Manuscript accepted on :24-Nov-2018
Published online on: --
Plagiarism Check: Yes
Reviewed by: Virendra S. Ligade
Second Review by: Abdullah Noohu
Amita Priya D1, Meena Kumari K2 ,Muralidhar Varma1
,Muralidhar Varma1 , Amberkar Mohanbabu V1, Shalini Adiga, Balaji O1
, Amberkar Mohanbabu V1, Shalini Adiga, Balaji O1  and Vanishree R1
and Vanishree R1
1Department of Pharmacology, Kasturba Medical College, Manipal Academy of Higher Sciences, Manipal, Karnataka, India.
2Department of Medicine, Kasturba Medical College, Manipal Academy of Higher Sciences, Manipal, Karnataka, India.
Corresponding Author E-Mail: Meena.kumari@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/1558
Abstract
Currently, the preferred treatment for chloroquine (CQ) resistant Plasmodium falciparum (Pf) is Artemisinin combination therapy (ACT). Our aim was to assess the artemisinin based treatment outcomes in patients with Plasmodium falciparum infection. Patients with falciparum infection from a tertiary health care centre in South India were enrolled in this study. It was a non-randomised observational study .The data regarding peripheral blood smear, complete blood count, liver, renal function tests and the treatment given was documented at admission and on the day of discharge. Patients with uncomplicated falciparum malaria were most common. Artesunate and doxycycline was the most common combination used at our centre (54.6%) followed by artemether –lumefantrine. All patients had peripheral smear negative for Plasmodium falciparum parasite by the end of treatment. There was improvement in blood count,liver and renal function tests. Artemisinin based combination therapy was effective in treatment of falciparum malaria.
Keywords
Artemisinin; Doxycycline; Haemoglobin; Lumefantrine; Malaria
Download this article as:| Copy the following to cite this article: Priya D. A, Kumari K. M, Varma M. Mohanbabu V. A, Adiga S, Balaji O, Vanishree R. Treatment Outcome Analysis of Artemisinin based therapy in Plasmodium falciparum infection: An observational study. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: Priya D. A, Kumari K. M, Varma M. Mohanbabu V. A, Adiga S, Balaji O, Vanishree R. Treatment Outcome Analysis of Artemisinin based therapy in Plasmodium falciparum infection: An observational study. Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=24839 |
Introduction
Mortality and morbidity associated with falciparum malaria is high , unfortunately, it has already developed resistance to most of the antimalarials. World health organisation recommended the use of multidrug therapy, a significant leap in malaria treatment to counteract the resistance of Plasmodium falciparum, improve the treatment outcomes and reduce the burden of malaria. The preferred treatment of choice is artemisinin based combination therapy (ACT).The ACTs used in India are artesunate –sulphadoxine (AS-SP) and artemether –lumefantrine for uncomplicated falciparum malaria. Artesunate-doxycycline combination are used as second line regimen for both uncomplicated and severe malaria.1
Subjects and Methods
Study Design/Site
An retroprospective observational study, performed in a single health centre at Kasturba Medical College and Hospital, Manipal. The study was conducted during 2015-2017. Study was undertaken after approval from the Institutional Ethics Committee (IEC 472/2015 and 832/2015) of Kasturba Medical College and Kasturba Hospital, Manipal.
Inclusion and Exclusion Criteria
Inclusion Criteria
An age of more than 18years of either sex
Diagnosed with falciparum malaria and were started on ACT.
Malaria was clinically confirmed by positive peripheral smear and Quantitative buffy coat(QBC), axillary temperature >99.5oF or history of fever during last 24 hours along with chills and rigors.
Exclusion criteria
Children and pregnant women
Patient allergic to ACT or doxycycline
Having received a previous treatment with ACT
Study Procedure
Data concerning patient demographics, presenting clinical symptoms, treatment and investigations were documented. Participants were required to provide a signed informed consent prior to collection of data form their respective medical records
Outcome Measures
Complete resolution of fever with a negative peripheral smear for falciparum parasite after the completion of course of treatment
Improvement or worsening in haematological parameters, liver and renal function tests from the baseline and documentation of an adverse event.
Stastical Analysis
Data was analysed using SPSS version 16. Continuous variables are expressed in mean or median with interquartile range .Both parametric and nonparametric tests were used for comparison of laboratory parameters. P value <0.05 was considered statistically significant.
