minute. The slides were then placed in eosin for 10-15 seconds after that washed again in water for 3 minute. The sections were then dehydrated in (70, 80, and 95%) alcohol few seconds for each and 2 changes of absolute alcohol. The final step put the slides in xylene after drying and covered by covered slip with Canada balsam, and examined under light microscope.
Statistical Analysis
The data were analyzed to obtain the level of significance. The least significant difference (LSD) test was used to compare between the means for the groups.21
Results and Discussion
Two types of extracts were PBVL and PBVF. These extracts were tested for general chemical identifications to verify the chemical constituents of each extract (Table 1).
The poly-phenolic fraction, the extracts were dark brown residue, and there were compatible with the positive results of phytochemical analysis. The dark brown color may be due to the presence of large amounts of poly-phenolic compounds and flavonoids.22,23
Table 1: The chemical identification of Bauhinia variegate two extracts.
| Type of Extract | Test | Result |
| PBVL
PBVF |
Lead acetate | White jelly residue |
| PBVL
PBVF |
Test for Flavonoids | Dark yellow color |
| PBVL
PBVF |
Liebermann Reaction | Blue-violet |
| PBVL
PBVF |
Ferric chloride | Green color |
Table (2) and Figure (1) the concentrations of serum CAT were significantly increased (p < 0.05) in the treated rats group by flowers purify for 3 weeks (9.49±0.10 Unit/ml) in comparison to the control group (5.60±0.43unit/ml) which indicate that of severe hepatotoxicity. The CAT is a haemoprotein, it protects the cells from the accumulation of H2O2 by dismutating it to form H2O and O2.24 The decrease in the level of catalase enzyme was detected in paracetamol of treated rat due to extreme creation of free radicals and triggering of lipid peroxidation.25 The biomarkers of oxidative stress include CAT, GST, and SOD.26
Table 2: Effect of leaves purified and flowers purified of Bauhinia vareigata L. on CAT, GSH, and SOD activities in different groups.
| Groups | Mean ± SD | ||
| CAT (unit/ml) | GSH (unit/ml) | SOD (unit/ml) | |
| Control | 5.60 ± 0.43 b | 7.31 ± 0.33 b | 1.44 ± 0.11 b |
| CCL4 | 10.17 ± 0.51 a | 11.59 ± 0.60 a | 3.19 ± 0.87 a |
| Leaves (purify) | 8.54 ± 0.33 a | 11.43 ± 0.46 a | 1.66 ± 0.59 b |
| Flowers (purify) | 9.49 ± 0.10 a | 13.10 ± 0.11 a | 3.65 ± 1.16 a |
| Vitamin C | 9.21 ± 0.26 a | 12.59 ± 0.24 a | 2.28 ± 0.43 ab |
| LSD value | 1.632 * | 2.194 * | 1.625 * |
| * p < 0.05 | |||
Different letters represent the significant difference at P < 0.05 between means of column.
These results suggest that the leaves and flowers purify of Bauhinia vareigata L. may have protective activity against toxic effects of hepatic damage-inducing agents. This evidence is in agreement with the previous studies.3
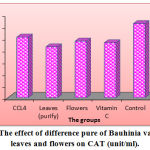 |
Figure 1: The effect of difference pure of Bauhinia variegate L. leaves and flowers on CAT (unit/ml).
|
The rats treated by CCL4 showed a markedly reduction (p < 0.05) in the liver enzymes’ level of serum GSH to (11.59 µmol/min/L) as paralleled to control rat (13.63 µmol/min/L) (Figure 2).
In the Figure (2), there was a markedly elevation (p < 0.05) in the level of serum GSH in rat treated with 200 mg/kg of flowers purified B. variegata for three weeks than in rats treated with leaves purified 200 mg/kg which reach (13.10 and 11.43 µmol/min/L respectively) as compared to control rats. Moreover, there was a significant reduce (p < 0.05) in the liver activity level of GSH in rats treated with 200 mg/kg of vitamin C to (12.54 µmol/min/L) for 3 weeks was significantly (p < 0.05) less than control treated group.27 It has been documented that aqueous extract of P.niruri can be act as a heptoprotection alongside paracetamol.