Results
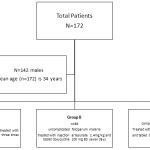
During the study period data from 172 patients was documented..Patients are divided into three groups based on the diagnosis and treatment as depicted in figure 1.The majority of the patients were male 142(82.5%). Sixty five percent of the patients belonged to the age group 20-40 years. The most common form of falciparum malaria was uncomplicated falciparum malaria . The most common symptom and sign was fever in all cases. The mean axillary temperature (0F) at baseline is 100.7±1.42. The mean axillary temperature (0F) after completion of therapy was 99.4±0.9.There was significant reduction in in comparison to the baseline (p<0.05), with no fever on discharge from the hospital. The other predominant symptoms and signs were headache, nausea, vomiting, icterus and hepatosplenomegaly (Table1).
 |
Scheme 1 |
Haematological abnormalities were present in both uncomplicated and severe falciparum malaria patients on admission (TABLE 2, 3, 4) The mean haemoglobin of patients of group A and B was 13.9±2.06and 13.05±2.18 mg/dL respectively . Haemoglobin levels <11g/dl, leukopenia and platelet count <1, 00,000 cells/cumm were observed in both groups. Serum bilirubin, liver and renal function tests were within normal limits in group A patients only. Mean haemoglobin on the day of discharge for group A and B was 12.6±1.9 and 12.1±1.9 mg/dL. Patients with Haemoglobin levels <11mg/dL increased in all groups (TABLE 5). Improvement in platelet counts was observed in all the groups. On comparing the laboratory parameters from the baseline, statistically significant differences were observed in haemoglobin, white blood cell count, platelet count, total bilirubin, serum amino transaminases, serum urea and creatinine. Except the haematological parameters, changes in total bilirubin, liver and renal function tests were just at the higher end of normal range in group A only.(TABLE 3,4)
In group B more number of patients had elevated liver and renal function tests.After the completion of therapy only few had elevated liver enzymes and renal function tests.Similar to group A patients group B patients had statistically significant differences in hematological parameters, liver and renal function tests.
In patients with complicated falciparum malaria, the mean haemoglobin on the day of admission was 12.4±2.2 (TABLE 2, 5, 6) and three patients had haemoglobin less than 11g/dL . Platelet counts less than 1, 00,000cells/cumm were observed in sixteen patients with four patients having platelet counts less than 20,000 cells/cumm. Leukopenia , leucocytosis elevated liver enzymes , urea and creatinine were observed. . Highest AST and ALT observed were 182 and 225 mg/dL respectively. Highest Alkaline phosphatase (ALP) observed was 278 mg/dL and highest total bilirubin observed was 32.50mg/dL. Abnormal serum creatinine was observed in seven patients with highest serum creatinine as 11.50mg/dL, five of them had oliguria and five patients presented with impaired consciousness. None of the patients had cerebral malaria or bleeding at the time of admission. After completion of course of treatment there was complete resolution of fever and nonspecific symptoms. Haemoglobin <11g/dl was observed in three patients (TABLE 5). Platelet counts showed improvement in all patients. Serum total bilirubin, renal function tests and liver function tests gradually improved by day 7 of discharge .Statistical significant differences were observed in haemoglobin, platelet count, total bilirubin, serum urea and serum creatinine (TABLE 7) as they were more than the normal range. Treatment was initiated from the day of admission and all patients recovered with peripheral blood smear and QBC negative for falciparum parasite on the day of discharge.