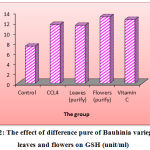 |
Figure 2: The effect of difference pure of Bauhinia variegate L. leaves and flowers on GSH (unit/ml).
|
Figure (3) showed that the CCL4 treated rat increase significantly (p < 0.05) in the liver activity level of SOD (3.19 unit/ml) as paralleled to the control group (1.44 unit/ml). Rats treated with 200 mg/kg of flowers purified B. variegata for 3 weeks revealed a significant increase (p < 0.05) in the level of SOD 3.65 unit/ml as paralleled to the control group. Also, rats treated with 200 mg/kg of vitamin C presented a markedly increase (p < 0.05) in the level of SOD 2.28 unit/ml as paralleled to the controls.
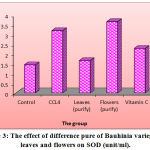 |
Figure 3: The effect of difference pure of Bauhinia variegate L. leaves and flowers on SOD (unit/ml).
|
In the table (3) and Figure (4) showed a significant increase (p < 0.05) in the levels of serum ALT than the healthy control group. The treatment of rat with leaves purify improved (p < 0.05) of elevated values. Treating rat with flowers purify in the diet was more efficient than the lower dose. Treatment with purify flowers extract (133.08±6.4 U/I) for 3 weeks showed markedly elevation (p < 0.05) in serum concentrations of ALT in paralleled to the CCL4 treated group. Additionally, the ALT levels were distinctly increase (p < 0.05) in the purify leaves extract (149.69 ± 4.40 U/I) for 3 weeks, this indicate that the effective role of purify flowers and leaves as hepatoprotective, which is in accordance with the study of Nasir.28
Biswas and his workers shown that liver enzymes levels (ALT, AST, and ALP) were significantly increase in all the groups of rats treated with different doses of diclofenac sodium as a result of injury in liver rat’s tissue.29
Table 3: Effect of purified leaves and flowers as hepatoprotective on serum ALT (U/L).
| Groups | Mean ± SD (unit/L) | ||
| Week 1 | Week 2 | Week 3 | |
| Control | 151.15 ± 2.44 b | 119.40 ± 9.77 b | 92.04 ± 0.49 b |
| CCL4 | 181.93 ± 4.39 a | 176.56 ± 17.10 a | 147.01 ± 3.17 a |
| Leaves (purify) | 157.99 ± 8.79 ab | 152.62 ± 10.75 a | 149.69 ± 4.40 a |
| Flowers (purify) | 137.23 ± 2.20 b | 150.18 ± 0.98 a | 133.08 ± 6.84 a |
| Vitamin C | 158.73 ± 8.55 ab | 153.11 ± 3.42 a | 110.85 ± 4.15 ab |
| LSD value | 25.839 * | 27.561 * | 32.677 * |
| * p < 0.05 | |||
Means bearing different letters within the same column within each period are significant at p < 0.05.
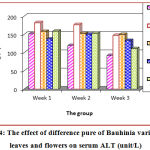 |
Figure 4: The effect of difference pure of Bauhinia variegate L. leaves and flowers on serum ALT (unit/L).
|
Rats treated by CCL4 revealed a markedly raise (p < 0.05) in serum ALP level as paralleled to control rats (Table 4) (Figure 5). While rats treated with 200 mg/kg of purified leaves extract revealed a markedly elevation (p < 0.05) in serum ALP level as paralleled to the control group, but significantly less than CCL4-only treated group. Rats treated with 200 mg/kg of purified flowers extract showed a significant (p < 0.05) decrease in serum ALP level as paralleled to the control group, but significantly it was less than CCL4-treated group.