Table 1: Clinical characteristics of patients with Plasmodium falciparum infection
| Signs and symptoms | Group A | Group B | Group C | Total |
| Fever | 59 | 89 | 24 | 172 |
| Headache | 20 | 40 | 18 | 78 |
| Nausea/Vomiting | 14 | 24 | 14 | 52 |
| Abdominal pain | 03 | 14 | 14 | 31 |
| Icterus | 02 | 01 | 12 | 15 |
| Hepatospleenomegaly | 02 | 02 | 09 | 13 |
| Altered consciousness | 0 | 0 | 5 | 05 |
Table 2: Number of patients with abnormal haematological and biochemical laboratory parameters at baseline
| Laboratory parameters | Group A (n=59) | Group B(n=89)
|
Group C(n=24) | Total (n=172) |
| Haemoglobin -<11g//dl | 6 | 11 | 3 | 20 |
| White blood count -<4000cells/cumm
White blood cell counts ->11,000 cell/cumm |
14
1 |
17
2 |
1
4 |
32
7 |
| Decrease in platelet counts -<1,00,000cells/cumm | 27 | 51 | 16 | 94 |
| Elevated Total bilirubin ->3mg/dL | 0 | 13 | 13 | 26 |
| Elevated AST- >2SD | 0 | 04 | 05 | 09 |
| Elevated ALT- >2SD | 0 | 06 | 03 | 09 |
| Elevated Serum urea ->40mg/dL | 01 | 10 | 10 | 21 |
| Elevated Serum creatinine ->3mg/dL | 0 | 0 | 7 | 7 |
Group A= Uncomplicated falciparum malaria – artemether lumefantrine
Group B=Uncomplicated falciparum malaria – artesunate and doxycycline
Group C=Severe malaria receiving artesunate and doxycycline
Table 3: Comparison of laboratory parameters in patients with uncomplicated falciparum malaria (group A) treated with artemether-lumefantrine
| Parameters | Day 1(n=59)
Median (Interquartile range) |
Day 4(n=59)
Median (Interquartile range) |
| White blood cell count(cells/cumm) |
4900(4100-6100) |
6400(5400-7800)* |
|
Platelet count(cells/cumm) |
111000(76000-1,71,000) |
1,67,000(1,27,000-2,17,000)* |
|
Total bilirubin(mg/dL) |
1.2(0.8-2.0) |
0.8(0.6-1.10)* |
|
Aspartate aminotransferase (IU/L) |
39(29-56) |
30(22-43) |
|
Alanine aminotransferase(IU/L) |
44(30-65) |
38(26-52) |
|
Serum Urea(mg/dL) |
24 (18-30) |
20(16-24)* |
|
Serum creatinine (mg/dL) |
1.0(0.8-1.2) |
0.9(0.8-1.0)* |
*P value <0.05 shows statistically significant difference and Wilcoxon signed rank test done
Table 4: Comparison of Laboratory parameters in patients with uncomplicated falciparum malaria (group B) treated with artesunate and doxycycline
| Parameters | On admission (n=89)
Median (Interquartile range) |
Day 7(n=89)
Median (interquartile range) |
| White blood cell count(cells/cumm) | 5700(4200-,6850) | 5900(5200-7350)* |
| Platelet count(cells/cumm) | 88,000(66500-,1,10,000) | 1,50,000(100500-2,41,000)* |
| Total bilirubin(mg/dL) | 1.3(0.8-2.4) | 0.8(0.5-1.1)* |
| Aspartate aminotransferase (IU/L) | 42(28-60) | 33(24-50) |
| Alanine aminotransferase(IU/L) | 40(25-61) | 41(29-67)* |
| Serum Urea(mg/dL) | 25(19-33) | 20(16-25)* |
| Serum creatinine (mg/dL) | 0.9(0.8-1.2) | 0.8(0.8-1.0)* |
* P value <0.05 shows statistically significant difference and Wilcoxon signed rank test was done
Table 5: Number of patients with abnormal haematological and biochemical laboratory parameters after completing treatment
| Laboratory parameters | GroupA
(n=59) |
Group B (n=89) | GroupC (n=24) | Total (n=141) |
| Haemoglobin -<11g//dl | 09 | 16 | 11 | 36 |
| White blood count -<4000cells/cumm
White blood cell counts ->11,000 cell/cumm |
1
2 |
0
2 |
0
3 |
1
7 |
| Decrease in platelet counts <1,00,000cells/cumm | 08 | 18 | 07 | 33 |
| Elevated Total bilirubin ->3mg/dL | 0 | 0 | 01 | 01 |
| Elevated AST- >2SD | 0 | 0 | 01 | 03 |
| Elevated ALT ->2SD | 0 | 0 | 01 | 06 |
| Elevated Serum urea ->40mg/dL | 0 | 0 | 04 | 04 |
| Elevated Serum creatinine ->3mg/dL | 0 | 0 | 03 | 03 |
Group A= Uncomplicated falciparum malaria – artemether lumefantrine
Group B=Uncomplicated falciparum malaria – artesunate and doxycycline
Group C=Severe malaria receiving artesunate and doxycycline
Table 6: Comparison of Laboratory parameters in complicated falciparum malaria (Group C) treated with artesunate and doxycycline
| Parameters | Day 1(n=24) | Day 7(n=24)
Mean ± S.D /Median(IQR) |
| Mean±SD | Mean±SD | |
| Haemoglobin(g/dL) | 12.7±2.37 | 11.5±2.5*a |
| Median (interquartile range) | Median (interquartile range) | |
| White blood cell count(cells/cumm) | 6450(4325-9550) | 7250(6000-8725) |
| Platelet count(cells/cumm) | 52000(24250-97750) | 153500(932500,213000)*b |
| Total bilirubin(mg/dL) | 4.65(2.0-6.25) | 1.35(0.6-1.9)*b |
| Aspartate aminotransferase (IU/L) | 60(41-90) | 39(28-54) |
| Alanine aminotransferase(IU/L) | 43.5(28.7- 78.2) | 40(28-59) |
| Alkaline phosphatase(IU/L) | 109.5(87.7,137.5) | 104(83.2,125.75) |
| Serum Urea(mg/dL) | 43..5( 21 -83.7) | 28(18 -44)*b |
| Serum creatinine (mg/dL) | 1.7(0.9,3.9) | 0.95(0.8-1.1)*b |
*P value <0.05 shows statistically significant difference
a Paired ‘t’ test
b Wilcoxon signed rank test
Discussion
First ACT that was registered for use in India was AS – SP. Currently, it serves to be the first line treatment for uncomplicated falciparum malaria in all areas where resistance to chloroquine is consistent. In 2010, this combination was made universal all over India as CQ resistance was widespread. Artemether-lumefantrine is another combination that is available 2. Other combinations are AS and Amodiaquine, AS and Mefloquine, Dihydroartemisinin and Piperaquine. Global reduction in malaria has been made possible by their use.2 Medications to treat malaria are a handful. The slow pace of new drug and preventive vaccines development against falciparum malaria, the only hope seems to be the artemisinin derivatives.