Table 4: Effect of purified leaves and flowers as hepatoprotective on serum ALP (unit/100ml).
| Groups | Mean ± SD (unit/100 ml) | ||
| Week 1 | Week 2 | Week 3 | |
| Control | 3.04 ± 0.16 b | 2.48 ± 0.15 a | 2.35 ± 0.05 b |
| CCL4 | 3.05 ± 0.13 b | 1.21 ± 0.05 b | 3.93 ± 0.07 a |
| Leaves (purify) | 4.88 ± 0.66 a | 2.25 ± 0.03 a | 3.60 ± 0.28 a |
| Flowers (purify) | 3.44 ± 0.06 b | 2.77 ± 0.09 a | 2.43 ± 0.11 b |
| Vitamin C | 3.73 ± 0.05 b | 2.50 ± 0.22 a | 3.52 ± 0.14 a |
| LSD value | 1.037 * | 0.749 * | 0.863 * |
| * p < 0.05 | |||
Means bearing different letters within the same column within each period are significant at p < 0.05.
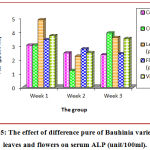 |
Figure 5: The effect of difference pure of Bauhinia variegate L. leaves and flowers on serum ALP (unit/100ml).
|
Figure (6) illustrates then in CCL4–treated group, there was a significant increase (p < 0.05) in serum AST level as paralleled to control rats (Table 5). Rats treated with 200 mg/kg of flowers purified showed a significant elevation (p < 0.05) in serum AST level as paralleled to control group. Rats treated with 200 mg/kg of leaves purified extract indicated a significant increase (p <0.05) in serum AST level as paralleled to the control group, but significantly it was less from CCL4 -treated group. Rats treated with 200 mg/kg of essential vitamin C a markedly elevation (p < 0.05) in serum AST level as paralleled to the control group, but significantly it was fewer than CCL4-treated group.
Table 5: Effect of purified leaves and flowers as hepatoprotective on serum AST (unit/L).
| Groups | Mean ± SD (unit/L) | ||
| Week 1 | Week 2 | Week 3 | |
| Control | 139.23 ± 0.41 a | 141.40 ± 0.37 a | 140.85 ± 0.48 a |
| CCL4 | 106.21 ± 6.36 b | 108.41 ± 2.68 c | 109.14 ± 0.01 b |
| Leaves (purify) | 123.56 ± 0.24 ab | 136.40 ± 4.01 a | 114.02 ± 9.28 b |
| Flowers (purify) | 119.15 ± 3.66 b | 116.22 ± 12.4 bc | 117.93 ± 1.96 b |
| Vitamin C | 114.27 ± 0.24 b | 129.38 ± 0.88 ab | 137.38 ± 2.00 a |
| LSD value | 19.448 NS | 16.209 * | 19.536 * |
| *p < 0.05 | |||
Means bearing different letters within the same column within each period are significant at p < 0.05, NS: Not significant.
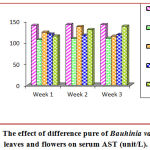 |
Figure 6: The effect of difference pure of Bauhinia variegate L. leaves and flowers on serum AST (unit/L).
|
Histopathological section in the kidney rat of normal animals (control group) showing the normal glomeruli, proximal convoluted tubules and distal convoluted tubules (Figure 7A). But when the rat treated with CCL4 the kidney section showing degeneration and necrosis of renal epithelial tubules (Figure 7B). However, when the rat treated with CCL4 and with vitamin C the kidney section tissue showing degenerative changes and necrosis of epithelial lining cells of renal tubules with congestion in between renal tubules and inside the glomeruli, and moderate inflammatory cells infiltration (Figure 7C). While in rat’s kidney treated by CCL4 and with Flowers purified shows look like normal renal tissue with mild degenerative changes of renal epithelial cells (Figure 7D). Finally the section in the kidney rat treated with CCL4 and with leaves purified shows congestion of blood vessels, degeneration of renal tubular epithelial cells (Figure 7E).