In our study, falciparum malaria infection was more common in males and more in the 3rd and 4th decade of life. Decrease in haemoglobin levels at baseline in all patients is because of the parasite infecting the red blood cells. The infected RBC undergo destruction in the reticuloendothelial cells. After completion of treatment with artemisinin derivatives the number of patients with decreased haemoglobin (<11g/dl) increased, showing that drug therapy cannot prevent the destruction of infected red blood cells.3Similarly decrease in platelet count could be because of peripheral pooling, splenic sequestration, disseminated intravascular coagulation, immunoglobulin -G mediated and platelet clumping.3There is significant increase in platelet counts after the therapy was completed. Therefore, by rapid clearance of parasites, further decrease in platelet counts is prevented with the help of artemisinin derivatives. Both leukopenia and leucocytosis were observed at the baseline, however counts returned to normal once treatment was completed.The haematological alterations observed in our study are consistent with the observations in other studies.3
The liver function tests and renal function tests in complicated falciparum malaria patients were elevated at baseline, however majority of them recovered on the day of discharge. None of the patients had worsening of both liver and renal function tests during therapy or after the completion. This is similar to observations made in seven patients with severe falciparum malaria, treated with intravenous AS and intravenous doxycycline in Norway.4
In India, continuous drug pressure since the use of ACT containing SP, resistant strains have developed in certain geographical regions. Under the National Drug Policy, AS-SP combination has been made the first line treatment for uncomplicated falciparum malaria in high endemic areas.2 It should not be used in North-eastern states because of high resistance to SP. As this study was carried out in a tertiary health centre located in Dakshina Kannada district where malaria is prevalent all throughout year, use of SP predisposes the parasites to develop resistance.5 The use of AS and mefloquine combination in the past has resulted in neuropsychiatric adverse effects at our health care centre that led to the preferential use of artemether and lumefantrine for uncomplicated falciparum malaria.