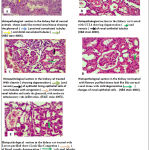 |
Figure 7: Hisptopathological sections in the kidney rat after treated with CC14 and treatment with leaves purified and flowers purified from Bauhinia variegate.
|
In liver section from each animal treated with CCL4 showed aggregation inflammatory cell particle neutrophil and macrophage in the liver parenchyma in addition to necrotic of hepatocyte which characteristic by dichotic of disappear of nuclei and also showed aggregation of inflammatory cell around a central vein (Figure 8B) as compared with control group (Figure 8A) Which shows normal structure appearance which consist of central vein and threads of hepatocyte with sinusoid. But when the rat treated with CCL4 and treatment with vitamin C shows dilatation of sinusoids with congestion of sinusoidal capillaries and Shrinkage of hepatocyte cells. (Figure 8 C). The (Figure 8D) has shown the rat lives treated with flowers purified extract look–like normal hepatic tissue which consists of central vein and threads of hepatocyte cells. Also (Figure 8E). shown section in the liver of animal treatment with Leaves purified extract shows look–like normal architecture of hepatic tissue with mild congestion and certain degeneration of hepatocyte cells. These results agree with Biswas et al., whom showed that the liver tissues histopathological changes, confirming hepatotoxicity by CCL4.29
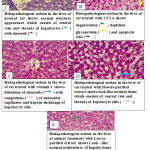 |
Figure 8: Histopathological sections in the liver of rat after treatment with CCl4, leaves purified, and flowers purified from Bauhinia variegate (H & E stain 400X).
|
The hepatotoxicity induced by CCL4 is owed to the creation of the active metabolite, trichloromethyl free radical (CCL3). This readily interrelates with molecular oxygen to form the trichloromethyl peroxy radical (CCL3OO). Both radicals are capable of binding to proteins and other macromolecules with concurrent attack on polyunsaturated fatty acids to yield lipid peroxidation foremost to hepatotoxicity.30
Shaikh et al., found that the measurement of marker enzymes like serum ALT and AST is a suitable method to screen oxidative cell injury.31 Inhibition of these raised enzymes levels detected in treated groups probably due to its compensation of bio-membranes of hepatic parenchymal cells.
All the results indicate that flavonoids presented in the plant part could be an important source of antioxidant molecules. The antioxidant capacity of flavonoids is based on their molecular structure. The hydroxyl group position and other characteristics in the chemical structure of flavonoids are more vital for their antioxidant and free radical scavenging actions. Plants phenolic in general are effective free radical scavengers and antioxidants. The remarkably strong A. nervosa leaf extract can be used as a powerful herbal antioxidant. The antioxidant activity should be regarded as an additional health promoting value for use as phytonutrients.32
Al-Sayed et al., indicated that the histological explanations established the highly hepatoprotective activity.33 The study recommend that a nutritional enhancement of BHE could employ a favorable result against oxidative stress and liver diseases by improving the antioxidant defense status, reducing lipid peroxidation, and protecting against the pathological fluctuations of the liver. Therefore, measurement the activities of serum marker enzymes like AST and ALT can make assessment of liver function and these enzymes is sensitive marker of liver injury and several fold increase in the release of these enzyme indicate severing of damage in chronic injury.
Biswas et al., shown that the phytochemical investigation of ethanolic extract of vaccaria pyrimadata medik have shown the presence of flavonoid. ethanol extract of vaccaria pyrimadata medik offers protective effect against CCL4–induced hepatotoxicity in experimental rats. The study, revealed that the group treated with CCL4 showed dramatic elevation in serum ALT, AST, ALP, (total and direct) bilirubin levels, and triglycerides levels.
Conclusions
From the current results, it can be concluded that the administration of purified PLBV and PFBV of prevented CCL4 induced elevation in different biochemical parameters representing the hepatoprotective activity of the purified leaves and flowers extract against CCL4 induced hepatotoxicity. This was also established by the result of histopathological examination, which revealed dose dependent diminish in prevalence and severity of histopathological changes.
Bauhinia variegate has differentiated pharmacological prospective and was used since ancient times. It has a strong future in the field of herbal medicine, thus the plant should be cultured in a large scale principally in unutilized and wasteland which will cooperative the financial of the farmers beside with the progress of study in the field of herbal medicine. Likewise, scientific research is requisite to discover the pharmacological impending of the plant.
Acknowledgements
We would like to thank the Genetic Engineering and Biotechnology Institute for Postgraduate Studies/ University of Baghdad for helping me to complete this research.
Conflict of Interest
There is no conflict of interest.
References
- Serafini M and Peluso I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Current Pharmaceutical Design. 2016;22(44):6701–6715.
CrossRef - Mani A. S., Prasad Y. R., Siddhanadham A. S and Aparna B. Phytochemical and antioxidant activity screening of chloroform leaf and Aerial part extracts of Tephrosia villosa. World J. of Pharmaceutical. 2017;13(9):181-184.
- Chaerunisa A. Y., Ramadhani F. N., Nurani T. D., Najihudin A., Susilawati Y and Subarnas. Hepatoprotective and antioxidant activity of the ethanol extract of Cassia fistula L. Barks. J. Pharm. Sci. & Res. 2018;10(6):1415-1417.
- Srivastava A and Shivanandappa T. Hepatoprotective effect of the root extract of Chemistry. 2010;118(2):411–417.
- Wagner H and Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16(2-3):97–110.
CrossRef - Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Molecular Nutrition & Food Research. 2008;52(5):507–526.
CrossRef - Filho V. C. Chemical composition and biological potential of plants from the genus Bauhinia,” Phytotherapy Research. 2009;23(10):1347–1354.
CrossRef - Bodakhe S. H and Ram. Hepatoprotective properties of Bauhinia variegata bark extract. The Pharmaceutical Society of Japan. 2007;127(9):1503-1507.
CrossRef - AL-Jumaily E. F and Fakhri S. M. Phytochemical Estimation of Some Active Molecules and Plant Insulin (Glucokinin) From the Bauhinia variegata Linn. Online International Interdisciplinary Research Journal. 2015;(Special Issue):1-13.
- Ozaki Y., Soedigdo S., Wattimena Y. R and Suganda A. G. Antiinflammatory effect of mace, aril of Myristica fragrans Houtt. and its active principles. Japan J. Pharmacol. 1989;49:155-163.
CrossRef - Harborne J. B. Phytochemical Methods. Chapman and Hall. London, New York. 1984.
CrossRef - Sharma K. K. An Introduction to Practical Chemistry. NewDelhi. India. 1992.
- Al-Shahaat N. A. Z. Plants and Medicinal Herbs. Dar Al-Behaar, Beirut. 1986;140-146.
- Odetola O., Adetola O., Ijadumola T., Adedeji O. Y and Adu O. A. Utilization of Moringa leaf meal as a replacement for soya bean meal in rabbit’s diets. Journal of Agricultural Science. 2012;2(12): 309313.
- Jollow D. J., Mitchell J. R., Zampoglione N., Gillete J. R. Phamacology. 1974;11:151169 .
- Shangari N. O and Brien P. J. Catalase activity assays. Current Protocols. Wiley online library. 2006.
- Malstrom B., Andreasson L and Reinhammer B. In the Enzymes. Boyer P., editor,XIIB, Academic Press: New York. 1975;533.
- Provan D and Krentz A. Oxford Handbook of Clinical and Laboratory Investigation (1st ed). Oxford. 2002;326.