Artemether with lumefantrine, a popular ACT regimen has been proven to be efficacious for the treatment of uncomplicated falciparum malaria.6,7,8,9 It was registered as a fixed dose combination by WHO in 2001. In the same year these co-formulated tablets containing 20 mg of artemether and 120 mg of lumefantrine were available. Using Polymerase chain reaction for malaria diagnosis, studies carried out in India using this combination documented cure rates of 98.4% and 98.6% respectively.10
Use of antibiotics as antimalarials is becoming pertinent especially when malaria continues to be a significant health problem and the resistance has developed to most of the drugs, thereby limiting the availability of potential new antimalarial drugs. Most common class of antibiotics that have been studied for their antimalarial properties are tetracycline and macrolides. Tetracyclines, are a family of antibiotics long known to inhibit the protein synthesis of bacteria and were active against all three asexual stages of Pf11. Initially tetracycline were shown to be effective as monotherapy for uncomplicated falciparum malaria, later a standard treatment was given for zones with multi resistance Pf which included the use of doxycycline along with quinine for 7 days. This bi- therapy has a therapeutic efficacy of 91-100% when given for 7 days .In 1985, WHO recommended 100 mg doxycycline every day from the first day in endemic areas upto four weeks after return as chemoprophylaxis.11
In a randomised controlled trial, wherein Artesunate and doxycycline and Artesunate and mefloquine were used to treat patients with uncomplicated falciparum malaria, it was observed that the Artesunate and doxycycline combination was more efficacious and safer than the latter.12A quasi experimental study carried out in Karachi, patients with uncomplicated falciparum malaria were given quinine and doxycycline as first line therapy because of cheap cost and ease of availability. The treatment was well tolerated with a mean parasite clearance time of 3 days.13 Doxycycline is also used in combination with SP, mefloquine and AS in the treatment of uncomplicated malaria. Doxycycline must be always used in combination with another antimalarial for treatment of malaria because of its slow schizonticidal action and the risk of uncomplicated malaria turning into severe malaria. It is most commonly given along with quinine as it reduces the likelihood of resistance and efficacious in killing the malarial parasite. This combination has its drawbacks in terms of patients adherence because of adverse effects to quinine and frequent dosing. Therefore, these limit the use of this combination as first line therapy although being cheaper compared to artemisinin derivatives.11,13
At our hospital setting both the uncomplicated and complicated falciparum patients received a combination of artesunate and doxycycline and all patients recovered clinically and had a negative peripheral smear on the day of discharge. This study has limitations. It is an observational non comparative study. It reinforces the dictum that artemisinin derivatives are the most effective antimalarials of choice currently available. Artesunate and doxycycline as second line regimens can be used to treat patients with uncomplicated falciparum malaria and severe malaria in endemic areas till better combinations are available.
Acknowledgement
We are thankful to the medical record department of Kasturba Hospital for providing data for retrospective analysis part of the study.
Conflict of interest
Nil
Funding source
It was a non-funded project
References
- Mohapatra B. N., Mohanty C. B. Antimalarial combination-current clinical practices. Editor Singal RK, Medicine update, Bombay. Association of Physicians of India. 2007;17:649-51.
- Anvikar A. R., Arora U., Sonal G. S., Mishra N., Shahi B., Savargaonkar D., et al.Antimalarial drug policy in India Past, present & future.Indian J Med Res. 2014;139:205–215.
- Kotepui M., Phunphuech B., Phiwklam N., Chupeerach C., Duangmano S. Effect of malarial infection on haematological parameters in population near Thailand-Myanmar border. Malar J. 2014;13:218.
CrossRef - Morch K., Strand O., Dunlop O., Berg Å et al. Severe malaria and artesunate treatmen. Emerg Infect Dis. 2008;14:1816–1818.
CrossRef - Dayanand K. K., Punnath K., Chandrashekar V et al. Malaria prevalence in Mangaluru city area in the southwestern coastal region of India. Malar J. 2017;1:492.
CrossRef - Eshetu T., Abdo N., Bedru K. H., et al. Open-label trial with artemether-lumefantrine against uncomplicated Plasmodium falciparum malaria three years after its broad introduction in Jimma Zone, Ethiopia. Malar J. 2012;11:240.
CrossRef - .Kinfu G. Therapeutic Efficacy of Artemether–Lumefantrine for the treatment of uncomplicated Plasmodium Falciparum Malaria in Northern Ethiopia. In. Malar Res Treat. 2012;5:84-92.
- .Elamin S. B., Awad A. I., Eltayeb I. B., et al. Descriptive study on the efficacy of artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Sudan. Eur J Clin Pharmacol. 2010;66:231-7.
CrossRef - Joseph D., Kabanywanyi A. M., Hulser R., et al. Exploration of in vivo efficacy of artemether-lumefantrine against uncomplicated Plasmodium falciparum malaria in under-fives in Tabora region, Tanzania. Malar J. 2013;12:60.
CrossRef - Valecha N., Srivastava P., Mohanty S. S., Mittra P., Sharma S. K., Tyagi P. K.,et al. Therapeutic efficacy of artemether-lumefantrine in uncomplicated falciparum malaria in India. Malar J. 2009;8:107.
CrossRef - Gaillard T., Madamet M., Pradines B. Tetracyclines in malaria. Malar J. 2015;14:445.
CrossRef - Looareesuwan S., Viravan C., Vanijanonta S., et al. Randomized trial of mefloquine-doxycycline, and artesunate-doxycycline for treatment of acute uncomplicated falciparum malaria. Am J Trop Med Hyg. 1994;50:784-9.
CrossRef - Ejaz A., Haqnawaz K., Hussain Z., Butt R., Awan Z. I., Bux H. Treatment of uncomplicated plasmodium falciparum malaria with quinine-doxycycline combination therapy.J Pak Med Assoc. 2007;57:502-5.