- Kind P. R and King E. J. Estimation of plasma phosphatase by Determination of hydrolyzed phenol with amino-antipyrine. J. Clin. Pathol. 1954;7(4):322-326.
CrossRef - Junqueira L. C and Carneiro J. Basic Hisology, Tenth Edition. The McGraw-Hill Companies. USA. 2003.
- SAS. Statistical Analysis System, User’s Guide. Statistical. Version 9.1th ed. SAS. Inst.Inc. Cary. N.C. USA. 2012.
- Nagulendran K. R., Velavan S., Mahesh R and Begum V. h. In vitro antioxidant activity and total polyphenolic content of Cyperus rotundus rhizomes. E.J. Chem. 2007;4:440-449.
CrossRef - Al-Jumaily E. F and Al-Obaida R. S. Plasma Biochemical Parameters Effect of Flavonoids and Ethanol extract from Iraqi propolis on CCl4 –induced liver damage in mice. Asian Academic Research Journal of Multidisciplinary. 2013;1(13):244-253.
- Bhakta T., Pulok K. M., Kakali M., Banerje S., Subhash C. M., Tapan K. M., Pal M and Saha B. P. Evaluation of hepatoprotective activity of Cassia fistula leaf extract. J. Ethnopharmacol. 1999;66: 227.
CrossRef - Kuriakose G. C and Kurup M. G. Antioxidant and hepatoprotective activity of Aphanizomenon flosaquae Linn. Against paracetamol intoxication in rats. Indian Journal of Experimental Biology. 2010;48:1123-1130.
- Radovanović T. B., Mitić S. S. B., Perendija B. R., Despotović S. G., Pavlović S. Z., Cakić P. D, Saičić Z. S. Superoxide dismutase and catalase activities in the liver and muscle of barbel (Barbus barbus) and its intestinal parasite (Pomphoryinchus laevis) from the Danube river’, Arch. Biol. Sci. Belgrade. 2010;62(1):97-105.
CrossRef - Makin M. S., Adanyeguh I. M and Nwatu L. I. Hepatoprotective effect of Phyllanthus niruri aqueous extract in acetaminophen sub-acutec exposure rabbits. J. of Veterinary Medicine and Animal Health. 2013;5(1):8-15.
- Nasir A. S. Biochemical and histological evaluation of diclofenac sodium induced acute hepatotoxicity in rats. J. Pharm. Sci. & Res. 2018;10(4):733-735.
- Biswas P., Trivedi N., Singh B. K and Jha K. K. Evaluation of heptoprotective activity of ethanolic root extract of “VACCARIA PYRAMIDATA” against CCL4-induced hepatotoxicity in wister rats. European Journal of Pharm.& Med. Res. 2017;4(9):532-538.
- Zeashan H., Amresh G., Singh S and Rao C. V. Hepatoprotective activity of Amaranthus spinosus in experimental animals. J. Food Chem. Toxicol. 2008;46:3417–3421.
CrossRef - Shaikh H., Bhosle D., Shaikh A., Bhagat A., Khan S and Quazi Z. To evaluate the hepatoprotective activity of ethanolic leaf extract of Moringa oleifera plant in albino wistar rats. European J. of Pharm. & Med. Res. 2017;4(9):601-604.
- Singh D., Sati C. S and Sati D. M. Total flavonolics, phenolics, flavonoids and antioxidant evaluation in the leaves Eria alba. European J. of Pharmaceutical and Medical Research. 2017;4(11):350-353.
- Al-Sayed E., Martiskainen O., Seifel-Din S. H., Sabra A. N. A., Hammam O. A., El-Lakkany N. M and Abdel-Daim M. M. Hepatoprotective and Antioxidant Effect of Bauhinia hookeri Extract against Carbon Tetrachloride-Induced Hepatotoxicity in Mice and Characterization of Its Bioactive Compounds by HPLC-PDA-ESI-MS/MS. Bio Med Research International. 2014;1:9.







